Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9768
Peer-review started: January 11, 2022
First decision: March 15, 2022
Revised: March 26, 2022
Accepted: August 14, 2022
Article in press: August 14, 2022
Published online: September 26, 2022
Processing time: 248 Days and 4.2 Hours
Giant cellulitis-like Sweet syndrome (SS) is a rare subtype of SS, and reports of the combined histiocytoid type of pathology are scarce. Here, we report a case of SS with distinctive clinical presentations and which was difficult to distinguish from cellulitis. By sharing this case and a discussion of the related literature in detail, we aim to provide clinicians with new insights into the characteristics of histiocytoid giant cellulitis-like (HGC)-SS and the pathogenesis of SS.
A 52-year-old male was admitted after experiencing progressive fatigue for 1 mo and tongue swelling with pain for 1 d. He was diagnosed with myelodysplastic syndrome (MDS) and angioneurotic edema of the tongue and floor of the mouth. However, 7 d after examination by sternal aspiration, a violaceous, tender, and swollen nodule developed at the site, with poorly demarcated erythema of the surrounding skin. Considering his profile of risk factors, the diagnosis of cellulitis was made and he was administered broad-spectrum antibiotics. When the lesion continued to worsen and he developed chills and fever, pathogenic and dermatopathological examination led to the diagnosis of HGC-SS. Treatment with prednisone led to the fever being relieved within 24 h and the skin lesion being resolved within 1 wk. The patient refused intensive treatment and was instead given thalidomide, erythropoietin, stanozolol, and supportive care. The prednisone was gradually tapered, with no signs of recurrence, but he died 2 mo later of severe pneumonia.
HGC-SS demonstrates unique manifestation. SS and leukemia cutis share cytological origin. Myelofibrosis and SS are adverse prognostic factors for MDS.
Core Tip: We describe the case of a 52-year-old male with myelodysplastic syndrome who developed histiocytoid giant cellulitis-like Sweet syndrome (SS) at a sternal puncture site. The patient had unique clinical presentations that have never been reported before, and the disease profile was difficult to distinguish from cellulitis. Cellulitis that has failed to respond to broad-spectrum anti-microbial therapy requires a skin biopsy. For patients with concurrent myeloid neoplasm and SS, intensive treatment is necessary since SS and myeloid leukemia cutis may be different stages of the same disease and both indicate a poor prognosis.
- Citation: Zhao DW, Ni J, Sun XL. Histiocytoid giant cellulitis-like Sweet syndrome at the site of sternal aspiration: A case report and review of literature. World J Clin Cases 2022; 10(27): 9768-9775
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9768.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9768
Giant cellulitis-like (GC-) Sweet syndrome (SS), a morphologically distinctive clinical variant of SS, is characterized by recurrent, multifocal, widespread, infiltrative plaques consisting mainly of mature neutrophils with bullous appearance, and can involve the arms, legs, abdomen, and/or trunk[1]. According to available case reports, GC-SS tends to occur in patients with obesity, autoimmune di
Histiocytoid (H-)SS is an uncommon histopathologic variant of SS, characterized by an infiltration mostly composed of immature myelomonocytic cells with histiocytoid morphology[6]. Clinical features of H-SS are mostly similar to those of classical neutrophilic (N-)SS, consisting of tender erythematous plaques and nodules on the extremities and trunk and often accompanied by systemic symptoms, including fever, arthritis, episcleritis, and diarrhea[7]. SS that has both histiocytoid pathology and features of a GC lesion is extremely rare. Here, we report the case of a 52-year-old male with MDS who developed HGC-SS at the sternal puncture site featuring a unique clinical manifestation profile that was difficult to distinguish from cellulitis; to our knowledge, such a case has never been reported before. We discuss the diagnosis and management of cellulitis, the clinical features of HGC-SS, the relationship between SS and leukemia cutis (LC), and their relationship between myeloid neoplasms (MNs) in the context of diagnostic and therapeutic procedures and relevant literature to facilitate timely diagnosis and effective treatment.
A 52-year-old male was admitted to our hospital after 1 mo of fatigue and 1 d of tongue swelling and pain.
The patient reported that he had started to feel fatigued 1 mo prior, and the feeling had worsened gradually over that time. He also reported that on the day prior, his tongue had become swollen and painful after eating conch flesh. He denied any coincident fever, rash, dyspnea, night sweats, weight loss, etc.
The patient had no significant past medical history.
The patient had no history of toxin or radiation exposure and had no family history of malignancy nor hereditary disease.
Positive physical examination findings were pallor, swollen tongue, and splenomegaly (3 cm below the left costal margin).
Routine blood tests yielded the following results: Low white blood cell count [1.82 × 109/L; normal range: (3.5-9.5) × 109/L]; slightly low absolute neutrophil count [1.07 × 109/L; normal range: (1.8-6.3) × 109/L]; low lymphocyte count [0.6 × 109/L; normal range: (1.1-3.2) × 109/L] with normal lymphocyte proportion (23.5%; normal range: 20%-50%); very low platelet count [19 × 109/L; normal range: (125-350) × 109/L]; and low hemoglobin level (53 g/L; normal range: 130-175 g/L). The red blood mean cell volume and mean corpuscular hemoglobin were within the normal ranges, as was the reticulocyte count [29.8 × 109/L; normal range: (25-112) × 109/L]. Peripheral blood (PB) smear showed erythroblasts, oval erythrocytes, and teardrop-shaped red blood cells that were readily visible, with 3% myeloblasts. In addition, lactate dehydrogenase and C-reactive protein were elevated (351 IU/L; normal range: 109-245 IU/L and 84.4 mg/L; normal range: 0-8 mg/L, respectively). Folic acid and vitamin B12 levels were normal. Direct antiglobulin (Coombs) testing and paroxysmal nocturnal hemoglobinuria clone testing yielded negative results.
There was high suspicion of hematologic malignancy because of pancytopenia, splenomegaly, and increased blast cell count in PB. Bone marrow aspiration and bone marrow biopsy were performed to clarify the diagnosis. The bone marrow aspiration yielded a “dry” tap, even at the sternal level. Bone marrow biopsy showed the marrow to be hypercellular, with erythroid hyperplasia and a slight increase in granulocytic precursors; moreover, megakaryocytes were normal in number, and micromegakaryocytes and nonlobulated megakaryocytes were readily visible. The reticulin fibrosis was grade 2 (designated MF-2), according to the World Health Organization grading for myelofibrosis. Flow cytometry and chromosomal and gene mutation testing were not performed due to the difficulties in obtaining bone marrow fluid.
There are none imaging examinations.
The patient had pancytopenia, hypercellular bone marrow, marked dysplasia of the megakaryocytic lineage, 2%-4% myeloblasts in PB, no Auer rods, and grade-2 myelofibrosis; in accordance with the World Health Organization classification of MNs and acute leukemia[8], the patient was diagnosed with MDS with excess blasts-1 associated with myelofibrosis. He was also diagnosed with an angioneurotic edema of the tongue and floor of the mouth.
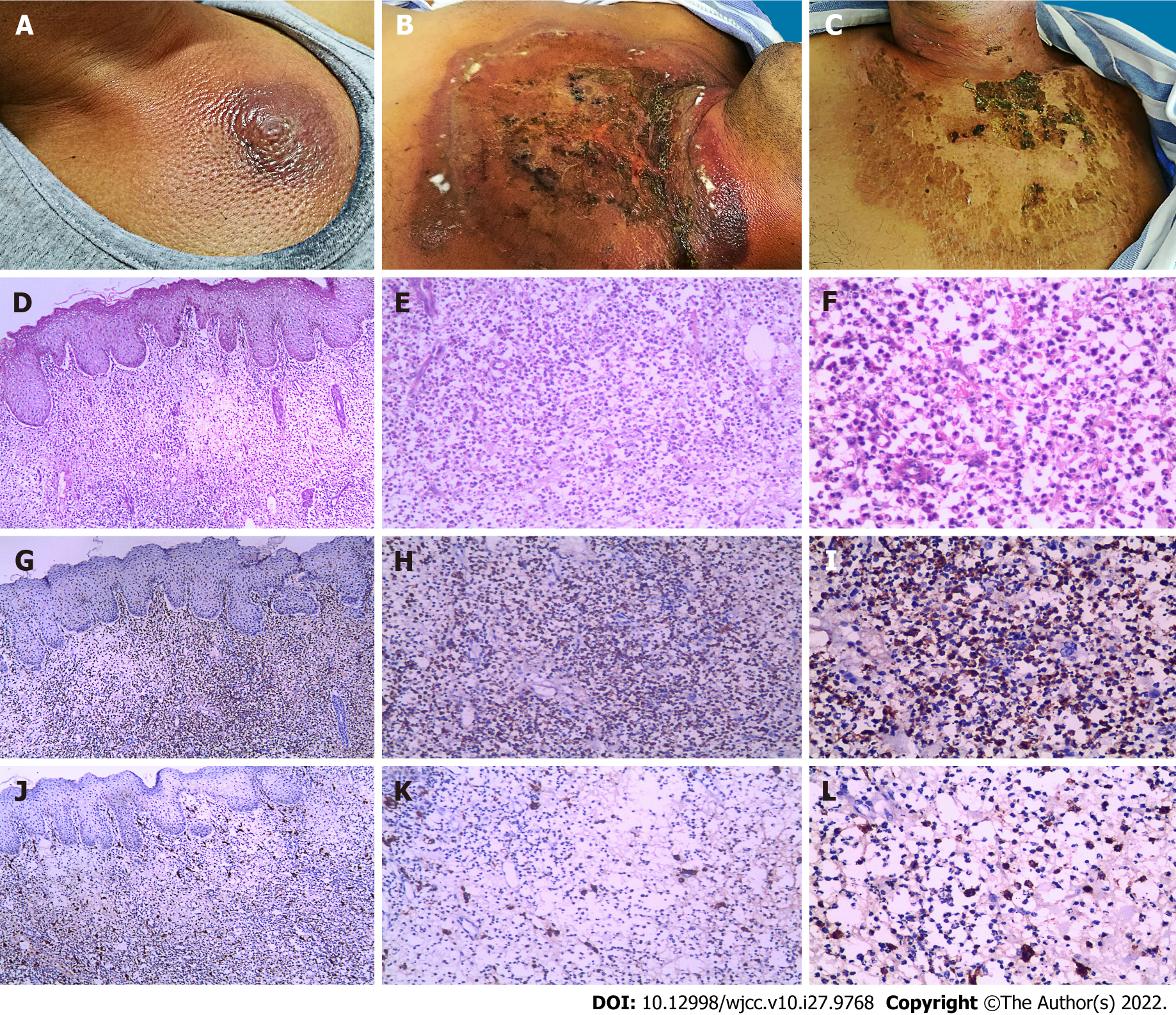
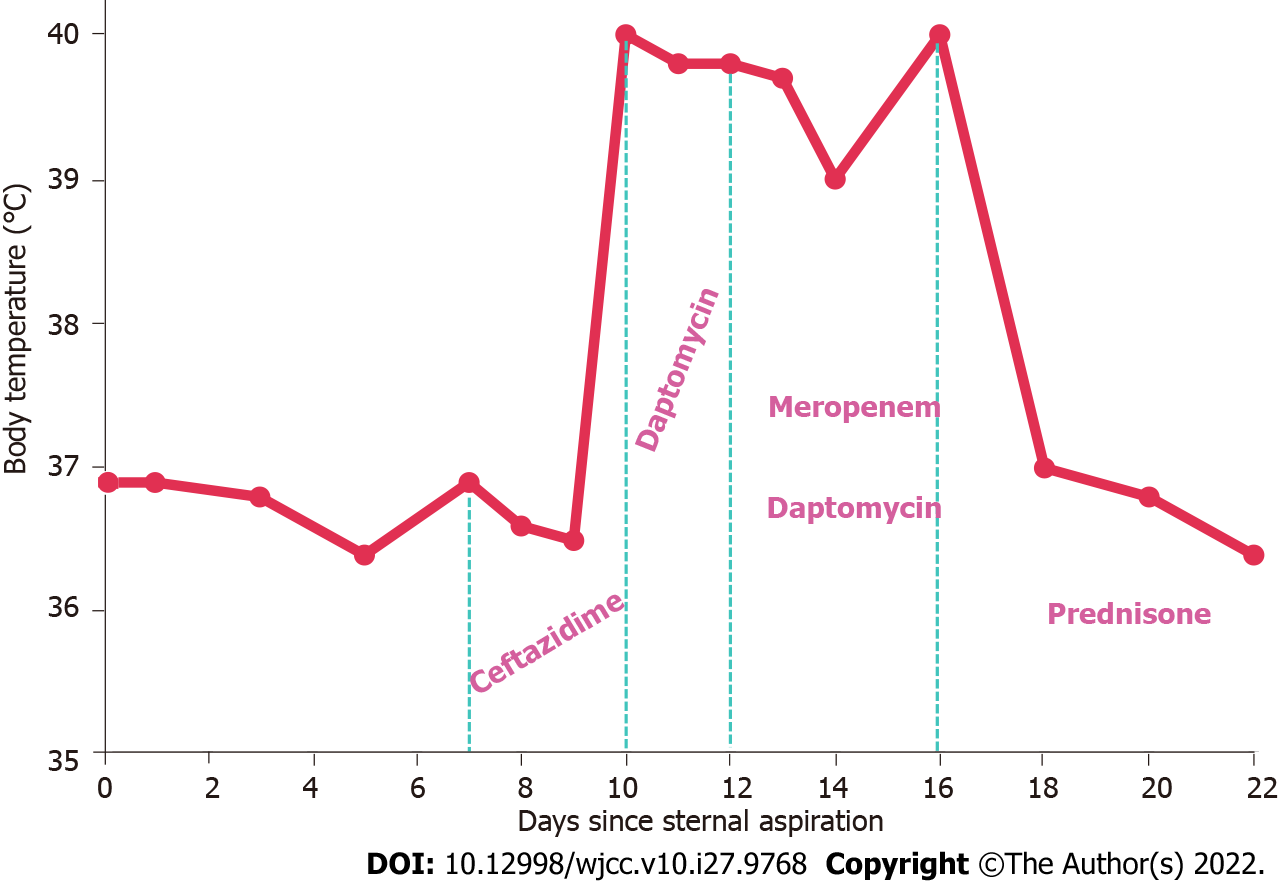
The patient refused cytoreductive therapy or hematopoietic stem cell transplantation. Therefore, thalidomide (50 mg/d), erythropoietin (10000 IU/d), and stanozolol (6 mg/d) were administered, along with supportive care. Dexamethasone (10 mg/d) and loratadine (10 mg/d) were also administered for 5 d to treat the angioneurotic edema. After 5 d of treatment, the tongue swelling was relieved. However, 7 d after the sternal aspiration (2 d after discontinuation of dexamethasone), a poorly demarcated area of erythema appeared on the skin around the puncture site (4 cm in diameter). The area was swollen, warm, painful, and tender, with a violaceous swollen nodule in the center (Figure 1A). Ultrasonography demonstrated subcutaneous edema without abscesses. Considering the predisposing factors for cellulitis, including skin barrier disruption, a history of systemic steroid use, neutropenia, and failure to maintain local hygiene of the puncture point, non-purulent cellulitis was diagnosed. The patient was administered ceftazidime [2 g intravenous (IV) every 8 h]. However, the lesion continued to expand, with bulla formation. On the 3rd d of anti-infective treatment, he developed chills and fever (maximum body temperature of 40 °C) and his procalcitonin was high (22.80 ng/mL; normal range: 0-0.05 ng/mL). To address the possibility of infection with methicillin-resistant Staphylococcus aureus (MRSA) and Gram-negative bacteria, the ceftazidime was replaced by daptomycin (4 mg/kg IV every 24 h) and meropenem (1 g IV every 8 h). However, his condition did not improve (Figures 1B and 2).
Blood cultures for bacteria and fungi were negative, and blister smears did not find any bacteria, fungi, or mycobacteria. To further differentiate the diagnosis, a skin biopsy was performed. Histopathology of the cutaneous lesion presented moderate edema in the papillary dermis, and dense, inflammatory infiltrate involving the superficial and mid-dermis, predominantly composed of myeloperoxidase (MPO)+ CD163- mononuclear cells with twisted vesicular nuclei and scant eosinophilic cytoplasm, but without evidence of vasculitis (Figures 1D-L). Next-generation sequencing of the biopsy specimen, based on the Illumina platform (iSeq 100 Sequencing System), covering 9694 bacteria, 1551 fungi, 6761 viruses, 144 mycobacteria, 305 parasites, and 107 mycoplasma/chlamydia, was negative.
The final diagnosis is HGC-SS.
The patient was treated with prednisone (1 mg/kg/d).
The fever relieved within 24 h and the skin lesion resolved within 1 wk (Figures 1C and 2). The prednisone was gradually tapered, with no recurrence. Because the combination of glucocorticoid and immunomodulatory therapy was effective in treating the lesion, and non-anti-leukemic regimens are not effective against LC, the diagnosis H-SS was confirmed while the possibility of LC was ruled out. This distinction is important because it is difficult to distinguish between H-SS and LC by clinical and pathological features alone. Unfortunately, the patient died of severe pneumonia 2 mo later.
Cellulitis is an infection involving the deep dermis and subcutaneous tissue, typically precipitated by the entry of bacteria through a breach in the skin barrier[9]. Predisposing factors to cellulitis infection include increasing age, obesity, barrier disruption, skin inflammation, pre-existing skin infection, chronic leg edema, immunosuppression, and previous cellulitis infection[10,11]. It typically presents with acute onset of poorly demarcated erythema, swelling, tenderness, and warmth, and can affect any area of the skin. Systemic manifestations, such as fevers, chills, and fatigue, indicate a more severe infection[12]. Cellulitis is a clinical diagnosis based on clinical history and findings from physical examination; however, a gold standard for diagnosis does not exist. As such, although it is a common disease, the misdiagnosis rate has been estimated to be 30.7%, even after a dermatology consult[13].
The patient described herein had risk factors for cellulitis, including hematological malignancies, neutropenia, and glucocorticoid-induced immunosuppression. Moreover, the skin lesion characteristic of cellulitis appeared after local skin puncture, supporting the initial diagnosis of cellulitis. The treatment of non-purulent cellulitis is most often initiated empirically due to the low clinical isolation rate of pathogens (< 20%), covering Streptococcus pyogenes and methicillin-susceptible Staphylococcus aureus, which are the most common pathogens[14]. In addition to treatment for the nosocomial infection, malignancy, and neutropenia, the patient received ceftazidime treatment. When there is no response after 24 h to 48 h of anti-infective therapy, drug-resistant bacteria, such as MRSA, Gram-negative bacteria, and atypical organisms should be considered[12]; therefore, we switched to a broad-coverage antibiotic regimen, but it was still ineffective. Combined with the negative results of blood and blister fluid pathogenic tests, the possibility of pseudocellulitis was considered.
SS is an uncommon inflammatory disorder characterized by an abrupt onset of painful erythematous plaques or nodules, histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis, usually accompanied by fever and elevated inflammatory markers, such as neutrophils’ count, erythrocyte sedimentation rate, and C-reactive protein[15]. Moreover, SS is often associated with a range of underlying disorders, such as hematologic or visceral malignancy, inflammatory disease, or pregnancy, or preceded by an upper respiratory or gastrointestinal infection or vaccination[16,17].
The lesions of GC-SS are characterized by recurrent, abrupt appearance of large, well-defined infiltrated plaques, with bullous appearance and involving multiple skin areas[1-5]. However, the characteristics of our patient’s lesion differed from those previously described; namely, it was solitary and progressively expanding, making it much more difficult to distinguish from cellulitis. The sequential occurrence of cellulitis and SS at the same site has been reported, and it is considered that SS may be induced by infection[18,19]. For this patient, the possibility of local infection-induced SS cannot be excluded.
After meeting the diagnostic criteria of SS, H-SS is diagnosed based on pathology results. The typical pathological feature of H-SS is a dense, band-like, inflammatory infiltrate predominantly composed of mononuclear cells with large, elongated, twisted, or kidney-shaped vesicular nuclei, inconspicuous nucleoli, and scant eosinophilic cytoplasm mimicking small histiocytes, which are MPO-positive immature nonblastic myeloid cells, and CD163-positive inflammatory cells, which are considered to be macrophages, scattered around[20]. The skin lesion infiltrate in cellulitis-like SS consists mainly of mature neutrophils. SS with both the pathological characteristics of H-SS and the lesion features of GC-SS is HGC-SS. The only reported case of HGC-SS involved an elderly woman with a MN, who also had a progressively enlarging solitary lesion, which, unlike in the present case, occurred without local irritation, and the lesion was sharply demarcated without bulla formation, making it easier to distinguish from cellulitis[21]. The clinical features of this particular type of SS require further investigation.
SS and LC may occur concurrently in the same skin lesion, especially in patients with myeloid malignancies[22,23]. LC features the specific cutaneous involvement of neoplastic leukocytes[24,25]. LC can also precede the transformation of MDS to acute myeloid leukemia, up to 12 mo in advance, which is termed as ‘aleukemic LC’. MDS combined with aleukemic LC predicts disease progression and poor prognosis, and patients may benefit from more aggressive therapeutic strategies, such as allogeneic bone marrow transplantation[26-28]. In HSS, it is difficult to identify whether myeloid LC (MLC) co-exists because they present overlapping features, and neither the cytologic nor the immunohistochemical characteristics of the cells can exclude the involvement of leukemia cells[29]. However, next-generation sequencing analysis has determined that neutrophils in the N-SS skin lesion samples of MN patients has a high clonal correlation with paired bone marrow or PB leukemia cells[30,31]. In MN patients with H-SS, fluorescence in situ hybridization detection of skin lesions has indicated that skin infiltrating cells have the same cytogenetic changes as neoplasm cells, highlighting the clonal correlation between the two[32]. These findings indicate that dermal infiltrating cells of SS and MLC have a common progenitor origin, the dysplastic myeloid cells in the skin lesions may transform from leukemia cells, and SS and MLC may be two different stages of the same disease. The local inflammatory response may be a common predisposing factor for both diseases[33-35]. This may explain why cells at different stages of maturation can appear in the same skin lesion, why SS has a high incidence in MN, and why MN patients with either of these two diseases have poor prognosis[36-39]. Therefore, for MN patients with SS, aggressive treatment is required to improve survival.
In this patient, the homology of the infiltrating cells in the lesion with the neoplasm cells could not be verified further because no cytogenetic nor molecular biological information of the neoplasm cells was available. MDS combined with myelofibrosis has a high risk of progression to acute leukemia or bone marrow failure, and the higher the fibrosis grade, the worse the prognosis[40-42]. Allogeneic hematopoietic stem cell transplantation may be able to overcome the poor prognosis associated with myelofibrosis[43]. For our patient, early skin biopsy may have led to a more timely diagnosis, and aggressive therapy may have improved his prognosis; however, the patient was unwilling to take such measures.
The clinical manifestation of HGC-SS is very different from that of GC-SS; however, whether the lesion is solitary and starts small and then gradually enlarges, and whether it is more likely to occur in MN patients remains to be concluded. When anti-microbial treatment for cellulitis is ineffective, skin biopsy and next-generation pathogen sequencing of the tissue are required to clarify the diagnosis. It is possible that SS and MLC may be different stages of the same disease, with a similar pathogenesis; however, this hypothesis requires further investigation. Both SS and MLC are poor prognostic factors for patients with MN and require intensive treatment to improve survival.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: China Medical Education Association; Chinese Society of Clinical Oncology; China Medical Women’s Association; China Anti-cancer Association.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee CY, Taiwan; Tajiri K, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Surovy AM, Pelivani N, Hegyi I, Buettiker U, Beltraminelli H, Borradori L. Giant cellulitis-like Sweet Syndrome, a new variant of neutrophilic dermatosis. JAMA Dermatol. 2013;149:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Georgesen C, Ahn C, Asgari M, Strowd L, Sangueza O. A Man With Fever and Bullous Plaques on the Thigh: Answer. Am J Dermatopathol. 2019;41:688-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Kaminska EC, Nwaneshiudu AI, Ruiz de Luzuriaga A, Tsoukas M, Bolotin D. Giant cellulitis-like Sweet syndrome in the setting of autoimmune disease. J Am Acad Dermatol. 2014;71:e94-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Mitaka H, Jammal R, Saabiye J, Yancovitz S, Perlman DC. Giant cellulitis-like Sweet syndrome: An underrecognized clinical variant mimicking skin and soft tissue infection. IDCases. 2020;21:e00874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Okuyama S, Nito T, Yanagawa N, Tajima K. Giant cellulitis-like Sweet syndrome as an initial clinical presentation of acute myeloblastic leukemia with t(6;9)(p23;q34): DEK-CAN and internal duplications of FMS-like tyrosine kinase 3. Ann Hematol. 2019;98:787-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Requena L, Kutzner H, Palmedo G, Pascual M, Fernández-Herrera J, Fraga J, García-Díez A, Yus ES. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Ghoufi L, Ortonne N, Ingen-Housz-Oro S, Barhoumi W, Begon E, Haioun C, Pautas C, Beckerich F, Robin C, Wolkenstein P, Cordonnier C, Chosidow O, Toma A. Histiocytoid Sweet Syndrome Is More Frequently Associated With Myelodysplastic Syndromes Than the Classical Neutrophilic Variant: A Comparative Series of 62 Patients. Medicine (Baltimore). 2016;95:e3033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5219] [Cited by in RCA: 6747] [Article Influence: 749.7] [Reference Citation Analysis (0)] |
| 9. | Rrapi R, Chand S, Kroshinsky D. Cellulitis: A Review of Pathogenesis, Diagnosis, and Management. Med Clin North Am. 2021;105:723-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | McNamara DR, Tleyjeh IM, Berbari EF, Lahr BD, Martinez J, Mirzoyev SA, Baddour LM. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Quirke M, Ayoub F, McCabe A, Boland F, Smith B, O'Sullivan R, Wakai A. Risk factors for nonpurulent leg cellulitis: a systematic review and meta-analysis. Br J Dermatol. 2017;177:382-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Raff AB, Kroshinsky D. Cellulitis: A Review. JAMA. 2016;316:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 13. | Ko LN, Garza-Mayers AC, St John J, Strazzula L, Vedak P, Shah R, Dobry AS, Rao SR, Milne LW, Parry BA, Kroshinsky D. Effect of Dermatology Consultation on Outcomes for Patients With Presumed Cellulitis: A Randomized Clinical Trial. JAMA Dermatol. 2018;154:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 964] [Article Influence: 96.4] [Reference Citation Analysis (0)] |
| 15. | Cohen PR. Sweet's syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 530] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 16. | Paydas S. Sweet's syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol. 2013;86:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Joshi TP, Friske SK, Hsiou DA, Duvic M. New Practical Aspects of Sweet Syndrome. Am J Clin Dermatol. 2022;23:301-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 18. | Dinh H, Murugasu A, Gin D. Sweet's syndrome associated with cellulitis. Australas J Dermatol. 2007;48:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Resende C, Santos R, Pereira T, Brito C. Sweet's syndrome associated with cellulitis - a challenging diagnosis. An Bras Dermatol. 2016;91:94-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, Rodríguez-Peralto JL, Alegre V, Cerroni L, Kutzner H, Requena L. Clinicopathologic, Immunohistochemical, and Molecular Features of Histiocytoid Sweet Syndrome. JAMA Dermatol. 2017;153:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | So JK, Carlos CA, Frucht CS, Cohen PR. Histiocytoid giant cellulitis-like Sweet's syndrome: case report and review of the literature. Dermatol Online J. 2015;21. [PubMed] |
| 22. | del Pozo J, Martínez W, Pazos JM, Yebra-Pimentel MT, García Silva J, Fonseca E. Concurrent Sweet's syndrome and leukemia cutis in patients with myeloid disorders. Int J Dermatol. 2005;44:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Dominguez ER, Greene JN, Sandin RL, DeGregorio R, Glass LF. Sweet Syndrome and Leukemia Cutis in the Same Patient: A Case Report and Review. Cancer Control. 1995;2:343-346. [PubMed] |
| 24. | Parsi M, Go MS, Ahmed A. Leukemia Cutis. 2022 Jul 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 25. | Barry D, Schmieder A. Leukemia Cutis. N Engl J Med. 2021;385:1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Longacre TA, Smoller BR. Leukemia cutis. Analysis of 50 biopsy-proven cases with an emphasis on occurrence in myelodysplastic syndromes. Am J Clin Pathol. 1993;100:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Findakly D, Amar S. A Rare Case of Leukemia Cutis as the First Presentation of a Myelodysplastic Syndrome to Acute Myeloid Leukemia Transformation. Cureus. 2020;12:e8698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Patel LM, Maghari A, Schwartz RA, Kapila R, Morgan AJ, Lambert WC. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Valerón-Almazán P, Bastida J, Vilar J, Santana N, Medina C, Carretero G. Utility of myeloperoxidase stain in the differential diagnosis of leukemia cutis vs. hystiocitoid Sweet syndrome. Dermatol Online J. 2011;17:11. [PubMed] |
| 30. | Passet M, Lepelletier C, Vignon-Pennamen MD, Chasset F, Hirsch P, Battistella M, Duriez P, Sicre de Fontbrune F, Boissel N, Legrand O, Raffoux E, Bagot M, Adès L, Clappier E, Bouaziz JD. Next-Generation Sequencing in Myeloid Neoplasm-Associated Sweet's Syndrome Demonstrates Clonal Relation between Malignant Cells and Skin-Infiltrating Neutrophils. J Invest Dermatol. 2020;140:1873-1876.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Mo W, Wang X, Wang Y, Li Y, Zhang R. Clonal neutrophil infiltrates in concurrent Sweet's syndrome and acute myeloid leukemia: A case report and literature review. Cancer Genet. 2018;226-227:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Chavan RN, Cappel MA, Ketterling RP, Wada DA, Rochet NM, Knudson R, Gibson LE. Histiocytoid Sweet syndrome may indicate leukemia cutis: a novel application of fluorescence in situ hybridization. J Am Acad Dermatol. 2014;70:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Ke H, Gong XP, Su HC, Su W, Cheng B. Leukemia cutis following herpes zoster infection: An unusual example of Wolf's isotopic response. Indian J Dermatol Venereol Leprol. 2019;85:539-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Wintzen M, Kluin PM, den Ottolander GJ. Specific cutaneous infiltrate caused by Staphylococcus aureus in a patient with chronic myelomonocytic leukemia. Ann Hematol. 2000;79:402-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Heath MS, Ortega-Loayza AG. Insights Into the Pathogenesis of Sweet's Syndrome. Front Immunol. 2019;10:414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 36. | Aractingi S, Bachmeyer C, Dombret H, Vignon-Pennamen D, Degos L, Dubertret L. Simultaneous occurrence of two rare cutaneous markers of poor prognosis in myelodysplastic syndrome: erythema elevatum diutinum and specific lesions. Br J Dermatol. 1994;131:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Lepelletier C, Bouaziz JD, Rybojad M, Bagot M, Georgin-Lavialle S, Vignon-Pennamen MD. Neutrophilic Dermatoses Associated with Myeloid Malignancies. Am J Clin Dermatol. 2019;20:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Marcoval J, Martín-Callizo C, Valentí-Medina F, Bonfill-Ortí M, Martínez-Molina L. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Nelson CA, Noe MH, McMahon CM, Gowda A, Wu B, Ashchyan HJ, Perl AE, James WD, Micheletti RG, Rosenbach M. Sweet syndrome in patients with and without malignancy: A retrospective analysis of 83 patients from a tertiary academic referral center. J Am Acad Dermatol. 2018;78:303-309.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Della Porta MG, Malcovati L. Myelodysplastic syndromes with bone marrow fibrosis. Haematologica. 2011;96:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Kröger N, Zabelina T, van Biezen A, Brand R, Niederwieser D, Martino R, Lim ZY, Onida F, Schmid C, Garderet L, Robin M, van Gelder M, Marks R, Symeonidis A, Kobbe G, de Witte T; MDS Subcommittee of the Chronic Leukemia Working Party (CLWP) of the European Group for Blood and Marrow Transplantation (EBMT). Allogeneic stem cell transplantation for myelodysplastic syndromes with bone marrow fibrosis. Haematologica. 2011;96:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Buesche G, Teoman H, Wilczak W, Ganser A, Hecker H, Wilkens L, Göhring G, Schlegelberger B, Bock O, Georgii A, Kreipe H. Marrow fibrosis predicts early fatal marrow failure in patients with myelodysplastic syndromes. Leukemia. 2008;22:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Zeng X, Xuan L, Fan Z, Zhang Y, Zhao K, Zhou Y, Xu J, Liu Q, Dai M. Allogeneic stem cell transplantation may overcome the adverse impact of myelofibrosis on the prognosis of myelodysplastic syndrome. Exp Hematol Oncol. 2021;10:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |