Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10742
Peer-review started: May 31, 2022
First decision: June 27, 2022
Revised: July 9, 2022
Accepted: August 23, 2022
Article in press: August 23, 2022
Published online: October 16, 2022
Processing time: 121 Days and 3.2 Hours
Vitamin K1 (phytomenadione) is a fat-soluble naturally occurring vitamin that is widely used to treat certain coagulation disorders. Adverse cutaneous reactions to vitamin K1 can occur; however, owing to its low incidence and considerable variability in presentation and morphology, its diagnosis can be easily over
Here we report the case of a 50-year-old woman with no pre-existing hepatic disease who developed a cutaneous allergic reaction to subcutaneous vitamin K1 that presented as localized eczematous plaques at the vitamin K1 injection site. The eruption developed within 5 d of the injection and persisted for 32 mo despite treatment with topical and intralesional steroids. Eczema was diagnosed based on the results of the pathological examination, immunohistochemical staining, and a skin biopsy. The patient was advised to take herbal medicines orally twice daily. After treatment and follow-up, the patient’s eczematous urticarial plaques improved and her condition stabilized.
Here we present the first case of a cutaneous allergic reaction to subcutaneous vitamin K1 that was successfully treated with Chinese medicine.
Core Tip: Adverse cutaneous reactions to vitamin K1 can occur; however, their diagnosis can easily be overlooked. Managing these reactions may be challenging for both patients and clinicians. Here we report a patient who developed a cutaneous allergic reaction to subcutaneous vitamin K1 that was successfully treated with herbal medicine, suggesting that Chinese medicine therapy may be a feasible way to treat adverse cutaneous reactions. We confirmed our clinical findings through physical examination, a skin biopsy, and immunohistochemical staining. We also conducted a literature review of eczematous-type reactions to clarify the possible pathogenesis and provoke new thinking regarding the clinical treatment of this disease.
- Citation: Zhang M, Chen J, Wang CX, Lin NX, Li X. Cutaneous allergic reaction to subcutaneous vitamin K1: A case report and review of literature. World J Clin Cases 2022; 10(29): 10742-10754
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10742.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10742
Vitamin K1, currently available for oral, intramuscular, subcutaneous, or intravenous administration, is generally well tolerated[1]. However, adverse effects of vitamin K1 have rarely been observed and were mostly reported in the early 1960s in the European literature. The most important adverse effects of vitamin K1 are hyperbilirubinemia and kernicterus in newborn infants[2], particularly in premature infants resulting from the competition for glucuronyl transferase in the presence of immature liver function[3].
Based on to the characteristics of skin rashes, three types of adverse events can be caused by vitamin K1 injection: (1) Localized pruritic erythematous, eczematous, indurated plaques (most common); (2) localized morphea-form eruptions (secondary to the eczematous type[4-8]); and (3) diffuse maculopapular eruptions (very rare), which were reported in early studies[9-11]. In accordance with the case report guidelines[12], here we present a case of a patient who developed an eczematous-type reaction to vitamin K1 injection. Residual pigmentation persisted after 18 mo of treatment; however, the patient responded well to treatment with Chinese medicine (CM).
Cutaneous reactions caused by vitamin K1 injections are mainly treated symptomatically. Most patients are treated with topical corticosteroids with or without occlusion and intralesional steroids; however, these treatments are ineffective. The topical application of tacrolimus (FK-506), a potent interleukin-2 and T-cell activation inhibitor, may become a future treatment option. Lauerma[13] demonstrated that topical tacrolimus pretreatment effectively suppresses allergic contact dermatitis caused by dinitrobutylphenol. However, its use for treating cutaneous hypersensitivity reactions to vitamin K1 has not been reported.
Some researchers believe that the exacerbation of persistent eruptions may be related to dietary vitamin K. However, no follow-up investigation of this possibility is available in the English literature. This is probably due to difficulty avoiding dietary vitamin K1, as small quantities are present in many different foods, with high concentrations in green leafy vegetables. Our patient failed to maintain a low vitamin K1 diet due to food preferences. Hence, whether the minute quantities of vitamin K1 present in some foods preclude the efficacy of dietary therapy remains unknown.
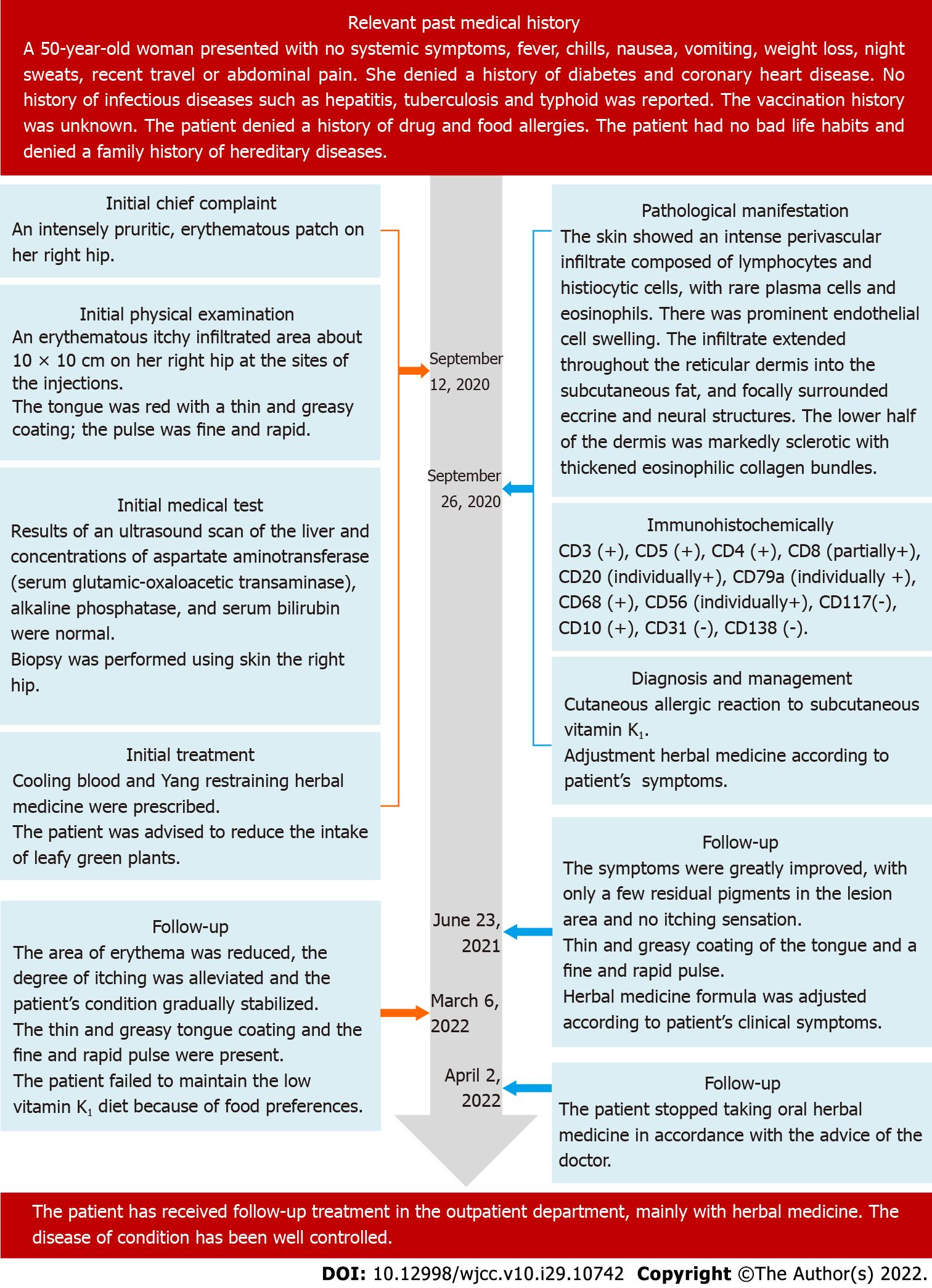
A 50-year-old woman was referred to our hospital on September 14, 2020 with the complaint of an intensely pruritic erythematous patch on her right hip.
The patient’s symptom of an intensely pruritic erythematous patch started 1 year before presentation.
One year prior, the patient had received subcutaneous injections of vitamin K1 40 mg before thyroid nodule surgery to prevent bleeding. Five days after the injections, she developed the abovementioned symptoms at the injection site that caused discomfort without any obvious burning, pain, or other problems. She had not been treated with phytonadione, cyclosporine, or other medications containing polyoxyethylated castor oil (PEO-CO). She was diagnosed with urticaria, for which topical chlo
The patient denied any family history of any hereditary disease.
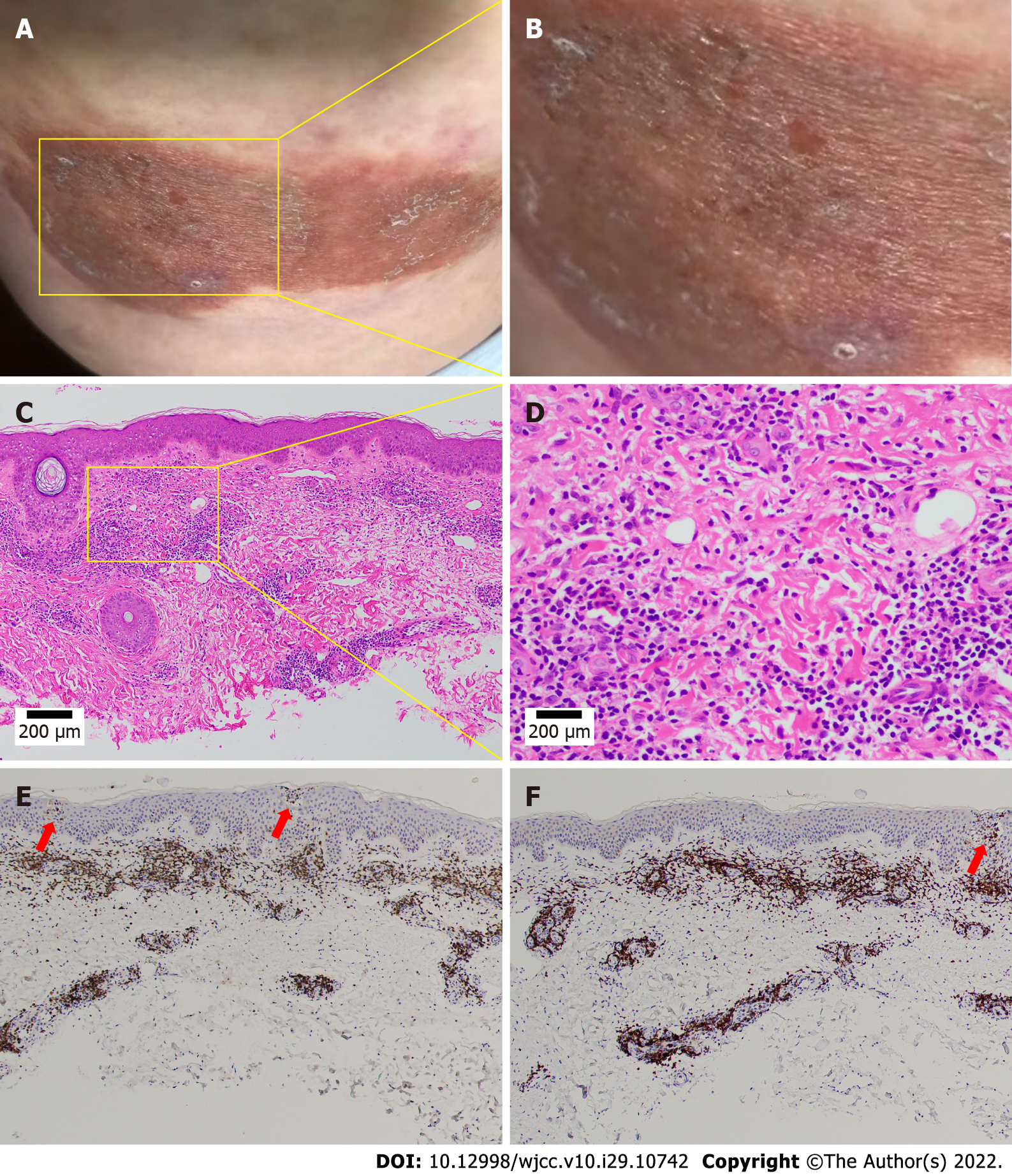
A physical examination revealed an erythematous itchy-infiltrated area of approximately 10 cm × 10 cm on the right hip at the injection sites. The patient had a dry mouth, no sweat, and normal urine and bowel movements. Her tongue was red and covered with a thin and greasy coating, and her pulse was fine and rapid. Since the onset of the disease, she had no fever, chills, joint pain, swollen lymph nodes, weight loss, trauma, foreign body contact, or suspicious drug use. Her clinical symptoms of intense pruritus and weeping, erythematous, eczematous, and indurated plaques are shown in Figure 1A and B.
Liver ultrasonography findings and aspartate aminotransferase (serum glutamic oxaloacetic transaminase), alkaline phosphatase, and serum bilirubin levels were normal.
A clear diagnosis of the disease could not be made based on the clinical manifestations of the erythematous and eczematous plaques. The patient’s skin lesions were nonspecific and could be easily confused with mycosis fungoides (MF). To ensure diagnostic accuracy, we performed a biopsy of the skin from the right hip during the examination, the results of which were available within a week. Considering the characteristics of the skin lesions, we temporarily suspected an adverse cutaneous reaction to vitamin K1 (eczematous type), and the CM diagnosis was “wet sores.” The tongue coating, pulse, and skin lesion characteristics were consistent with those of blood-heat syndrome. We considered blood heat and Yang floating as the main pathogeneses according to the CM theory.
The erythema area was reduced, the degree of itching was alleviated, and the patient’s condition gradually stabilized by November 12, 2020. The patient’s tongue was reddish with a white and greasy coating and her pulse was slippery. A skin biopsy was performed (Figure 1C and D), and a histological examination showed an intense perivascular infiltrate composed of lymphocytes and histiocytic cells with rare plasma cells and eosinophils. Prominent endothelial cell swelling was also observed. The infiltrate extended through the reticular dermis into the subcutaneous fat and focally surrounded the eccrine and neural structures. The lower half of the dermis was markedly sclerotic with thickened eosinophilic collagen bundles. These findings were consistent with our diagnosis.
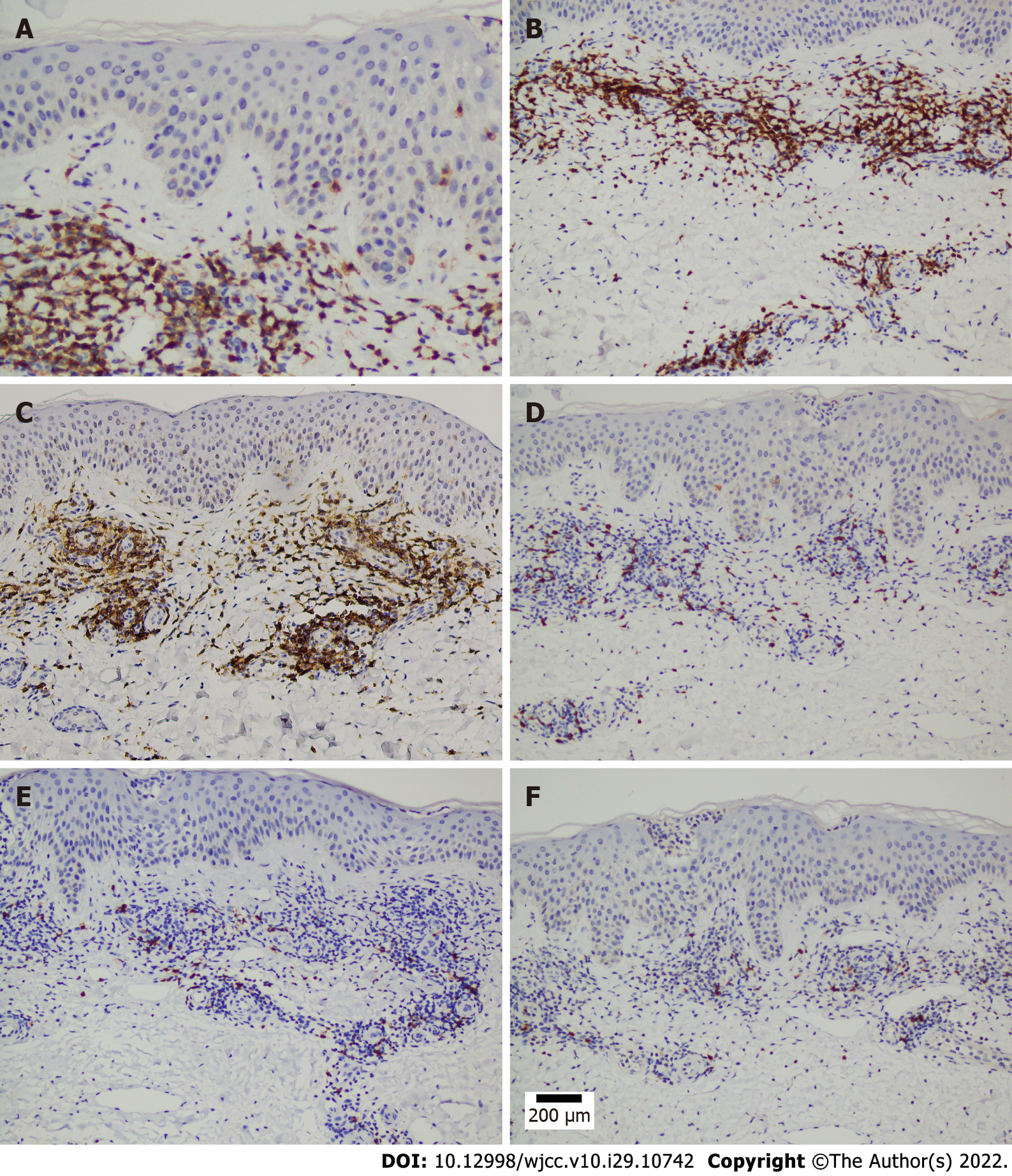
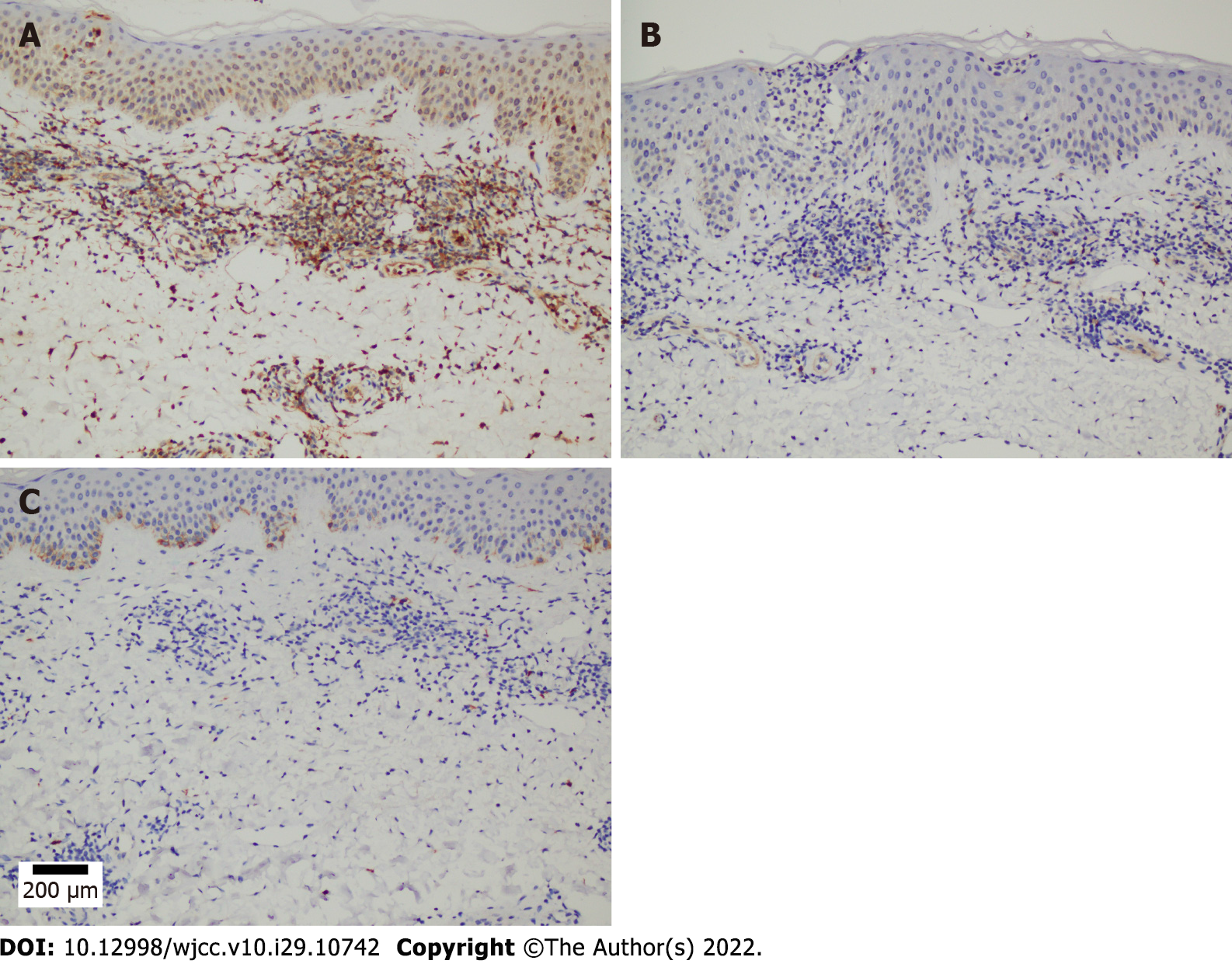
A microscopic examination revealed a normal epidermis and more lymphocytes infiltrating the blood vessels in the dermis, several of which mimicked Pautrier microabscesses (Figure 1E and F). Immunohistochemical (IHC) staining showed that T cells were positive for CD3, CD5, and CD4 and partially positive for CD8 (Figure 2A-D). B cells were positive for CD20 and CD79a (Figure 2E and F). Histocytes were positive for CD68, natural killer cells were positive for CD56, and mastocytes were negative for CD117 (Figure 3A-C). B cells tested positive for common acute lymphoblastic leukemia antigen (CD10), negative for platelet-endothelial cell adhesion molecule-1 was negative for (CD31), and negative for syndecan-1 (CD138) (Supplementary Figure 1). We ruled out MF because the IHC results suggested that the cells in this case were multi-lineage and polyclonal.
According to the clinical picture, histopathology results, and IHC results, the final diagnosis was a cutaneous allergic reaction to the vitamin K1 injections.
The patient was treated with an oral herbal medicine for “cooling blood and restraining Yang” twice daily, 30 min after meals. Our team previously reported that blood-cooling and Yang-restraining herbs had a positive clinical effect in the treatment of skin diseases such as blood-heat syndrome, eczema, and psoriasis vulgaris[14,15]. Other authors showed that the drug for cooling blood and restraining Yang may upregulate the expressions of PD-1 mRNA, PD-L2 mRNA, and their proteins in patients with psoriasis vulgaris, which manifests as blood-heat syndrome[16]. The specific compositions, dosages, and medicinal sources of these herbal decoctions are listed in Table 1.
| Main composition | Chinese pinyin | Latin scientific name | Medicinal source | Crude drug content (g) |
| Radix Cyathulae | Chuan Niu Xi | Cyathula officinalis Kuan | Root | 12 |
| Rhizoma Alismatis | Ze Xie | Alisma plantago-aquatica L | Tuber | 12 |
| Belvedere fruit | Di Fu Zi | Fructus Kochiae | Fruit | 15 |
| Cortex | Bai Xian Pi | Cortex dictamni | Root bark | 12 |
| Mother of pearl | Zhen Zhu Mu | Concha Margaritifera Usta | Shell | 30 |
| Magnetite | Ci Shi | Magnetium | Fe3O4 | 30 |
| Liquorice | Gan Cao | Glycyrrhiza uralensis Fisch | Root | 3 |
| Herba Lycopi | Ze Lan | Aconitum gymnandrum Maxim | Leaf | 9 |
| Scutellaria baicalensis | Huang Qin | Scutellaria baicalensis Georgi | Root | 12 |
| Rehmannia | Di Huang | Rehmannia glutinosa | Root | 15 |
| Prepared rehmannia | Shu Di Huang | Rehmannia glutinosa | Root | 15 |
| Cortex Moutan | Mu Dan Pi | Cortex Moutan Radicis | Root bark | 12 |
| Red-rooted Salvia | Sheng Dan Shen | Salvia miltiorrhiza Bge | Root | 15 |
| Red peony | Chi Shao | Radix Paeoniae Rubra | Root | 12 |
| Sophorae Flavescentis | Ku Shen | Sophora flavescens | Root | 12 |
| Angelicae Sinensis | Guan Huang Bai | Phellodendron amurense Rupr | Bark | 12 |
The previous misdiagnosis was corrected in a timely manner. Nevertheless, the main components of the blood-cooling and yang-restraining herbal medicine remained unchanged. The dose of the drug was adjusted appropriately, and the patient continued to reduce her intake of leafy green vegetables.
The patient was followed up on June 23, 2021, March 6, 2022, and April 2, 2022. After taking the herbal medicine, the patient’s erythema area was reduced, the degree of itching was alleviated, and her symptoms improved significantly. Her tongue was still red with a thin and greasy coating and her pulse was fine and rapid; hence, the composition of the herbal medicine was adjusted accordingly. After taking the herbal medicine for 18 mo, her symptoms greatly improved with only a few residual pigments in the lesion area and no itching sensation. She stopped taking the oral herbal medicine in April 2022 in accordance with the doctor’s advice.
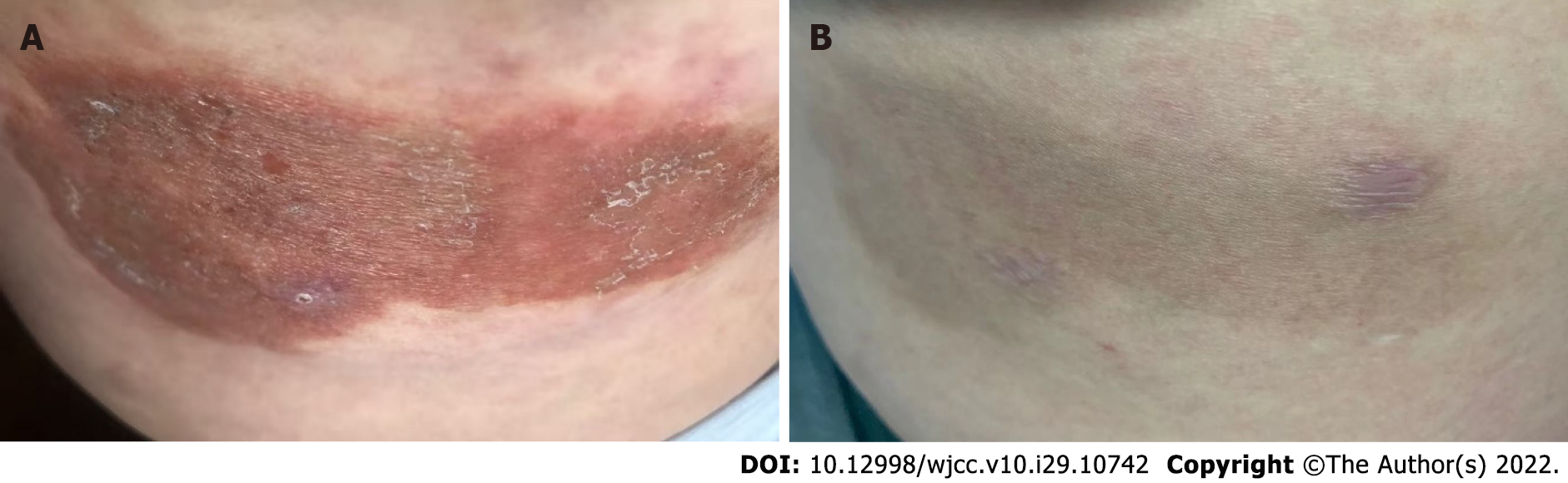
At presentation, the patient showed some inconspicuous pigment residues with no itching sensation, and the lesions did not involve the internal organs or lymph nodes. Throughout the treatment and follow-up period, the patient showed good adherence to and tolerance of the CM therapy, and no adverse or unanticipated events occurred during this period. The patient’s condition was well controlled, and her quality of life improved. She was regularly followed up at a local clinic, with no complaints of discomfort or adverse events during treatment. At the 18-mo follow-up, the eczematous erythema plaques on her right hip had notably improved compared to the pretreatment condition. However, the eruption persisted with occasional pruritus for approximately 32 mo. The patients’ skin lesions before and after treatment are shown in Figure 4A and B. The full timeline of diagnosis, medical treatment process, physical examination findings, clinical characteristics, and laboratory findings are shown in Figure 5.
Our patient developed severe localized reactions at the site of the subcutaneous injections of vitamin K1, and the lesions slowly subsided over a 32-mo period. Although the early changes resembled urticaria, they were not transient. Most importantly, the swollen red plaques involved underlying dermal involvement and epidermal changes. The diagnosis was confirmed by clinical imaging, histopathology, and IHC staining. The persistence of the pruritus in our patient may imply that the drug may have remained locally at the injection site or that an altered host tissue reaction in the skin may have provided a continued antigen stimulus.
We summarized the results of the 20 cases reported to date of eczematous-type reactions in Table 2, including the administration route, dose, reaction, and diagnostic assessment, with the aim of determining the pathogenesis of this hypersensitivity. Several features of the eczematous reaction to vitamin K1 suggest that it was a manifestation of delayed-type hypersensitivity, a prototype of type IV cell-mediated immunity. In most cases, lesions appeared approximately 7-10 d after the first dose of vitamin K1[17-36]. In addition, subsequent patch and intradermal testing produce a reaction within 3-5 and 1-2 d, respectively[17-21,23,25,26,31,32,34,36]. The time sequence of the development of these reactions corresponded well with type IV cell-mediated hypersensitivity. The positive results of sensitivity testing using patch test methods implied that sufficient antigens may have been absorbed to generate an immune response. Several patients exhibited a recall reaction[21,23,30] in previously affected areas compatible with the recall phenomenon in allergic contact eczema when antigens applied in new areas precipitate an eczematous flare-up. Several histopathological studies of biopsy specimens from the lesion and the intracutaneous test site showed spongiosis of the epidermis, dermal edema, and perivascular mononuclear and eosinophilic cellular infiltrates consistent with delayed-type hypersensitivity[18,20-25,27-31,33,36].
| First author | Country | Date | Sex | Age (yr) | Adm. | Dose (mg) | Onset | Reaction | Patch | I.c. | Other assessments | Comments | Outcome |
| Location/duration | |||||||||||||
| Piguet et al[17] | France | 1964 | F | 33 | IM | Before surgery | Buttock/wk | K1- | K1+ | Alcoholic cirrhosis | |||
| IM | Buttock, chest, face/8 d | ||||||||||||
| Barnes et al[18] | United Kingdom | 1976 | F | 31 | IM | 10 | 2 wk | Buttock/2 mo | ND | K1+ | Biopsy | Budd–Chiari syndrome, PCV | Plaques disappeared |
| F | 30 | IM | 100 | Several weeks | Upper arm, thigh/2 mo | Drug overdose, renal failure, generalized rash | |||||||
| Heydenreich et al[19] | Denmark | 1977 | F | 51 | IV | 20 daily | 8–10 d | Arms | K1+1 | K1+ | ST | Hepatitis, RA, GN | No skin problems |
| Intracutaneous test, epicutaneous test | |||||||||||||
| Bullen et al[20] | United Kingdom | 1978 | F | 17 | INJ | 340 | 13 d | Buttock/11 d | ND | Biopsy | CAH | Resolution with scaling | |
| F | 24 | INJ | 360 | 9 d | Buttock/9 d | ND | Biopsy | CAH | |||||
| F | 39 | INJ | 270 | 9 d | Buttock/19 d | ND | K1+, K4- | Biopsy | CAH, RA | ||||
| F | 50 | INJ | 440 | 16 d | Buttock/18 d | ND | ND | Biopsy | CAH, liver congestion, RA | ||||
| F | 48 | INJ | 300 | 10 d | Buttock/22 d | ND | K1+, K4- | Alcoholic cirrhosis | |||||
| F | 12 | INJ | 280 | 7 d | Buttock/9 d | ND | K1+, K4- | Biopsy | Congenital hepatic failure | ||||
| Robison et al[21] | United States | 1978 | M | 63 | IM | 30 | 10 d | Upper arm | K1+1 | K1+1 | Biopsy | Alcoholic cirrhosis | Temporarily alleviated |
| Epicutaneous test | |||||||||||||
| Jean-Pastor et al[22] | France | 1981+ | |||||||||||
| Finkelstein et al[23] | Canada | 1987 | F | 26 | SC | 280 | 11 d | Months | K1-, K3- | K1+, K3- | Biopsy | Metastatic cholangiocarcinoma | Hyperpigmentation remained |
| F | 42 | SC | 40 | 14 d | Months | K1+, K3- | ND | Biopsy | Primary biliary cirrhosis | Total bilirubin remained elevated | |||
| F | 39 | IM | 1750 | 2 d | Months | K1-, K3- | K1+, K3- | Biopsy | Chronic myeloid leukemia | Residual erythema scaling, pruritus, and hyperpigmentation | |||
| M | 51 | SC | 10 | 14 d | Months | K1+, K3- | K1+, K3- | Biopsy | Amyloidosis | Mild improvement | |||
| F | 64 | SC | 10 | 5 d | Months | K1+, K3- | ND | Biopsy | Cardiac cirrhosis | Persistent pruritus | |||
| F | 32 | SC | 10 | 14 d | Months | K1-, K3- | K1+, K3- | Biopsy | Preeclampsia with DIC | ||||
| Joyce et al[24] | United States | 1988 | F | 36 | IM | 20 | 14 d | Right arm/4 yr | Biopsy | Long-term warfarin therapy | Intermittent pruritus and persistent induration | ||
| F | 61 | IM | 10 | 6 d | Left arm/6 mo | Biopsy | Severe cardiac disease | Skin lesion persisted unchanged | |||||
| No significant medical abnormalities | |||||||||||||
| Sanders et al[25] | United States | 1988 | F | 18 | SC | 100 | > 10 d | Buttock, upper arm/weeks | K1+, K4- | Biopsy | Anorexia, N/V | Lesions resolved | |
| F | 28 | SC | 10 | 2 wk | Arm/1 mo | K1+, K4- | Dilated cardiomyopathy | Slowly resolved | |||||
| F | 31 | SC | 10 d | Upper arm/2 wk | Healthy | ||||||||
| F | 25 | SC | 40 | After the injection | Weeks | K1+, K4- | Biopsy | Drug overdose, elevated LFT results | Rash cleared | ||||
| Pigatto et al[26] | Italy | 1990 | F | 9 | IM | 10 d | Buttocks | K1+, K2-, K3- | Minor beta thalassemia | Some residual pigmentation | |||
| Factor X deficiency | |||||||||||||
| Lee et al[27] | United States | 1992 | F | 49 | SC | 110 | During treatment | Upper arms, thighs, and buttocks/2 mo | Biopsy | CAH, variceal bleeds | Total regression of lesions | ||
| Tuppal et al[28] | Canada | 1992 | M | 45 | SC | 330 | Few days | Upper arms and abdomen/2 mo | Biopsy | Long history of ethanol abuse | Resolution of skin eruption | ||
| Lemlich et al[29] | United States | 1993 | F | 43 | 30 | 2 wk | 6 mo | Biopsy | Budd–Chiari syndrome, PCV | No sclerodermatous changes | |||
| F | 62 | 20 | 2 wk | 1 mo | Biopsy | Stage Ⅳ endometrial carcinoma | Mild postinflammatory hyperpigmentation remained | ||||||
| F | 57 | 2 wk | Right arm/1 mo | Biopsy | Stage Ⅱ ovarian cancer | ||||||||
| F | 50 | 2 wk | Left upper arm | Biopsy | Healthy | Remained symptomatic | |||||||
| Bruynzeel et al[30] | Netherlands | 1995 | F | 32 | SC | 2 wk | Right upper arm/2 mo | K1-, K- | K+ | Biopsy | Pelvic vein thrombosis | Resolution | |
| F | 44 | SC | 4 wk | Left upper leg/mo | Acute pancreatic Ascaris lumbricoides infection | Lesion persisted | |||||||
| Balato et al[31] | Italy | 1998 | F | 49 | IM | 30 | Right buttock/4 mo | K- | K+ | Biopsy | Lesion healed spontaneously | ||
| Scratch-patch test | |||||||||||||
| Prick test | |||||||||||||
| Wong et al[32] | Australia | 1999 | F | 40 | IM | 40 | 5 d | Thighs/mo | K1+, K3+ | K1+ | Cholelithiasis, asthma, duodenal ulcer, and esophagitis | Residual erythema persisted | |
| Wilkins et al[33] | Canada | 2000 | F | 50 | SC | 15 | 1 wk | Left arm/18 mo | Biopsy | Hypothyroidism, superficial phlebitis | Eruption persisted with occasional flares | ||
| Sommer et al[34] | United Kingdom | 2002 | F | 27 | IM | 1 d | Thigh/2 yr | K1+, K4+ | CF, CF-related DM | Symptoms persisted | |||
| Bui et al[35] | United States | 2004 | F | 21 | SC | 65 | 10 d | Arms, abdomen/19 d | N/V, Wilson’s disease | Rash disappeared | |||
| Giménez-Arnau et al[36] | Spain | 2005 | F | 64 | IM | 1 wk | Buttock | K1- | K1+ | Biopsy | Chronic hepatitis C virus liver disease | Skin healed | |
| Immunoallergic study | |||||||||||||
| Prick test | |||||||||||||
| Our case | China | 2022 | F | 50 | SC | 40 | 5 d | Right hip | Biopsy | Multiple thyroid nodules | A few residual pigments in the lesion area and no itching sensation | ||
| IHC |
To identify the allergen, we summarized the reported cases that used patch and intradermal testing to delineate the mechanism of skin hypersensitivity to vitamin K1. Previous studies consistently reported that only the whole preparation of vitamin K1 (in its vehicle) or vitamin K1 alone produced positive test results[19-21]. When the individual components of the vehicles were tested, they almost always yielded negative results. In some cases, patch tests showed negative results, while intradermal tests showed opposite results[23,30], which may, to some degree, reflect insufficient percutaneous absorption. Page et al[37] found that dermatitis from topically applied vitamin K1 was caused by the chemical rather than the base. One study in Australia[32] illustrated the importance of testing with appropriate concentrations of reagents, as patch testing with Konakion Cremophor-EL 2 mg/mL showed a negative result, whereas testing with a 10 mg/mL concentration (the concentration administered at hospitals) showed a strongly positive result. Another study showed relatively high rates of intradermal positivity in control participants (4 of 10 controls)[20]; however, the reactions in controls were not as florid, and in two cases, the reaction was delayed to day 6, which may indicate sensitization.
Previous reports suggested that the underlying cause of the reaction is not phytonadione but rather the vehicle, PEO-CO, which is used as a solubilizer or diluent for several drugs. Although experimental and clinical data are limited, these reactions have been classified as anaphylactoid reactions[38]. Experimental and other evidence suggested that PEO-CO may cause anaphylaxis by releasing histamine, as demonstrated by studies showing its pharmacological action in dogs[39], a memory-mediated reaction involving complement activation[40], and the presence of immunoglobulin G steroid sulfatase antibodies as confirmed by appropriate immunological tests[41]. However, PEO-CO may not be responsible for all anaphylactic events, as demonstrated by two cases of anaphylaxis after the administration of formulations that did not containing PEO-CO[42,43].
Several vitamin K preparations are available that contain different inactive ingredients. Most previous investigators did not use patches or intracutaneous testing for these additional ingredients of vitamin K1. Five investigators tested the components of their particular preparations and obtained negative results[19,21,23,25,30]. These results suggest that the antigen that causes adverse cutaneous reactions is not the inactive ingredient in the mixture but vitamin K itself. Because of the potential for exacerbating the long-lived reactions in our patient, no patch or intracutaneous tests were performed.
Liver disease was previously believed to play a role in the pathogenesis of this allergic reaction[44]; however, six cases of cutaneous reactions to vitamin K1 have been described in patients with no known liver disease[21,24,25]. One study in Japan showed, in 94 documented cases of cutaneous hypersensitivity reactions to vitamin K1, no correlation between vitamin K1 hypersensitivity and liver disease[10]. Nevertheless, as patients with liver disease account for the majority treatment demand for vitamin K1, they may be at an increased risk of developing cutaneous reactions to it. An investigation into the underlying liver dysfunction may be indicated when a patient develops a local eczematous reaction to vitamin K1.
In all reported cases of skin reactions, the dose range was 10-1750 mg[17-36]. One study[20] hypothesized that even a minimum dose can cause an eruption. Some researchers have noted that patients in some of the more recalcitrant cases received the smallest doses[33]. In addition, the dosage of vitamin K seems to have no correlation with the onset of the allergic reaction as confirmed by a study[45] that described sclerodermatous reactions associated with large doses of vitamin K1 100-500 mg. This finding suggests that neither injections nor large doses are required for a sclerodermatous reaction to occur[46]. Our patient had no evidence of preexisting hepatic disease and received a total dose of 40 mg.
Although the incidence of adverse reactions to the subcutaneous injection of vitamin K1 is considerably low, such adverse effects can be devastating. In our case, the patient experienced discomfort only in the area of the local lesions and the disease did not have a particularly serious impact on her daily life. Overall, the disease progressed slowly and was well controlled from onset to follow-up. However, it remains difficult to make an accurate clinical conclusion about this case of hypersensitivity, which creates new challenges and requirements for the diagnosis and treatment of such conditions that, in the future, may be avoided with a more judicious use of this form of vitamin K1 in the clinical setting. In contrast to the previously reported cases, our patient was treated its CM. The key to CM treatment is to accurately identify the syndrome and treat the symptoms. CM may achieve unexpectedly good results in terms of improving the patient’s condition. However, the efficacy, safety, and mechanisms of CM therapy require further investigation. Additionally, whether CM therapy alone or combined with Western medicine has better clinical efficacy for the treatment of these adverse reactions requires confirmation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng TH, Taiwan; Jovandaric M, Serbia S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | World Health Organization International Agency for Research on Cancer (IARC). Vitamin K substances: IARC monograph. IARC Monogr Eval Carcinog Risks Hum. 2000;76:417-486. |
| 2. | De Jonge GA. Vruchrbeschadiging door farmaca. Folia Medica Neerlandica. 1965;8:65. |
| 3. | Wynn RM. The obstetric significance of factors affecting the metabolism of bilirubin, with particular reference to the role of vitamin K. Obstet Gynecol Surv. 1963;18:333-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Morell A, Betlloch I, Sevila A, Bañuls J, Botella R. Morphea-like reaction from vitamin K1. Int J Dermatol. 1995;34:201-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Guidetti MS, Vincenzi C, Papi M, Tosti A. Sclerodermatous skin reaction after vitamin K1 injections. Contact Dermatitis. 1994;31:45-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Pang BK, Munro V, Kossard S. Pseudoscleroderma secondary to phytomenadione (vitamin K1) injections: Texier's disease. Australas J Dermatol. 1996;37:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | García-Gavín J, Goossens A, Tennstedt D. Allergic contact dermatitis due to cosmetics containing vitamin K1 oxide. Contact Dermatitis. 2010;62:248-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Alonso-Llamazares J, Ahmed I. Vitamin K1-induced localized scleroderma (morphea) with linear deposition of IgA in the basement membrane zone. J Am Acad Dermatol. 1998;38:322-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Tsuboi R, Ogawa H. Skin eruption caused by fat-soluble vitamin K injection. J Am Acad Dermatol. 1988;18:386-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Honda M, Hattori Y, Moriyama M. Skin eruptions caused by vitamin K. Hifuka no Rinsho. 1970;12:295-301. |
| 12. | Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 982] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 13. | Lauerma AI. Immunomodulation of contact dermatitis. Curr Probl Dermatol. 1995;22:51-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Ru Y, Xu XZ, Kuai L. Study on the Drugs Used in the Treatment of Eczema by Li Bin Liangxue Qianzhen. Zhong Yi Jichu Yi Xue Zazhi. 2018;24:1773-1776. |
| 15. | Kuai L, Xu XZ, Ru Y. Analysis of Li Bin's formula for treating psoriasis vulgaris with the method of cooling blood and burying Yang. Zhong Yi Jichu Yi Xue Zazhi. 2018;24:1474-1477. |
| 16. | Xu R, Miao X, Li X, Chen J, Zhang YN, Li B. Effects of cooling blood restrain Yang drugs on programmed death protein-1 and its ligands in patients with psoriasis blood heat syndrome. World Clin Drugs. 2018;39:247-252. |
| 17. | Piguet B, Bertheuil F. Accidents cutanes allergiques provoques par une preparation injectable de vitamine K1 synthetique. Bull Soc Fr Dermatol Syphiligr. 1964;71:486-490. |
| 18. | Barnes HM, Sarkany I. Adverse skin reaction from vitamin K1. Br J Dermatol. 1976;95:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Heydenreich G. A further case of adverse skin reaction from vitamin K1. Br J Dermatol. 1977;97:697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Bullen AW, Miller JP, Cunliffe WJ, Losowsky MS. Skin reactions caused by vitamin K in patients with liver disease. Br J Dermatol. 1978;98:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Robison JW, Odom RB. Delayed cutaneous reaction to phytonadione. Arch Dermatol. 1978;114:1790-1792. [PubMed] |
| 22. | Jean-Pastor MJ, Jean Ph, Gamby T. Accidents cutanes consecutifs it l'administration parenterale de vitamine K. Therapie. 1981;36:369-374. |
| 23. | Finkelstein H, Champion MC, Adam JE. Cutaneous hypersensitivity to vitamin Kt injection. J Am Acad Drmaxol. 1987;16:540-545. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Joyce JP, Hood AF, Weiss MM. Persistent cutaneous reaction to intramuscular vitamin K injection. Arch Dermatol. 1988;124:27-28. [PubMed] |
| 25. | Sanders MN, Winkelmann RK. Cutaneous reactions to vitamin K. J Am Acad Dermatol. 1988;19:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Pigatto PD, Bigardi A, Fumagalli M, Altomare GF, Riboldi A. Allergic dermatitis from parenteral vitamin K. Contact Dermatitis. 1990;22:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Lee MM, Gellis S, Dover JS. Eczematous plaques in a patient with liver failure. Fat-soluble vitamin K hypersensitivity. Arch Dermatol. 1992;128:260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Tuppal R, Tremaine R. Cutaneous eruption from vitamin K1 injection. J Am Acad Dermatol. 1992;27:105-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Lemlich G, Green M, Phelps R, Lebwohl M, Don P, Gordon M. Cutaneous reactions to vitamin K1 injections. J Am Acad Dermatol. 1993;28:345-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Bruynzeel I, Hebeda CL, Folkers E, Bruynzeel DP. Cutaneous hypersensitivity reactions to vitamin K: 2 case reports and a review of the literature. Contact Dermatitis. 1995;32:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Balato N, Cuccurullo FM, Patruno C, Ayala F. Adverse skin reactions to vitamin K1: report of 2 cases. Contact Dermatitis. 1998;38:341-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Wong DA, Freeman S. Cutaneous allergic reaction to intramuscular vitamin K1. Australas J Dermatol. 1999;40:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Wilkins K, DeKoven J, Assaad D. Cutaneous reactions associated with vitamin K1. J Cutan Med Surg. 2000;4:164-168. [PubMed] |
| 34. | Sommer S, Wilkinson SM, Peckham D, Wilson C. Type IV hypersensitivity to vitamin K. Contact Dermatitis. 2002;46:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Bui L, Huynh T, Lam V. Skin reaction to subcutaneous phytonadione injections. Am J Health Syst Pharm. 2004;61:407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Giménez-Arnau AM, Toll A, Pujol RM. Immediate cutaneous hypersensitivity response to phytomenadione induced by vitamin K in skin diagnostic procedure. Contact Dermatitis. 2005;52:284-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Page RC, Bercovitz Z. Dermatitis from topical application of 2-methyl- 1-4-naphthoquinone (synthetic vitamin K analogue). Am J Med Sci. 1942;203:566-569. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Executive summary of disease management of drug hypersensitivity: a practice parameter. Joint Task Force on Practice Parameters, the American Academy of Allergy, Asthma and Immunology, the American Academy of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1999;83:665-700. [PubMed] |
| 39. | Lorenz W, Reimann HJ, Schmal A, Dormann P, Schwarz B, Neugebauer E, Doenicke A. Histamine release in dogs by Cremophor E1 and its derivatives: oxethylated oleic acid is the most effective constituent. Agents Actions. 1977;7:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 152] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Dye D, Watkins J. Suspected anaphylactic reaction to Cremophor EL. Br Med J. 1980;280:1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Moneret-Vautrin DA, Laxenaire MC, Viry-Babel F. Anaphylaxis caused by anti-cremophor EL IgG STS antibodies in a case of reaction to althesin. Br J Anaesth. 1983;55:469-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Aziz NA, Kamaruddin Z, Hassan Y, Jaalam K. Vitamin K1 induced anaphylactic shock. J Pharm Technol. 1996;12:214-216. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Havel M, Müller M, Graninger W, Kurz R, Lindemayr H. Tolerability of a new vitamin K1 preparation for parenteral administration to adults: one case of anaphylactoid reaction. Clin Ther. 1987;9:373-379. [PubMed] |
| 44. | Carton FX. Reaction allergique au cours d'un traitement: vitamine K, + extrait de foie. Bull Soc Fr Dermatol Syph. 1965;72:228. |
| 45. | Texier L, Gendre P, Gauthier O. Hypodermites sclerodermiformes lombo-fessires induites par des injections medicamenteuses intramusculaires associees it la vitamine K. Ann Dermatol Syph. 1972;99:363-371. |
| 46. | Rommel A, Saurat JH. Hypodermite Fessi~re sclerodermiforme et injections de vitamine K, /~ la naissance. Ann Pediatr (Paris). 1982;29:64-66. |