Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9851
Peer-review started: May 2, 2022
First decision: May 30, 2022
Revised: June 11, 2022
Accepted: August 15, 2022
Article in press: August 15, 2022
Published online: September 26, 2022
Processing time: 136 Days and 21.9 Hours
Polyarthritis is the most frequent clinical manifestation in antisynthetase syndrome (ASS) forms of idiopathic inflammatory myositis and may be misdiagnosed as rheumatoid arthritis (RA), particularly in patients with seronegative RA (SNRA). It is unclear whether there is an overlap between ASS and RA, or if ASS sometimes mimics RA. Pulmonary hypertension (PAH) is common in connective tissue diseases (CTDs). However, published reports on CTD-PAH do not include overlapping CTDs, and its incidence and impact on patient prognosis are unclear.
We report the case of a 63-year-old woman who presented with a 3-mo history of symptom aggravation of recurrent symmetrical joint swelling and pain that had persisted for over 10 years. The patient was diagnosed with RA and interstitial lung disease. The patient repeatedly presented to the hospital’s respiratory and rhe
Overlap of RA and ASS may be missed. Further research is necessary to facilitate early diagnosis, effective evaluation, and prognosis.
Core Tip: The joint manifestations of antisynthetase syndrome are usually difficult to distinguish from rheumatoid arthritis (RA), particularly seronegative RA (SNRA); consequently, rheumatologists and respiratory pathologists should be aware of this rare and underrecognized special clinical phenotype. Whether this phenotype is more prone to pulmonary hypertension than a single connective tissue disease remains unknown. However, further research into anti-Jo1 antibodies, anti-RO-52, and other extractable nuclear antigen autoantibodies is necessary to facilitate the early diagnosis, evaluation, and prognosis of this overlapping clinical syndrome.
- Citation: Huang CY, Lu MJ, Tian JH, Liu DS, Wu CY. Pulmonary hypertension secondary to seronegative rheumatoid arthritis overlapping antisynthetase syndrome: A case report. World J Clin Cases 2022; 10(27): 9851-9858
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9851.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9851
Rheumatoid arthritis (RA) is a multifactorial, chronic, autoimmune disease characterized by significant heterogeneity in clinical presentations and outcomes among individuals with the same formal diagnosis[1]. The 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA include autoantibodies [rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (ACPA)] as biomarkers of the disease[2]. However, a sizeable subgroup of RA patients is negative for both ACPA and rheumatoid factor (seronegative RA, SNRA)[3]. Fur
Antisynthetase syndrome (ASS) was first designated in 1990 as the first clinical serological syndrome described in patients with polymyositis and dermatomyositis[4]. This syndrome is characterized by the binding of an autoanthexadese antibody to an aminoacyl-transferred RNA synthase, most commonly the Jo-1 antibody[4]. Clinical manifestations of ASS are diverse, including idiopathic inflammatory myositis (IIM), arthralgia and arthritis, interstitial pneumonia, Raynaud’s phenomenon, and skin lesions characterized by “mechanic’s hands[5].” The pathogenesis of ASS is unclear, but it is thought to be associated with chronic immune system activation[6]. It is also unclear whether there is an overlap between ASS and RA, or if ASS in some cases mimics RA[4]. In this report, we describe the case of a rare association of SNRA with ASS. Overlap between RA and ASS has only been reported previously in a few case reports[7].
Pulmonary hypertension (PAH) is a life-threatening complication associated with connective tissue diseases (CTD) such as RA[8]. While most patients with CTD have relatively stable interstitial changes in their lungs during follow-up, a disproportionate number of patients with PAH have gradually emerged[9]. Although PAH is relatively common in most forms of CTD, there are currently no reports of PAH secondary to RA overlapping with ASS[10]. In this report, we describe the case of a rare overlap of SNRA with ASS in a patient with PAH.
A 63-year-old woman presented with a 3-mo history of aggravation of recurrent symmetrical joint swelling and pain, which she had experienced episodically over 10 years. She first presented to our hospital in April 2018 for these symptoms.
The patient had a history of painful metacarpophalangeal joints in both hands with no obvious cause, with symmetrical and persistent attacks, starting from the interphalangeal and metacarpophalangeal joints of both hands and gradually involving both wrist joints, elbow joints, shoulder joints, and knee joints. Her symptoms were accompanied by morning stiffness lasting more than 1 h, which could be relieved by routine daily activities.
The patient was diagnosed with depression more than four years prior and was treated with antidepressant medication (medication type, dose, and duration were unknown). She denied any other disease history, including heart or lung diseases.
Aside from the depression noted above, the patient’s medical history was uneventful. There was no history of a similar illness in the family.
On initial evaluation, the patient’s vital signs were as follows: Body temperature, 36.0 °C; pulse, 93 beats/min; respiration, 20 times/min; and blood pressure, 108/72 mmHg. The patient also exhibited anxiety, slightly coarse breath sounds in both lungs, inspiratory dry rales in both lower lung fields, swan neck-like deformity in both hands, localized swelling, elevated skin temperature, abduction deformity of both thumbs, and slightly restricted movement of both knees. No significant abnormalities were observed during cardiac and abdominal examinations. No edema in the lower extremities was observed, and no pathological signs were found.
Initial laboratory examinations were as follows: Erythrocyte sedimentation rate, 74 mm/h; hypersensitivity C-reactive protein, 17.7 mg/L; creatine kinase, 1002.1 U/L; antinuclear antibody cytoplasmic granular positive (1:100); J0-1 +++; and Ro-52 +++. She was negative for anti-CCP and RF. Arterial blood gas analysis under oxygen absorption (nasal cannula 2 L/min) revealed a partial pressure of carbon dioxide (PCO2) of 29.7 mmHg and a partial oxygen pressure (PaO2) of 70.6 mmHg. Liver and kidney function, coagulation and D-dimer levels, and white blood cell and neutrophil ratios were normal.
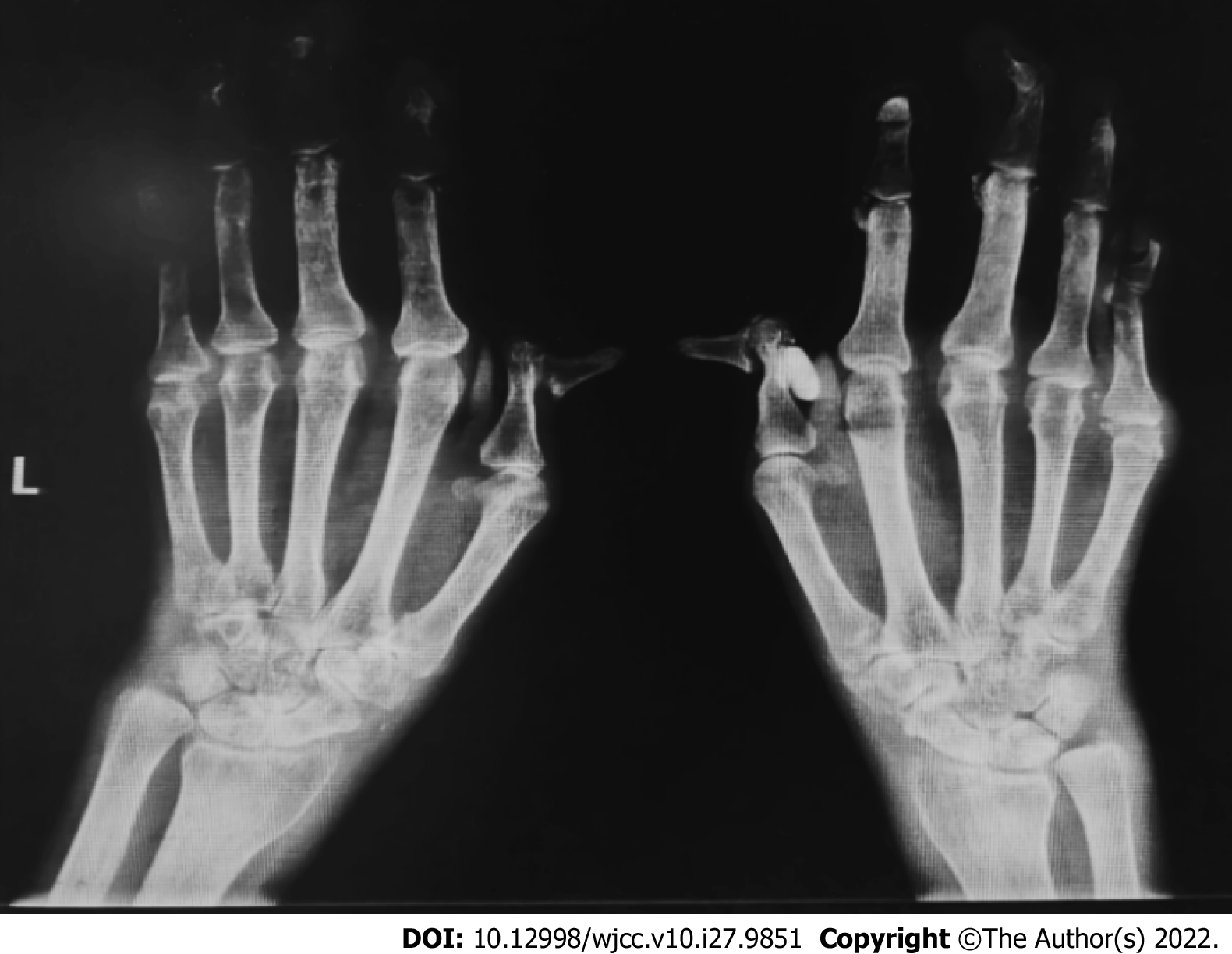
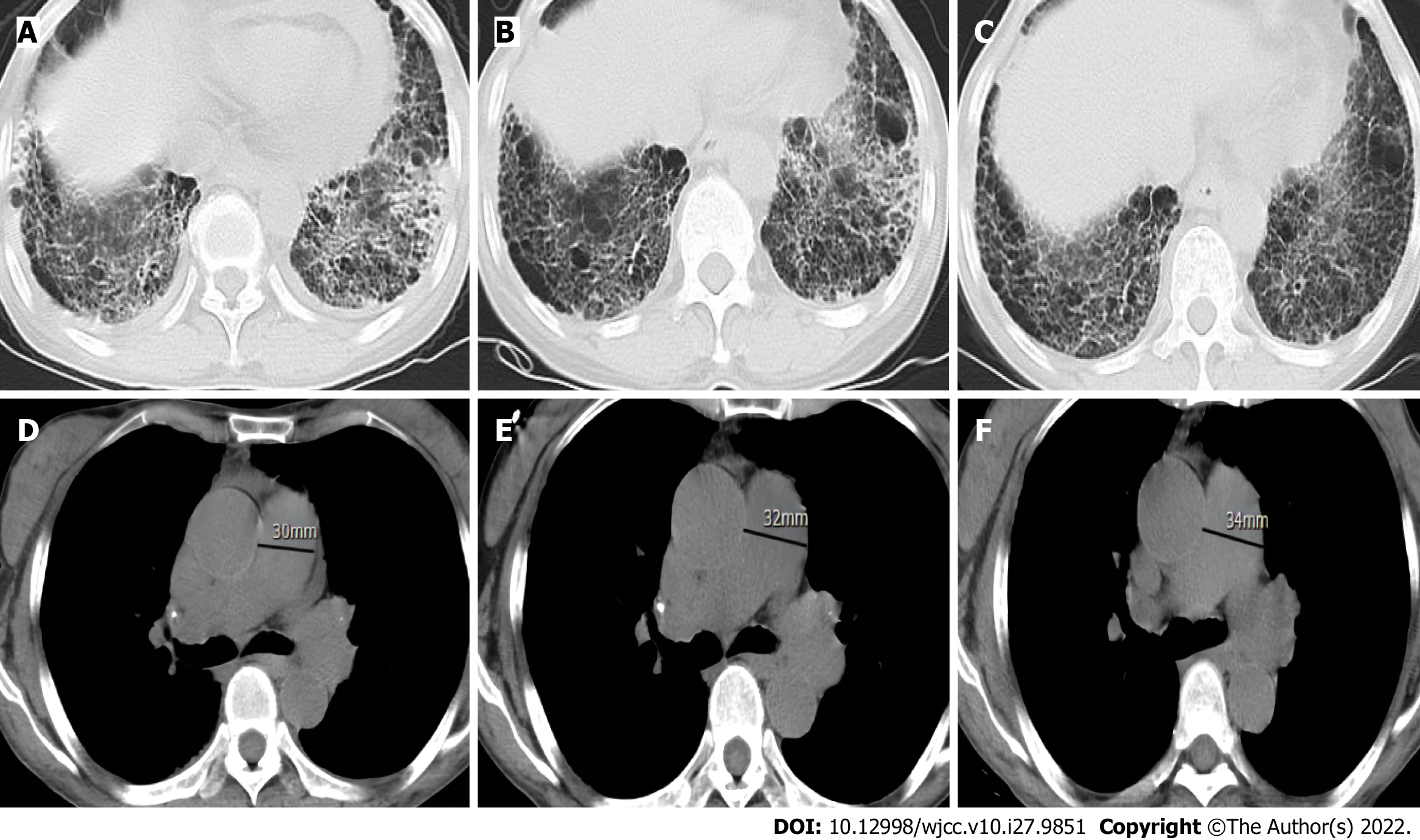
Plain radiographs of both hands (Figure 1) suggested degeneration, marked osteoporosis of the wrists, partial interphalangeal joint dislocation, and subluxation consistent with RA. Ultrasound of both knees suggested a luminal effusion and synovitis. Chest computed tomography (CT) revealed emphysema and interstitial degeneration in both lungs and interstitial pneumonia in both lower lung fields (Figure 2A-C). Pulmonary function tests suggested moderate restrictive ventilation dysfunction (forced vital capacity < 80%) and moderate diffuse function (diffusion capacity for carbon monoxide < 80%).
Considering the patient’s previous medical history and the results of our recent examinations, the patient was diagnosed with RA and interstitial lung disease (ILD).
The patient refused hormone and anti-rheumatic drug therapy. After providing analgesic and oxygen therapy, the patient’s symptoms were relieved, and she was discharged uneventfully.
After 3 mo, the patient was readmitted to the hospital due to a pulmonary infection. Her inflammatory and rheumatic immune indices had not changed significantly compared with the first hospitalization. However, cardiac ultrasound indicated PAH, and she was started on an anti-infective and anti-rheumatic treatment plan of leflunomide 20 mg QD, sulfasalazine enteric-coated tablets 0.5 g TID, and triptolide tablets 10 mg TID. She was instructed to have the treatment plan adjusted at regular follow-up visits with the rheumatology department.
Over the next 2 years, the patient repeatedly presented to the hospital’s respiratory and rheu
| Lab | Apr-18 | Apr-19 | Sep-20 | Apr-21 | Reference values |
| ESR | 74.0 | 88.0 | 56.3 | 82.1 | 0-20 mm/h |
| CRP | 17.7 | 55.8 | 28.7 | 36.8 | < 10 mg/L |
| Rheumatoid factor | < 20 | 20.3 | < 20 | < 20 | < 20 U/mL |
| Anti-cyclic citrullinated peptide antibody | 0.9 | 0.9 | < 0.5 | < 0.5 | < 5.0 U/mL |
| Antinuclear antibody titer | 1/100 | 1/100 | 1/100 | 1/1000 | Negative |
| C3 | 0.82 | 0.9 | 1.01 | 1.08 | 0.79-1.52 g/L |
| C4 | 0.13 | 0.18 | 0.20 | 0.19 | 0.16-0.38 g/L |
| R0-52 antibody | Positive | Positive | Strong positive | Strong positive | Negative |
| JO-1 antibody | Positive | Positive | Strong positive | Strong positive | Negative |
| p-ANCA | Negative | Negative | Negative | Negative | Negative |
| c-ANCA | Negative | Negative | Negative | Negative | Negative |
| Creatine kinase | 700.8 | 159.5 | - | 358.0 | 26-140 U/L |
| PaCO2 | 70.6 | 68.4 | - | 62.8 | 83-108 mmHg |
| PaO2 | 29.4 | 29.4 | - | 29.0 | 35-45 mmHg |
| Pulmonary artery systolic pressure | 47 | 55 | - | 72 | 15-30 mmHg |
RA is a heterogeneous systemic inflammatory disease characterized by painful polyarthritis, which has a significant impact on a patient’s quality of life, morbidity, and mortality[11]. Serological typing divides this condition into seropositive and seronegative RA based on the presence or absence of RF and anti-CCP antibodies, and both RA subtypes may have similar clinical manifestations despite different etiologies[12].
This report describes the case of a patient with SNRA who was previously treated for depression. Her diagnosis of ASS was delayed for 2 years because the joint manifestations of this condition were indistinguishable from RA, despite the presence of positive antibodies related to ASS. A review of cardiac ultrasound and chest CT images also revealed that the patient had progressively developed disproportionate PAH, based on stable interstitial lung changes. The following sections discuss these puzzling clinical presentations.
It has been reported that 18%-55% of patients with IIM have arthritis. As with RA, the most typical manifestation of joint disease in patients with IIM is symmetric non-erosive polyarthritis, primarily affecting the small joints of the hand[13]. Arthritis is particularly common in the ASS-associated form of IIM (20%-70%), with the highest prevalence associated with anti-Jo-1 positivity[13]. Radiological erosions are occasionally found in ASS, particularly in patients with overlapping RA[13]. RF and ACPA are relatively common in ASS patients with arthritis, as seen in 27.0%-31.5% and 11.0%-13.5% of patients with active arthritis, respectively[14]. The absence of arthritis is associated with a much lower pre
PAH is a life-threatening complication associated with CTD, particularly in systemic sclerosis and systemic lupus erythematosus. RA is the most common CTD, but the prevalence of PAH in patients with RA is lower than that in patients with CTDs such as scleroderma, mixed collagen tissue disease, and dermatomyositis/polymyositis[17]. Fayed et al[18] provided an overview of CTD-associated PAH types and reported that post-capillary PAH is most common in patients with RA, while lung disease-associated PAH is commonly seen in patients with IIM and sarcoidosis. The case of RA-associated PAH presented by Szturmowicz et al[17] illustrates the complexity of PAH in a single individual, with ILD-PAH dominating the early stage and CTD-PAH developing later.
Among patients with ASS, ILD is the main clinical feature and prognostic factor with an incidence of up to 80%, while PAH secondary to ILD is also frequently reported[9]. Approximately 7.9% of patients with ASS develop PAH[19]. It has been shown that PAH is usually severe and is believed to be related to Raynaud’s phenomenon, which is commonly associated with ASS[19]. A case report and literature review by García-Fernández et al[9] explored the relationship between ASS and PAH, and revealed that while their patients had stable and mild ILD, PAH at follow-up was disproportionate and unrelated to ILD severity. This study suggested that direct vascular involvement may lead to PAH in patients with ASS; both anti-Jo-1 positive and anti-PL-12 positive cases were found to have intimal proliferation on histological analysis of pulmonary arterial muscles, further supporting this pathophysiological theory[9].
PAH is common in most forms of CTD, but there are no reports of PAH secondary to RA overlapping ASS[10]. Current reports on CTD-PAH do not include overlapping CTDs, and its incidence and impact on patient prognosis are unclear[10]. There are currently no specific recommendations or guidelines for screening or treating patients with CTD, particularly those with PAH and ASS; however, routine echocardiography, electrocardiograms, pulmonary function tests, and timely initiation of specific treatments may improve prognosis[10].
There is no standardized protocol for the treatment of RA-ASS. While our patient’s case primarily manifested as joint and pulmonary conditions, for such an unusual clinical phenotype of SNRA overlapping with ASS, more clinical studies are required to specify and confirm an appropriate treatment protocol. One strength of our case study is that it is the first report of this particular phenotype, but a limitation is that although cardiac ultrasound can screen for PAH, our patient did not undergo the “gold standard” test.
The joint manifestation of ASS is usually difficult to distinguish from RA, particularly in patients with SNRA; as a result, rheumatologists and respiratory pathologists should be aware of this rare and underrecognized clinical phenotype. Whether patients with this phenotype are more prone to PAH compared with those with a single CTD remains unknown. However, further research into anti-Jo1 antibodies, anti-RO-52 antibodies, and other extractable nuclear-antigen autoantibodies is necessary to facilitate the early diagnosis, effective evaluation, and prognosis of this overlapping clinical syndrome.
We thank The First People’s Hospital of Zunyi (The Third Affiliated Hospital of Zunyi Medical University) for providing clinical information and patient assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Faraji N, Iran; Pradhan A, India; Tangsuwanaruk T, Thailand S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | De Stefano L, D’Onofrio B, Manzo A, Montecucco C, Bugatti S. The Genetic, Environmental, and Immunopathological Complexity of Autoantibody-Negative Rheumatoid Arthritis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6830] [Cited by in RCA: 6215] [Article Influence: 414.3] [Reference Citation Analysis (0)] |
| 3. | Erre GL, Mundula N, Colombo E, Mangoni AA, Sechi LA, Oggiano M, Irde R, Zinellu A, Passiu G, Carru C. Diagnostic Accuracy of Anticarbamylated Protein Antibodies in Established Rheumatoid Arthritis: A Monocentric Cross-Sectional Study. ACR Open Rheumatol. 2019;1:433-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Marco JL, Collins BF. Clinical manifestations and treatment of antisynthetase syndrome. Best Pract Res Clin Rheumatol. 2020;34:101503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 5. | Alfraji N, Mazahir U, Chaudhri M, Miskoff J. Anti-synthetase syndrome: a rare and challenging diagnosis for bilateral ground-glass opacities-a case report with literature review. BMC Pulm Med. 2021;21:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Gallay L, Gayed C, Hervier B. Antisynthetase syndrome pathogenesis: knowledge and uncertainties. Curr Opin Rheumatol. 2018;30:664-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Emad Y, Ragab Y, Abd-Elsalam M, Rasker JJ. Antisynthetase syndrome complicating the course of established case with rheumatoid arthritis: A rare and under-recognized overlapping disease. Reumatol Clin (Engl Ed). 2020;16:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Hernandez-Gonzalez I, Tenorio-Castano J, Ochoa-Parra N, Gallego N, Pérez-Olivares C, Lago-Docampo M, Palomino Doza J, Valverde D, Lapunzina P, Escribano-Subias P. Novel Genetic and Molecular Pathways in Pulmonary Arterial Hypertension Associated with Connective Tissue Disease. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | García-Fernández A, Quezada-Loaiza CA, de la Puente-Bujidos C. Antisynthetase syndrome and pulmonary hypertension: report of two cases and review of the literature. Mod Rheumatol Case Rep. 2021;5:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Xiao YS, Zhu FY, Luo L, Xing XY, Li YH, Zhang XW, Shen DH. [Clinical and immunological characteristics of 88 cases of overlap myositis]. Beijing Da Xue Xue Bao Yi Xue Ban. 2021;53:1088-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Yoshida K, Lin TC, Wei MY, Malspeis S, Chu SH, Camargo CA Jr, Raby BA, Choi HK, Tedeschi SK, Barbhaiya M, Lu B, Costenbader KH, Karlson EW, Sparks JA. Roles of Postdiagnosis Accumulation of Morbidities and Lifestyle Changes in Excess Total and Cause-Specific Mortality Risk in Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2021;73:188-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Paalanen K, Puolakka K, Nikiphorou E, Hannonen P, Sokka T. Is seronegative rheumatoid arthritis true rheumatoid arthritis? Rheumatology (Oxford). 2021;60:2391-2395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Klein M, Mann H, Vencovský J. Arthritis in Idiopathic Inflammatory Myopathies. Curr Rheumatol Rep. 2019;21:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | González-Gay MA, Montecucco C, Selva-O'Callaghan A, Trallero-Araguas E, Molberg O, Andersson H, Rojas-Serrano J, Perez-Roman DI, Bauhammer J, Fiehn C, Neri R, Barsotti S, Lorenz HM, Doria A, Ghirardello A, Iannone F, Giannini M, Franceschini F, Cavazzana I, Triantafyllias K, Benucci M, Infantino M, Manfredi M, Conti F, Schwarting A, Sebastiani G, Iuliano A, Emmi G, Silvestri E, Govoni M, Scirè CA, Furini F, Lopez-Longo FJ, Martínez-Barrio J, Sebastiani M, Manfredi A, Bachiller-Corral J, Sifuentes Giraldo WA, Cimmino MA, Cosso C, Belotti Masserini A, Cagnotto G, Codullo V, Romano M, Paolazzi G, Pellerito R, Saketkoo LA, Ortego-Centeno N, Quartuccio L, Batticciotto A, Bartoloni Bocci E, Gerli R, Specker C, Bravi E, Selmi C, Parisi S, Salaffi F, Meloni F, Marchioni E, Pesci A, Dei G, Confalonieri M, Tomietto P, Nuno L, Bonella F, Pipitone N, Mera-Valera A, Perez-Gomez N, Gerzeli S, Lopez-Mejias R, Matos-Costa CJ, Pereira da Silva JA, Cifrian J, Alpini C, Olivieri I, Blázquez Cañamero MÁ, Rodriguez Cambrón AB, Castañeda S, Cavagna L; AENEAS (American and European NEtwork of Antisynthetase Syndrome) collaborative group. Timing of onset affects arthritis presentation pattern in antisyntethase syndrome. Clin Exp Rheumatol. 2018;36:44-49. [PubMed] |
| 15. | Noguchi E, Uruha A, Suzuki S, Hamanaka K, Ohnuki Y, Tsugawa J, Watanabe Y, Nakahara J, Shiina T, Suzuki N, Nishino I. Skeletal Muscle Involvement in Antisynthetase Syndrome. JAMA Neurol. 2017;74:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Kumar RR, Jha S, Dhooria A, Naidu GSRSNK, Minz RW, Kumar S, Sharma SK, Sharma A, Jain S, Dhir V. Anti-Jo-1 Syndrome Often Misdiagnosed as Rheumatoid Arthritis (for Many Years): A Single-Center Experience. J Clin Rheumatol. 2021;27:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Szturmowicz M, Franczuk M, Jędrych ME, Wyrostkiewicz D, Oniszh K, Darocha S, Kasperowicz K, Kurzyna M. Dominating Cause of Pulmonary Hypertension May Change Over Time-Diagnostic and Therapeutic Considerations in a Patient with Pulmonary Hypertension Due to Rheumatoid Arthritis with Lung Involvement. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Fayed H, Coghlan JG. Pulmonary Hypertension Associated with Connective Tissue Disease. Semin Respir Crit Care Med. 2019;40:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Kay D, Kadri F, Fitzpatrick G, Alnuaimat H, Reddy R, Ataya A. Anti-synthetase syndrome-associated pulmonary veno-occlusive disease. Pulm Circ. 2020;10:2045894020935289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |