Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10543
Peer-review started: August 24, 2021
First decision: September 29, 2021
Revised: December 13, 2021
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: October 16, 2022
Processing time: 400 Days and 23.9 Hours
Klippel-Trenaunay-Weber syndrome (KTWS) is a very rare syndrome that involves three conditions: Cutaneous hemangiomas, varicosities, and soft-tissue hypertrophy of the affected limb. There are few cases of ischemic infarction with KTWS. Here, we describe a case of KTWS with ischemic stroke.
A 43-year-old man was diagnosed with KTWS with ischemic stroke. His chief complaints were worsening weakness and spasticity in the right leg. These symptoms had been present for 1 year, but the patient did not receive comprehensive rehabilitation until he underwent a 3-week integrated inpatient rehabilitation program at our center. After the program, his muscle strength, walking ability, and exercise endurance improved. Although relatively rare, clinicians should consider the possibility of a thromboembolic event in KTWS patients. Integrated rehabilitation can help such patients to recover function.
In conclusion, although rare, patients with KTWS may experience central nervous system vascular malformations and accompanying stroke. It is necessary to investigate whether such patients have any neurological or comorbid abnor
Core Tip: Klippel-Trenaunay-Weber syndrome (KTWS) is a very rare syndrome that involves three conditions: Cutaneous hemangiomas, varicosities, and soft-tissue hypertrophy of the affected limb. There are few cases of ischemic infarction with KTWS. Here, we describe a 43-year-old man who was diagnosed with KTWS with ischemic stroke. His chief complaints were worsening weakness and spasticity in the right leg. These symptoms had been present for 1 year, but the patient did not receive comprehensive rehabilitation until he underwent a 3-wk integrated inpatient rehabilitation program at our center. After the program, his muscle strength, walking ability, and exercise endurance improved. Although relatively rare, clinicians should consider the possibility of a thromboembolic event in KTWS patients. Integrated rehabilitation can help such patients to recover function.
- Citation: Lee G, Choi T. Klippel-Trenaunay-Weber syndrome with ischemic stroke: A case report. World J Clin Cases 2022; 10(29): 10543-10549
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10543.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10543
Klippel-Trenaunay-Weber syndrome (KTWS) is a rare vascular disorder distinguished by hemihypertrophy, variceal veins, and port-wine stains. Klippel and Trenaunay first described Klippel-Trenaunay syndrome (KTS) in 1900 as hemihypertrophy and varicose veins[1]. In 1907, Weber described KTS in more detail and called the disease KTWS when a port-wine stain was present with KTS. The vascular malformations caused by KTWS generally influence the capillary, venous, and lymphatic systems of the legs[2]. Central nervous system (CNS) vascular anomalies, especially rapid blood flow vascular anomalies such as arteriovenous brain malformations and arteriovenous fistulas, are common in KTWS patients, whereas slow blood flow vascular anomalies, also known as CNS-associated cavernous angiomas, are rare[3]. In addition to vascular malformation or hemimegalencephaly, hemorrhagic or ischemic strokes have rarely been reported in KTWS[5].
Here, we report a case of cerebral infarction related to KTWS, with a literature review.
A 43-year-old man was admitted to our rehabilitation center for weakness, spasticity, and claudication of his right leg. The symptoms had been present for about 1 year and worsened recently.
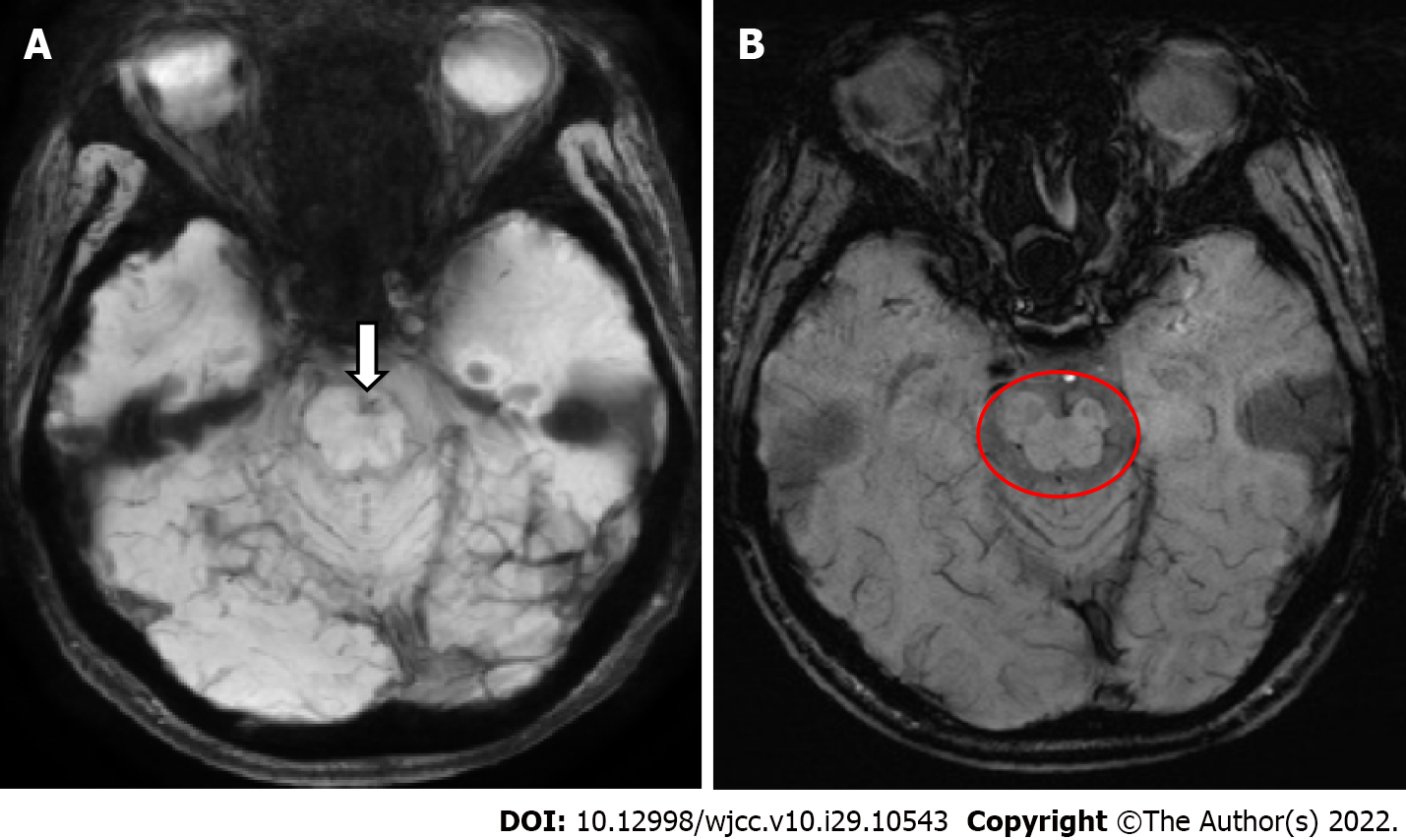
One year ago, motor weakness developed in the right leg, and the patient visited another hospital where he was diagnosed with KTWS. There was no other medical history associated with this weakness. Brain magnetic resonance imaging at that time revealed left midbrain atrophy and infarction in the left pons (Figure 1).
There were no special findings in the family history.
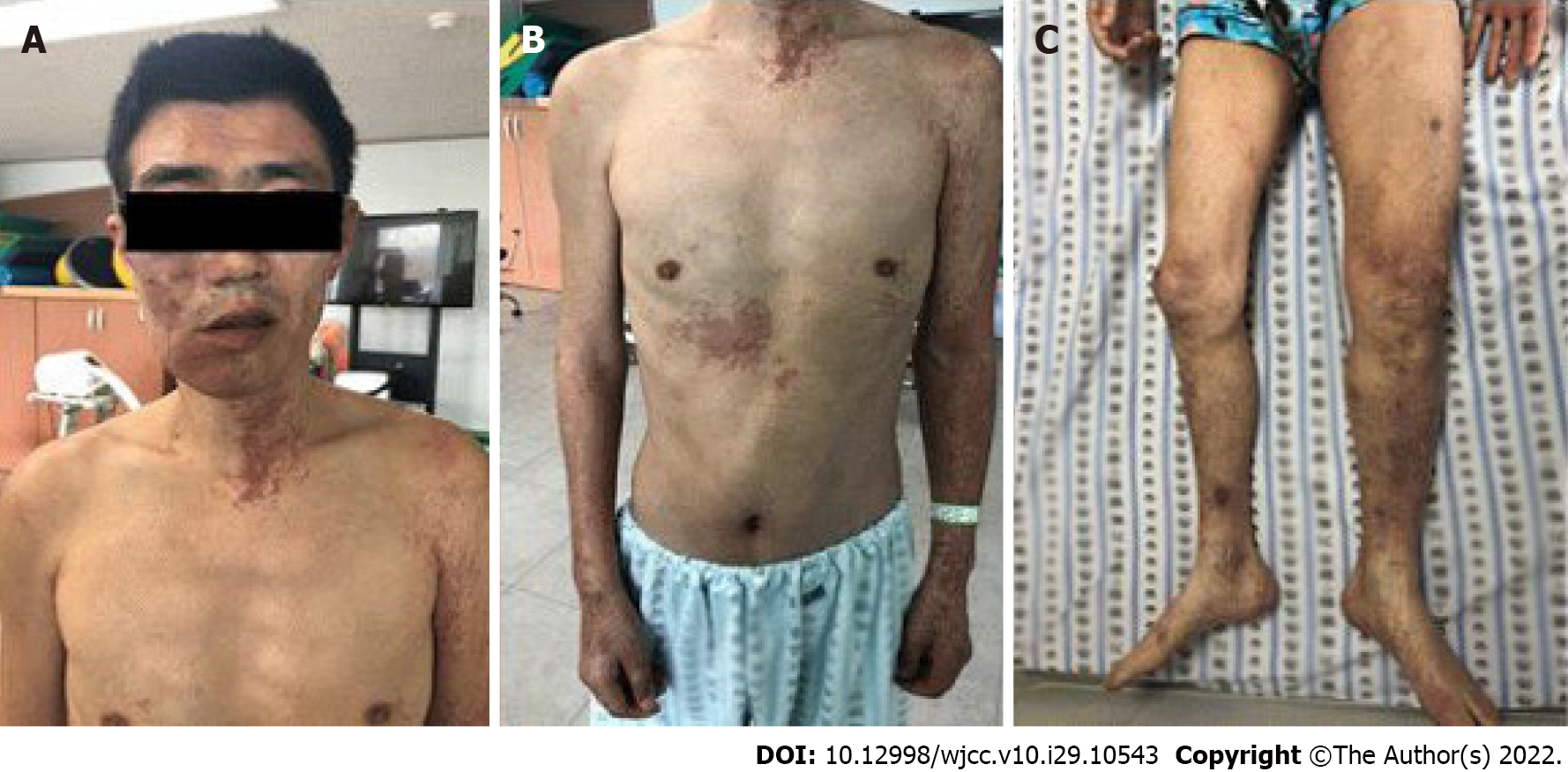
On physical examination, paresthesia was present in the right leg and the strength levels of the right knee and ankle were fair (3/5 on a manual muscle test). The deep tendon reflexes were increased (National Institute of Neurological Disorders and Stroke scale, grade 4), and right ankle clonus was observed. There was no motor weakness or paresthesia in the left leg. However, the left leg was hypertrophied, with multiple port-wine stains related to painless varicose veins (Figure 2). The hypertrophy, varicose veins, and port-wine stains had been present since childhood but never evaluated.
The results of transthoracic echocardiography and laboratory tests, including those related to coagulation factors and cholesterol levels, were normal.
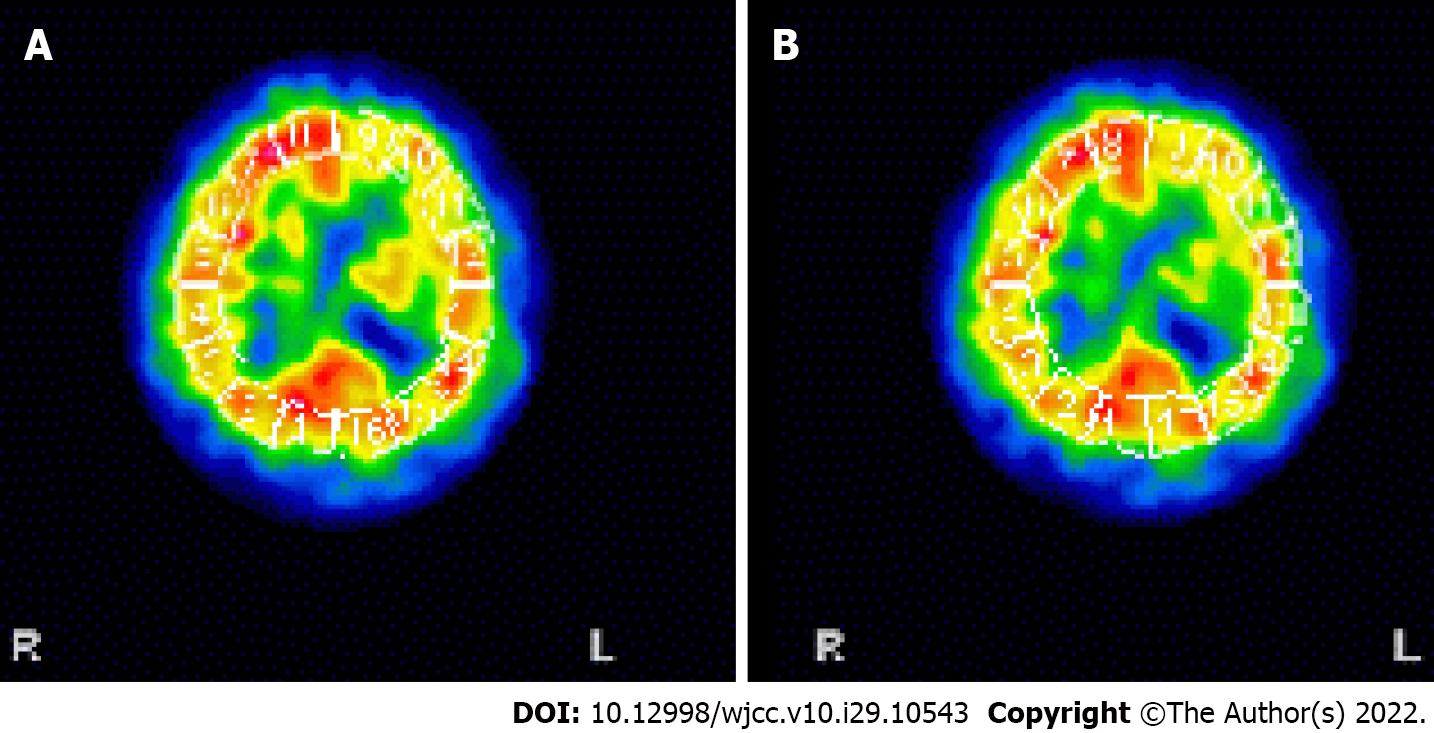
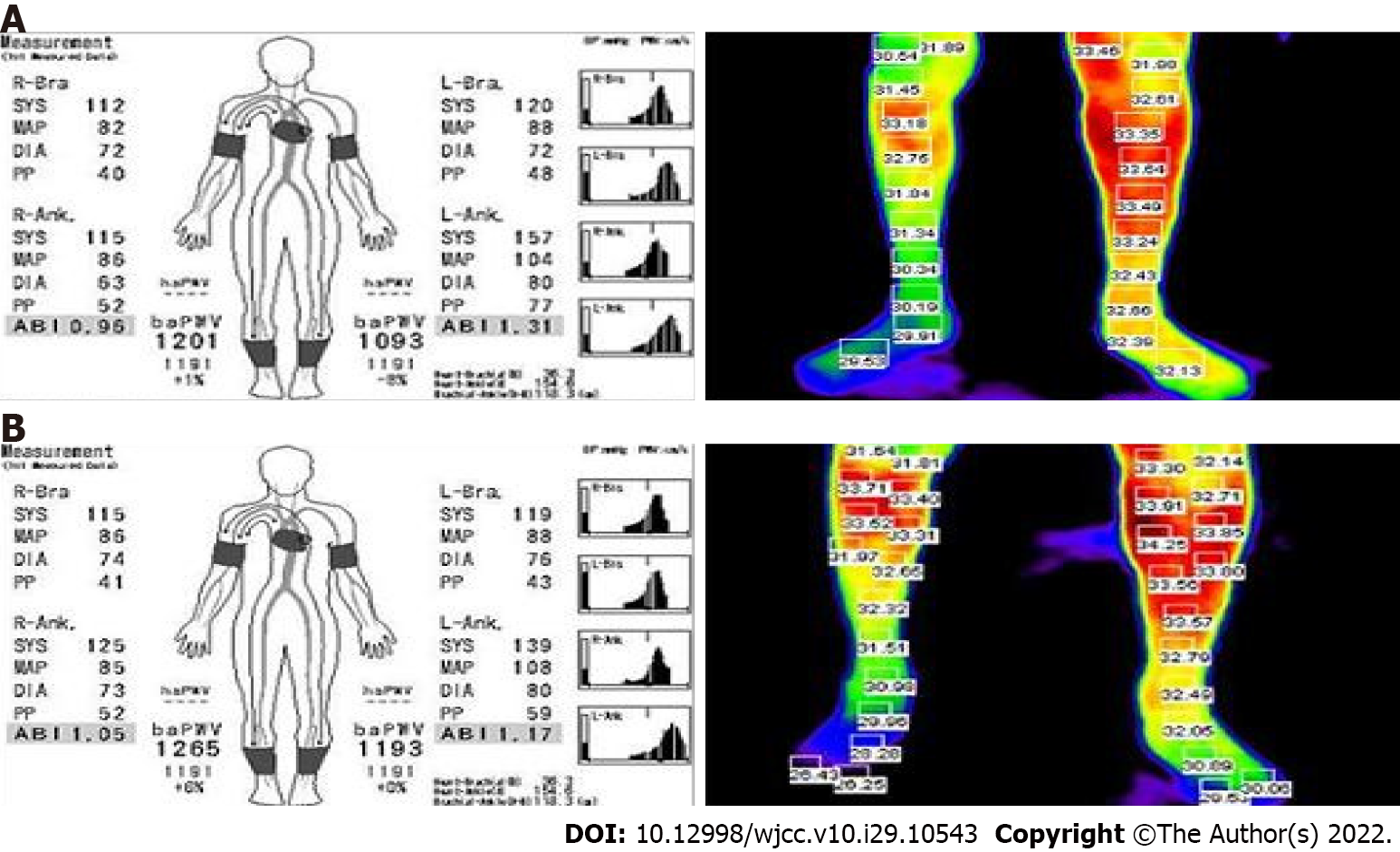
To evaluate the patient’s symptoms, brain single-photon emission computed tomography was performed, revealing moderate hypoperfusion in the left temporal and frontal cortices (Figure 3). Brain magnetic resonance angiography (MRA) did not show any cerebral or carotid artery stenosis. As cerebral perfusion asymmetry was observed, the Ankle-Brachial index (ABI) was calculated and infrared thermography was performed to evaluate the perfusion asymmetry in the peripheral extremities, which might have been influenced by cerebral blood flow. The ABI was 0.96 on the right and 1.31 on the left. The systolic blood pressure was 42 mmHg higher in the left leg than in the right leg. On infrared thermography, the right leg was 2.04 ℃ cooler than the left leg (Figure 4). Computed tomography angiography (CTA) of the legs showed no peripheral artery stenosis.
KTWS with ischemic stroke.
The patient was given 10 mg of oral baclofen daily. We developed an exercise program to improve his muscle strength and blood flow of the right leg, consisting of resistance and aerobic exercises (Table 1). The heavy resistance exercise consisted of knee extensions after fastening a 1.3-kg sandbag around the right ankle. The patient performed three sets of 10 extensions at 50% of his maximum walking speed, 5 d per week, for 3 wk. In another resistance exercise, a Thera-Band was applied to the ankle while the patient was lying on the floor, with the metatarsal heads of his right foot fixed. The knee was fully stretched with the Thera-Band extended to 170% of its resting length, as has been done elsewhere[4]. Each resistance exercise session took around 30 min to complete. The aerobic exercise included 20 min on a treadmill at 50%-70% of the patient’s maximum walking speed for 5 d per week for 3 wk. The patient’s maximum walking speed was based on the result of a 1-min walk test. He was instructed to walk as fast as he could without running. After 3 wk of the exercise program, the ABI, thermographic outcomes, strength level, and gait function were reassessed. The strength of the patient’s right leg as well as his walking speed and walking endurance all improved (Table 2), and the differences in leg temperature and ABI decreased (Figure 4).
| Exercise | Velocity | Duration, min | Sets | Resistance | Rest, s |
| Aerobic | Walking movement is retained to 50%-70% of walking speed1 | 20 | 1 | X | |
| Resistance | Resistance exercise is retained to 50% of 1 repetition maximum2 | 30 | 3 | Fastening 1.3 kg sandbag around ankle | 90 |
| Resistance exercise using the Thera-Band | 30 | 3 | Tying Thera-Band around metatarsal heads | 90 |
The patient’s resistance and aerobic exercises focused on his right leg. As a result, his muscle strength, gait speed, and exercise endurance improved (Table 2). We think that these results were related to improved vascular factors, although the improvement in vascular factors was measured indirectly using the ABI before and after the training regimen.
KTWS is a congenital disease that is typically discovered in childhood, although some patients may not be diagnosed until adulthood due to the varying degrees of severity. Our patient was diagnosed with KTWS late in life due to his mild symptoms. The first and most common sign of KTWS is capillary hemangiomas or port-wine stains. The exact etiology and pathogenesis of KTWS remain unclear. It may be associated with RASA1 gene mutations in some patients, but genetic abnormalities are not present in most cases of KTWS[1,5].
The CNS abnormalities reported in KTWS include hemorrhage, infarction, hemimegalencephaly, cavernomas, and arteriovenous malformations[5]. Deep vein thrombosis and pulmonary embolism have been reported with KTS, but cerebral infarction related to KTWS is rare, and the stroke mechanism remains unclear. A few KTWS patients have had transient ischemic attacks and patent foramen ovale[6]. Grira et al[7] reported a patient with KTWS presenting with stroke, who also had antithrombin III deficiency. In a KTWS patient with recurrent cerebral infarction, fibromuscular dysplasia in the carotid artery was observed[5]. Arterial vascular malformations such as intracranial aneurysms and carotid artery dissection have also been seen in KTWS-related stroke[8]. In the cases described above, the KTWS patients had risk factors for stroke. There is additionally a report of a KTWS patient with both ischemic stroke and cerebral hemorrhage; the cerebral infarction occurred first, and cerebral hemorrhage occurred after the administration of 100 mg of acetylsalicylic acid. The underlying etiology of both events remained undetermined, but increased vascular fragility might have been a cause[9].
In this study, there were no abnormal findings suggesting the focus of the thromboembolic event, including those from the blood coagulation tests and cardiac evaluation. The patient experienced asymmetry from childhood but received no medical services. We could not determine exactly when the stroke occurred, and it might have been a cryptogenic stroke. Although time-resolved MRA did not entirely exclude a small arteriovenous malformation or cavernous angioma, the only specific findings were infarction, a small presumed capillary telangiectasia in the left pons, and hypotrophy in the left midbrain. Anticoagulation was not considered because there were no abnormal findings that could cause a thromboembolic event, and cerebral hemorrhage had been observed to follow aspirin administration for cerebral infarction in a KTWS patient[9].
The ABI is the ratio of the blood pressure in an ankle to that in an arm and is a sensitive, non-invasive marker for diagnosing peripheral arterial disease (PAD). Without PAD, increased arterial impedance due to narrowed distal arteries increases the arterial blood pressure farther from the heart. Consequently, the systolic pressure in the ankle is usually higher than those of brachial arteries. People with no PAD have an ABI between 1.10 and 1.40[10]. We examined the differences in ABI and temperature in both legs although there were no stenotic lesions on CTA. To improve perfusion in the right leg, resistance and aerobic exercises were prescribed. After the exercise program, the ABI improved, reflecting improved peripheral artery stiffness and blood flow[11]. Resistance exercise training can increase muscle fiber size and tissue capillary networks[12]. The increased metabolites from contracting muscle diffuse to resistance arterioles and directly induce vasodilation; prolonged exercise prompts angiogenesis and increases peripheral blood volume[13]. The blood flow capacity of skeletal muscle increases with training, causing structural vascular remodeling within skeletal muscle, such as angiogenesis and remodeling of the arterial tree within skeletal muscle[12].
In conclusion, although rare, patients with KTWS may experience CNS vascular malformations and accompanying stroke. It is necessary to investigate whether such patients have any neurological or comorbid abnormalities. Even in the subacute or chronic period after neurological insult, integrated rehabilitation programs can lead to structural and functional enhancement.
Dr. Deng has nothing to disclose.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rehabilitation
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cao X, China; Ding N, China; Mzhavanadze ND, Russia S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Aradag A, Senoglu M, Sayhan S, Okromelidze L, Middlebrooks EH. Klippel-Trenaunay-Weber Syndrome with Atypical Presentation of Cerebral Cavernous Angioma: A Case Report and Literature Review. World Neurosurg. 2019;126:354-358. |
| 2. | Ang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trenaunay syndrome. J Vasc Surg Venous Lymphat Disrd. 2017;5(4):587-595. |
| 3. | Yoshinaga T, Yagi K, Morishita T, Abe H, Nonaka M, Inoue T. Cerebral and spinal cavernomas associated with Klippel-Trenaunay syndrome: case report and literature review. Acta Neurochir (Wien). 2018;160:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Kaminski TW, Buckley BD, Powers ME, Hubbard TJ, Ortiz C. Effect of strength and proprioception training on eversión to inversion strength ratios in subjects with unilateral functional ankle instability. Br J Sports Med. 2003;37(5):410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Renard D, Larue A, Taieb G, Jeanjean L, Labauge P. Recurrent cerebral infarction in Klippel-Trenaunay-Weber syndrome. Clin Neurol Neurosurg. 2012;114:1019-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Sakai K, Sibazaki K, Kimura K, Kobayashi K, Matsumoto N, Iguchi Y. Paradoxical brain embolism with Klippel-Trenaunay syndrome. Intern Med. 2011;50:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | rira M, Ben-Jemaa H, Lammouchi T, Benammou S. Syndrome de Klippel-Trenaunay et déficit en antithrombine III A propos d'une observation [Klippel-Trenaunay syndrome associated with antithrombin III deficiency]. Rev Neurol (Paris). 2008;164(10):855-858. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Star A, Fuller CE, Landas SK. Intracranial aneurysms in klippel-trenaunay/weber syndromes: case report. Neurosurgery. 2010;66:E1027-8; discussion E1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Beume LA, Fuhrmann SC, Reinhard M, Harloff A. Coincidence of ischemic stroke and recurrent brain haemorrhage in a patient with Klippel-Trenaunay Syndrome. J Clin Neurosci. 2013;20:1454-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | McDermott MM, Applegate WB, Bonds DE, Buford TW, Church T, Espeland MA, Gill TM, Guralnik JM, Haskell W, Lovato LC, Pahor M, Pepine CJ, Reid KF, Newman A. Ankle brachial index values, leg symptoms, and functional performance among community-dwelling older men and women in the lifestyle interventions and independence for elders study. J Am Heart Assoc. 2013;2:e000257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Fahs CA, Heffernan KS, Fernhall B. Hemodynamic and vascular response to resistance exercise with L-arginine. Med Sci Sports Exerc. 2009;41:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Verdijk LB, Snijders T, Holloway TM, VAN Kranenburg J, VAN Loon LJ. Resistance Training Increases Skeletal Muscle Capillarization in Healthy Older Men. Med Sci Sports Exerc. 2016;48:2157-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand. 1998;162:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 158] [Article Influence: 5.9] [Reference Citation Analysis (0)] |