Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11853
Peer-review started: May 11, 2022
First decision: June 27, 2022
Revised: July 12, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: November 16, 2022
Processing time: 181 Days and 1.1 Hours
Inflammatory myofibroblastic tumor in the liver (IMTL) is a rare borderline mesenchymal tumor. Neither clinical symptoms nor laboratory tests have absolute specificity for the diagnosis of IMTL, and imaging also lacks obvious specificity. Although there are sporadic reports of recurrence after surgical treatment, surgical resection is the mainstay of treatment.
A 29-year-old man complained of general weakness, slight discomfort in the upper abdomen, with a history of upper respiratory tract infection for 1 wk before admission. Plain and enhanced upper abdominal magnetic resonance imaging showed a mass in liver segments II and III (48 mm × 53 mm). He was treated by laparoscopic left lateral segmentectomy. Postoperative pathological examination with hematoxylin and eosin staining suggested that the mass in liver segments II and III was IMTL. During 21 mo postoperative follow-up, no obvious residual or recurrent lesions were observed.
There is a risk of malignant degeneration in IMTL. The principal choice of treatment is laparoscopic left lateral segmentectomy.
Core tip: Inflammatory myofibroblastic tumor (IMT) is a rare borderline mesenchymal tumor with myofibroblastic proliferation and varying number of inflammatory cells that can occur in the lungs, stomach, intestines, gallbladder and nervous system. We present a rare case of IMT in the liver (IMTL) and its treatment with laparoscopic left lateral segmentectomy. This was a rare case of IMTL that was difficult to diagnose and treat, which was unresponsive to pharmacological treatment. This case highlights the importance of surgery for IMTL located in segments II and III. Laparoscopic treatment of IMTL is effective and minimally invasive.
- Citation: Li YY, Zang JF, Zhang C. Laparoscopic treatment of inflammatory myofibroblastic tumor in liver: A case report. World J Clin Cases 2022; 10(32): 11853-11860
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11853.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11853
Inflammatory myofibroblastic tumor (IMT) was first found in the lungs[1] and then in the stomach, intestines, gallbladder and nervous system. The first case of IMT in the liver (IMTL) was found by Pack and Baker in 1953[2]. The World Health Organization defined IMTL in 2021, according to its pathological characteristics, as a borderline stromal tumor infiltrated by plasma cells and lymphocytes that is often composed of spindle-shaped differentiated myofibroblasts[3]. IMTL can occur in children and young adults[4,5], but it is more common in young adults. The average age of onset is about 37 years[6,7]. IMTL is a rare disease. Since Li et al[8] reported it in China in 1989, few other cases have been reported. Its clinical manifestations and imaging features are not specific. These factors increase the difficulty of clinical diagnosis and misdiagnosis rate. Currently, complete resection of the tumor, followed by pathological diagnosis of tissue specimens is recommended as the mainstay therapeutic modality[9]. Although there are sporadic reports of recurrence after surgical treatment[6], most patients have a good prognosis without recurrence or obvious complications. We report the case of a 29-year-old man with IMTL. He was treated by laparoscopic left lateral segmentectomy and the postoperative diagnosis was IMTL after pathological diagnosis. During 21 mo postoperative follow-up, no obvious abnormalities were observed.
A 29-year-old man complained of general weakness, slight discomfort in the upper abdomen, and there was no obvious tenderness and mass in the abdomen on the physical examination.
No symptoms related to the hepatic mass were found, and no related treatment or management had been performed.
He had a history of upper respiratory tract infection for 1 wk before admission. He denied a history of other diseases, trauma or surgery.
There was no abnormality in his medical history, such as surgical trauma, allergy, personal and family history, and history of hepatitis.
The physical examination of the abdomen revealed no abnormalities. The patient had a flat abdomen with no pigmentation of the abdominal skin. The patient had no abdominal tenderness or rebound pain, and no palpable abdominal mass.
Laboratory tests after admission were as follows. Routine blood tests: white blood cell (WBC) count (13.58 × 109/L, normal range: 3.5–9.5), platelet count (568 × 109/L, normal range: 125–350) and C-reactive protein (CRP) (115.6 mg/L, normal range: 0–10). Tumor markers: -fetoprotein (AFP) (1.21 ng/mL, normal range: 0.89–8.78), abnormal-prothrombin (APT) (19.31 mAU/mL, normal range: 11.12–32.01), carcinoembryonic antigen (CEA) (3.81 ng/mL, normal range: 0–5), carbohydrate antigen (CA)125 (16.60 U/L, normal range: 0–35), and CA19-9 (3.94 U/L, normal range: 0–43). Hepatitis virus test was negative. IgG was normal (13.20 g/L, normal range: 7.0–16.0).
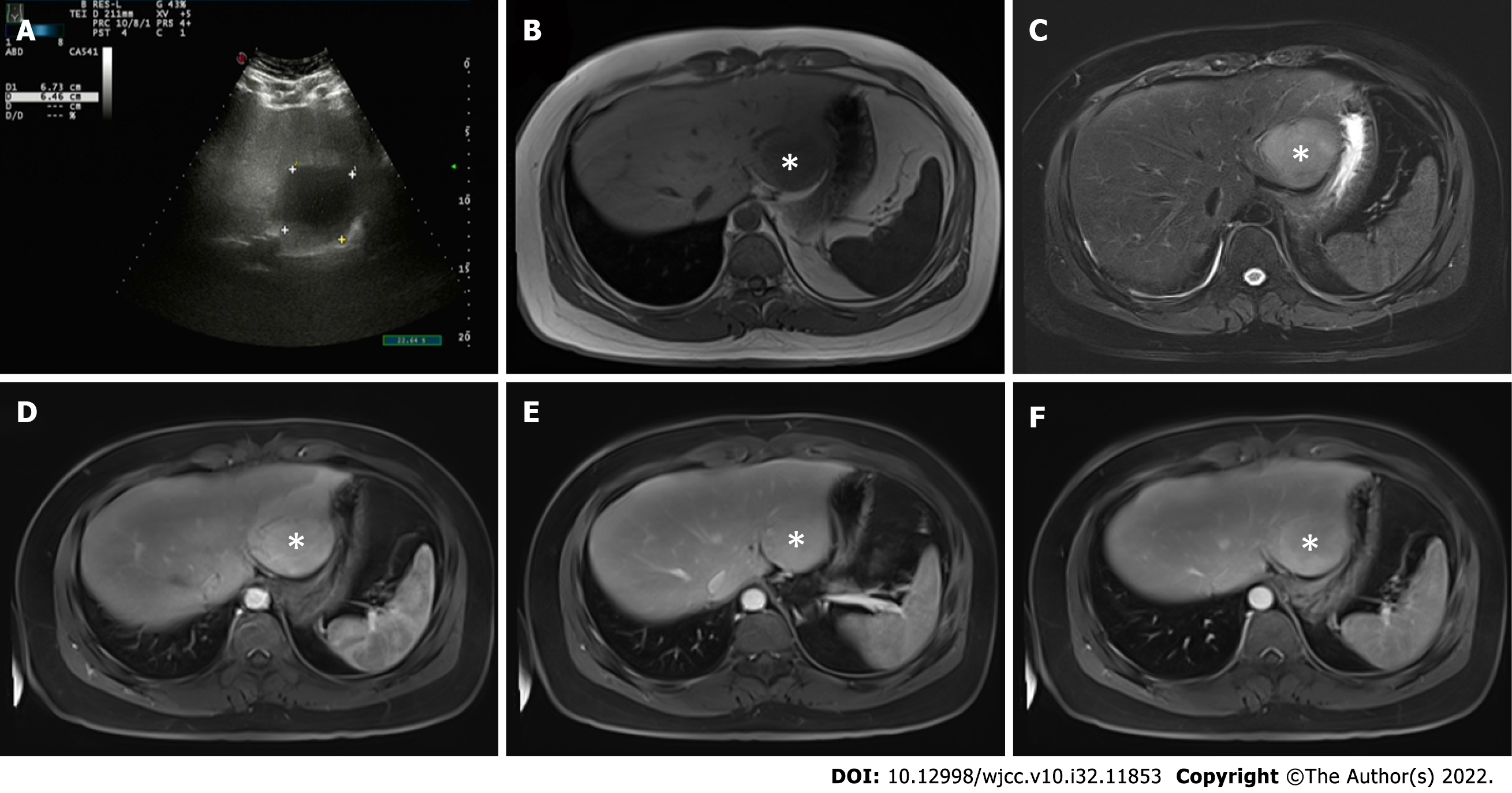
Abdominal ultrasound (Figure 1A) showed that the liver capsule was intact, and a low-echo area of 67 mm × 64 mm could be seen in segments II and III. Plain and enhanced upper abdominal magnetic resonance imaging (MRI) showed that T1-weighted imaging of liver segments II and III (48 mm × 53 mm) was low, and T2-weighted imaging revealed medium and high signal shadows. The boundary was clear and smooth. The lesions in the enhanced arterial phase were significantly enhanced. Some signals in the lesions in the venous phase were reduced, and showed uneven enhancement after delay. There was obvious patchy enhancement in the liver at the edge of the lesions in the arterial phase. The enhanced signal decreased slightly (Figure 1B–F). Abdominal ultrasound showed a low-echo area of 67 mm × 64mm in segments II and III of the liver.
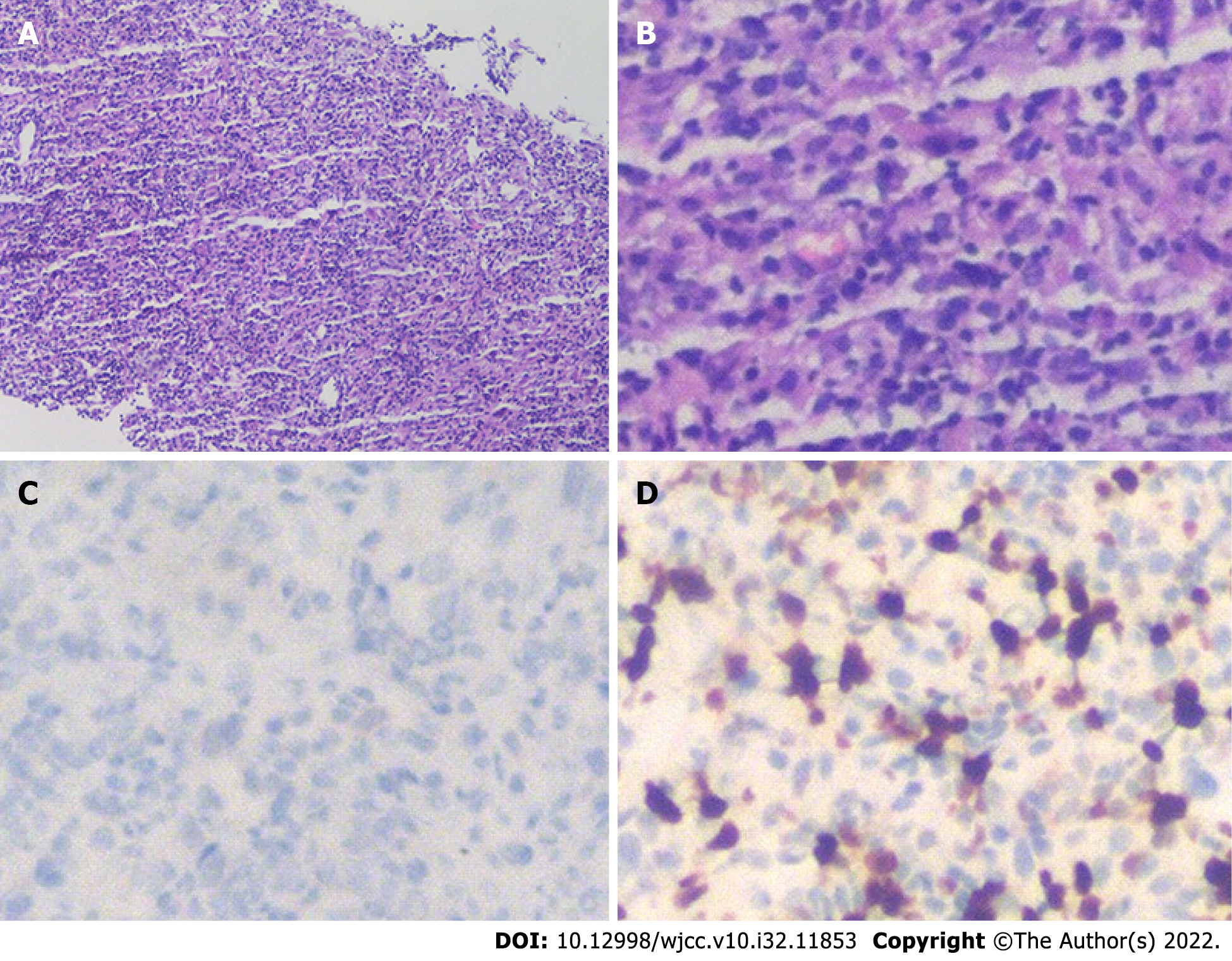
To evaluate further the nature of the mass, liver biopsy was performed under ultrasound guidance. The mass was composed of inflammatory cells, myofibroblasts, spindle cells and plasma cells (Figure 2). Routine hematoxylin and eosin (HE) staining and immunohistochemistry showed that there was no obvious monoclonal proliferation of cells and suggested IMTL, with the following manifestations: anaplastic lymphoma kinase (ALK): D5F3+/, endomysial antibody partial +, CD20 lymphocytes partial +, CD79 α lymphocytes partial +, CD38 plasma cells +, interferon regulator 4 partial +, CD3 lymphocytes partial +, CD5 lymphocytes partial +, CD43+, CD138 plasma cells +, κ partial +, λ partial +, desmin, vimentin+, and Ki-67+.
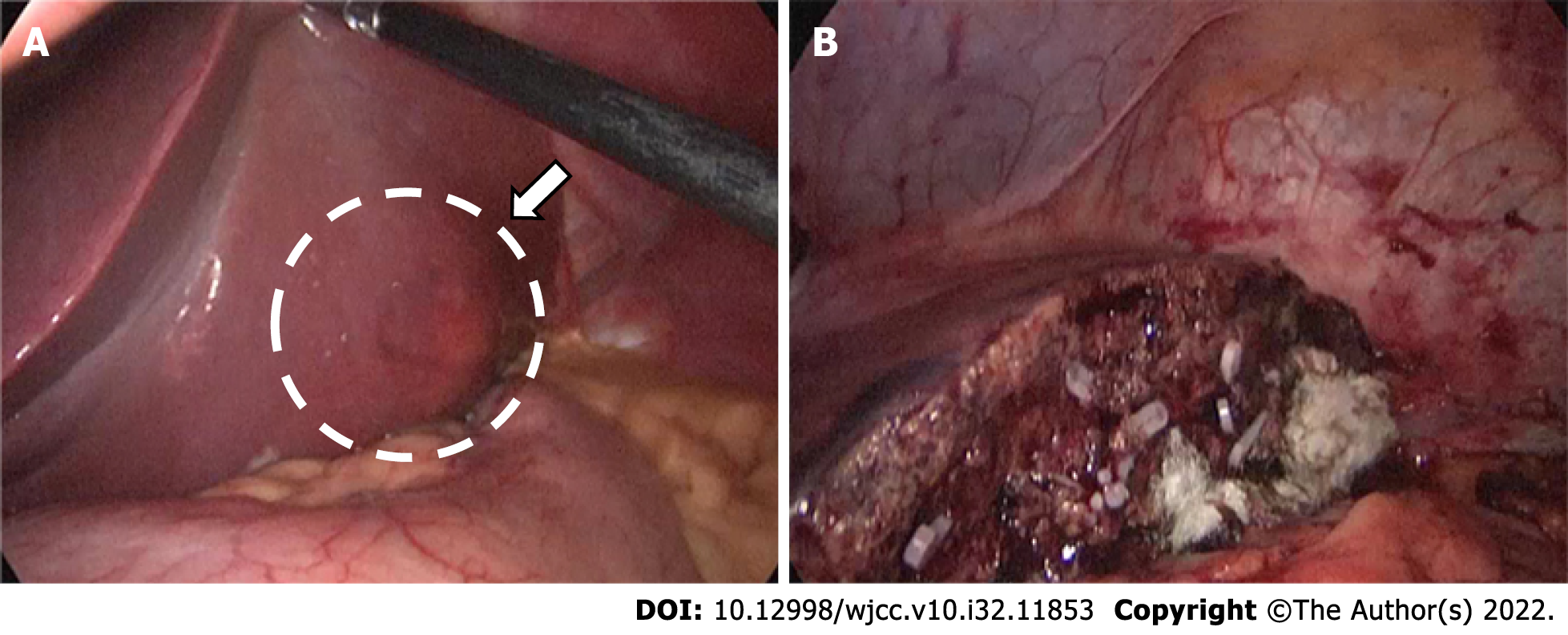
Preoperative treatment of the patient’s disease using prednisone acetate was ineffective. After symptomatic treatment, fatigue improved slightly, and MRI showed no significant changes in the tumor. Laparoscopic left lateral segmentectomy was performed under general anesthesia. During the operation, a nodular bulge with a diameter of about 5 cm was seen on the visceral surface of liver segments II and III (Figure 3). The operation lasted 3.5 h, and intraoperative blood loss was 500 mL. During the operation, the Pringle maneuver (clamping the hepatoduodenal ligament) was performed three times, at intervals of 10 min, each time lasting 15 min.
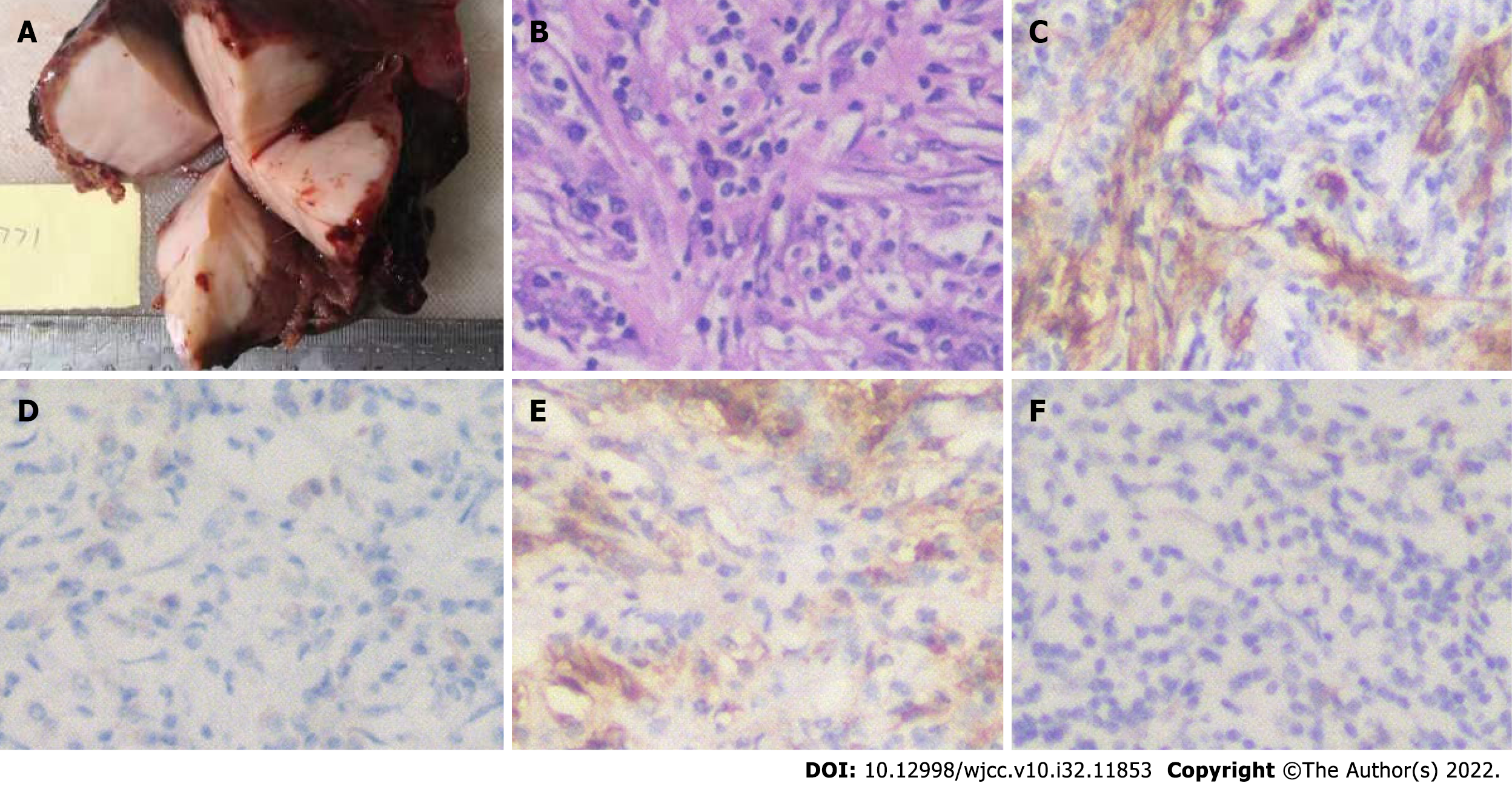
Postoperative pathological examination with HE staining suggested that the mass in liver segments II and III was IMTL. The size of the tumor was 6.5 cm × 6.2 cm × 5.0 cm, without a capsule, and showed expansive and invasive growth. CRP level on postoperative day 1 was 96.9 mg/L. Immunohistochemistry showed that tumor cells had smooth muscle actin+, vimentin+, ALK+/, desmin, CD68+ and CD163+ in tissue cells, blood vessels CD34+, plasma cells CD38+, CD138+, κ+, λ+ and Ki-67+ (Figure 4).
Postoperative diagnosis was IMTL.
The patient recovered well and was discharged 7 d after surgery. During 21 mo postoperative follow-up, no obvious abnormalities were observed in blood routine examination (CRP: 0.499 mg/L), liver function, tumor markers and abnormal prothrombin. Abdominal MRI showed postoperative changes without residual or recurrent lesions. The follow-up is continuing. At present, the patient is in good condition, and there is no obvious tumor recurrence or metastasis.
The most common site of IMT is the lungs. The incidence outside the lungs and in the liver is about 8%[10]. IMTL is a rare borderline tumor[11]. In recent years, IMTL has been simply divided into lymphoplasmacytic and fibrous histiocytic types[12]. The lymphoplasmacytic type is defined as a large number of lymphoplasmacytes and eosinophils infiltrating around the hilar region. The fibrous histiocytic type is characterized by mass formation in the liver parenchyma or inflammation of yellow granuloma, and some refer to macrophage and neutrophil infiltration. The pathological diagnosis of this patient was inflammatory fibrous proliferative tumor like lesions in segments II and III of the liver, with a large amount of plasma cell infiltration, which roughly accorded with the lymphoplasmacytic type. The most standard classification is to divide IMTL into three types: I, mucinous vasculitis; II, fibrous tissue spindle cell type; and III, collagen fiber type[13]. In the present case, the tumor was accompanied by plasma cell and inflammatory cell infiltration, which is consistent with type II. Histological or pathological typing of IMTL is helpful in determining the type of IMTL, and plays an important role in identifying the risk factors of tumorigenesis and early pathological diagnosis of the disease. At the same time, ALK expression is found in about 50% of IMTL patients, and IMTL is more likely to occur in tissues with positive ALK expression. That means that in IMTL, ALK-negative tumors have higher malignancy, invasive ability and metastatic rate than ALK-positive tumors have.
The etiology and risk factors of IMTL are unclear. At present, the following factors are considered to be related to IMTL. (1) Infection with Escherichia coli, Staphylococcus and some Gram-positive bacteria can cause the release of inflammatory mediators, gradually resulting in fibrous tissue lesions, and eventually forming IMTL[14]. (2) Some trauma and surgery may cause inflammatory reactions and then formation of IMTL. (3) Some low-grade fibrous nodules or fibrous components with inflammatory cell infiltration may be transformed into IMTL[15]. (4) Autoimmunity: plasma cells that are IgG positive in the tumor foci, especially IgG4, are increased in IMTL patients[10,16]. Research on the relationship between IMTL and IgG4 is being carried out[17]. (5) Genes: ALK is associated with IMTL, but the exact relationship between them has not been reported[18].
The present patient was a young man with a history of upper respiratory tract infection before admission, which further indicates that IMTL might be related to infection. Clinical symptoms in IMTL patients included abdominal pain and even jaundice[19]. Some patients have fever, abdominal pain and other combined symptoms[20], but about 20% of patients are asymptomatic[21]. The admission examination did not show increased IgG, indicating that it was unhelpful for diagnosis. Laboratory examination may reveal inflammatory indicators, such as increased WBC count, CRP and erythrocyte sedimentation rate, while the liver-function-related enzymes and the tumor markers have no obvious abnormalities[6]. The tumor markers in this patient were normal, and WBC count and CRP were increased. Neither clinical symptoms nor laboratory tests have absolute specificity for the diagnosis of IMTL, thus increasing the difficulty with the choice of diagnosis. Computed tomography (CT), MRI, ultrasound and even positron emission tomography/CT can assist in diagnosis[22], but imaging also lacks obvious specificity. Contrast-enhanced ultrasound showed a variety of echoes in the arterial, portal and delayed phases. There are different manifestations in the case of hyperechogenicity in the three phases, such as overall uniform hyperechogenicity, edge encapsulated hyperechogenicity, or thin line ring hyperechogenicity[23]. The CT findings of IMTL show low-density shadows, but uniform or uneven enhancement, and peripheral and central enhancement can also appear. Similarly, in MRI, the signal of IMTL in T1 is lower than that in surrounding normal liver tissue, and the signal in T2 is equal or higher, without obvious specificity.
Because there is no obvious specificity in clinical manifestations, laboratory and imaging examination, IMTL is difficult to diagnose and determine the treatment. At present, antibiotics, nonsteroidal anti-inflammatory drugs and corticosteroids can be used for treatment of IMTL[24,25], but the best treatment regimen has not been determined. Some researchers have suggested that in case of abnormal liver function, surgical resection should be performed as soon as possible[26]. In addition, there is a risk of malignant degeneration[27]; therefore, it is accepted that the preferred treatment is still early surgical resection, which has a satisfactory outcome and good prognosis. Considering that there are a few reports of postoperative recurrence[6], regular follow-up in case of the disease recurrence or related complications is necessary.
There is a risk of malignant degeneration in IMTL; therefore, early diagnosis and treatment are required. Currently, as the mainstay therapeutic method, complete resection of the tumor is recommended. Laparoscopic left lateral segmentectomy should be the principal choice of treatment in patients with IMTL.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta R, India; Wang CC, Taiwan S-Editor: Chen YL L-Editor: Kerr C P-Editor: Chen YL
| 1. | Copin MC, Gosselin BH, Ribet ME. Plasma cell granuloma of the lung: difficulties in diagnosis and prognosis. Ann Thorac Surg. 1996;61:1477-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | PACK GT, BAKER HW. Total right hepatic lobectomy; report of a case. Ann Surg. 1953;138:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 240] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv Anat Pathol. 2021;28:44-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 252] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 4. | Al-Hussaini H, Azouz H, Abu-Zaid A. Hepatic inflammatory pseudotumor presenting in an 8-year-old boy: A case report and review of literature. World J Gastroenterol. 2015;21:8730-8738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Thavamani A, Mandelia C, Anderson PM, Radhakrishnan K. Pediatric Inflammatory Myofibroblastic Tumor of the Liver: A Rare Cause of Portal Hypertension. ACG Case Rep J. 2019;6:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Chang SD, Scali EP, Abrahams Z, Tha S, Yoshida EM. Inflammatory pseudotumor of the liver: a rare case of recurrence following surgical resection. J Radiol Case Rep. 2014;8:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Sürer E, Bozova S, Gökhan GA, Gürkan A, Elpek GO. Inflammatory myofibroblastic tumor of the liver: a case report. Turk J Gastroenterol. 2009;20:129-134. [PubMed] |
| 8. | Li GH, Li JQ, Lin YZ. Inflammatory pseudotumor of the liver. J Surg Oncol. 1989;42:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Saleem MI, Ben-Hamida MA, Barrett AM, Bunn SK, Huntley L, Wood KM, Yelbuz TM. Lower abdominal inflammatory myofibroblastic tumor -an unusual presentation- a case report and brief literature review. Eur J Pediatr. 2007;166:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Patel H, Nanavati S, Ha J, Shah A, Baddoura W. Spontaneous Resolution of IgG4-Related Hepatic Inflammatory Pseudotumor Mimicking Malignancy. Case Rep Gastroenterol. 2018;12:311-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Zhou P, Chen YH, Lu JH, Jin CC, Xu XH, Gong XH. Inflammatory myofibroblastic tumor after breast prosthesis: A case report and literature review. World J Clin Cases. 2022;10:1432-1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Yamaguchi J, Sakamoto Y, Sano T, Shimada K, Kosuge T. Spontaneous regression of inflammatory pseudotumor of the liver: report of three cases. Surg Today. 2007;37:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1030] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 14. | Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 624] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 15. | Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, Koniaris LG. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Yamamoto H, Yamaguchi H, Aishima S, Oda Y, Kohashi K, Oshiro Y, Tsuneyoshi M. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. Am J Surg Pathol. 2009;33:1330-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Legkiy O, Wajda J, Ćwierz A, Wysocka J, Komorowski AL. Hepatic inflammatory pseudotumor related with IgG4. Gastroenterol Hepatol. 2019;42:176-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Vroobel K, Judson I, Dainton M, McCormick A, Fisher C, Thway K. ALK-positive inflammatory myofibroblastic tumor harboring ALK gene rearrangement, occurring after allogeneic stem cell transplant in an adult male. Pathol Res Pract. 2016;212:743-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Arora KS, Anderson MA, Neyaz A, Yilmaz O, Pankaj A, Ferrone CR, Zen Y, England J, Deshpande V. Fibrohistiocytic Variant of Hepatic Pseudotumor: An Antibiotic Responsive Tumefactive Lesion. Am J Surg Pathol. 2021;45:1314-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Zhao J, Olino K, Low LE, Qiu S, Stevenson HL. Hepatic Inflammatory Pseudotumor: An Important Differential Diagnosis in Patients With a History of Previous Biliary Procedures. ACG Case Rep J. 2019;6:e00015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Yin L, Zhu B, Lu XY, Lau WY, Zhang YJ. Misdiagnosing hepatic inflammatory pseudotumor as hepatocellular carcinoma: A case report. JGH Open. 2017;1:76-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Liao M, Wang C, Zhang B, Jiang Q, Liu J, Liao J. Distinguishing Hepatocellular Carcinoma From Hepatic Inflammatory Pseudotumor Using a Nomogram Based on Contrast-Enhanced Ultrasound. Front Oncol. 2021;11:737099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Chougule A, Bal A. IgG4-related inflammatory pseudotumor: A systematic review of histopathological features of reported cases. Mod Rheumatol. 2017;27:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Ballester-Pla N, García-Domínguez R, Pérez-Girbes A, Orbis-Castellanos JF, Pareja E. Conservative treatment of hepatic inflammatory pseudotumor. Cir Esp. 2016;94:422-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Imazu N, Shibata M, Koya Y, Morino K, Honma Y, Senju M, Watanabe T, Harada M. Hepatic Inflammatory Pseudotumor Protruding from the Liver Surface and Directly Penetrating the Colon. Intern Med. 2020;59:527-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Zhang GH, Guo XY, Liang GZ, Wang Q. Kidney inflammatory myofibroblastic tumor masquerading as metastatic malignancy: A case report and literature review. World J Clin Cases. 2019;7:4366-4376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |