Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9703
Peer-review started: April 3, 2022
First decision: May 11, 2022
Revised: May 17, 2022
Accepted: August 15, 2022
Article in press: August 15, 2022
Published online: September 26, 2022
Processing time: 165 Days and 22 Hours
Gemcitabine plus nab-paclitaxel (GA) is a commonly used first-line treatment regimen for metastatic pancreatic cancer, and many studies will add a novel targeted agent to this regimen for improving patient survival rate. However, the clinical effectiveness of GA is the most controversial issue.
To compare the efficacy and safety of GA regimen with a targeted agent and GA regimen.
Up to 1 December 2021, the eligible randomized controlled trials (RCTs) relating to GA and GA with a targeted agent were searched on PubMed, EMBASE and Cochrane Library for eligible data. We screened out appropriate studies for overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and toxicity, which had been pooled and finally analyzed by using Stata version 15.1. In addition, we use Reference Citation Analysis (https://www.referencecitationanalysis.com/) to collect the latest related literature to improve the latest cutting-edge research results.
Seven RCTs involving 1544 patients (848 men and 696 women) were included. There were no significant differences between GA with a targeted agent and GA in PFS [hazard ratio (HR): 1.18 95% confidence interval (CI): 0.91-1.53], OS (HR: 1.12 95%CI: 0.99-1.27), and ORR (HR: 0.96 95%CI: 0.71-1.29). There was no notable difference in the two groups in grade 3/4 toxicity (fatigue, anemia, vomiting and neutropenia), whereas the incidence of grade 3/4 diarrhea considerably increased in GA with a targeted drug.
Adding a novel targeted agent to the GA regimen did not improve survival rate of patients with metastatic pancreatic cancer.
Core Tip: Gemcitabine plus nab-paclitaxel (GA) is a commonly used first-line treatment regimen for metastatic pancreatic cancer, and many studies will add a novel targeted agent to this regimen to improve patient survival. However, the clinical effectiveness of GA is more controversial. We conducted a meta-analysis to compare the effectiveness and safety of GA combined with a targeted agent regimen and GA regimen.
- Citation: Li ZH, Ma YJ, Jia ZH, Weng YY, Zhang P, Zhu SJ, Wang F. Meta-analysis of gemcitabine plus nab-paclitaxel combined with targeted agents in the treatment of metastatic pancreatic cancer. World J Clin Cases 2022; 10(27): 9703-9713
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9703.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9703
Pancreatic cancer is a highly malignant disease with annual incidence rates ranging from 0.5% to 1.0%[1]. One study predicted that by 2040, pancreatic cancer will be the second most common cause of cancer-related deaths in the United States[2]. The current treatment outcome of pancreatic cancer is still not ideal, and an important reason is the shortage of effective screening and diagnosing modalities, which causes most patients to be in locally advanced (30%-35%) or metastatic (50%-55%) stage when diagnosed. In addition, surgery is the only treatment modality to cure pancreatic cancer, but 80%-85% of pancreatic cancer patients have missed the opportunity for surgical resection when diagnosed[3]. Even if surgical treatment improves patient outcomes, the 5-year survival rate is still < 10%[4]. Currently, gemcitabine and albumin combined with paclitaxel or modified FOLFIRINOX (fluorouracil, calcium folinate, irinotecan and oxaliplatin) are the standard regimen for treating metastatic pancreatic cancer. According to the results of a phase III study on 861 patients with metastatic pancreatic cancer, Von Hoff et al[5] showed that gemcitabine plus nab-paclitaxel (GA) had a higher survival rate than single-agent gemcitabine [median survival 8.5 vs 6.7 mo; hazard ratio (HR): 0.72 95% confidence interval (CI): 0.62-0.83; P < 0.001].
The effectiveness of GA has been fully demonstrated by its widespread application in clinical practice and systematic analysis[6]. However, systematic analyses for effectiveness and safety in GA and FOLFIRINOX have shown that FOLFIRINOX has longer median overall survival (OS) and few differences in the overall risk of death and progression between FOLFIRINOX and GA[7]. Previous studies have compared gemcitabine with gemcitabine combinations, and meta-analysis has indicated that gemcitabine combined with targeted drugs does not improve OS and progression-free survival (PFS) in patients compared with gemcitabine alone[8]. Nevertheless, there has been no meta-analysis on whether GA regimens can improve effectiveness when combined with a targeted drug.
In clinical settings, targeted medicines with distinct modes of action, which could dramatically increase patients’ survival rate, play a critical role in cancer treatment. For instance, tarextumab, a novel anticancer stem cell antibody against Notch 2/3, has been demonstrated to decrease tumor stem cell growth, promote cell differentiation, disrupt tumor angiogenesis, and prevent tumor development[9]. Ibrutinib (pci-32765) is an irreversible inhibitor with high selectivity for Bruton tyrosine kinase (BTK) and high potency, and ibrutinib plus gemcitabine considerably improved survival rate in preclinical trials in pancreatic cancer[10-11]. A phase Ib study evaluated the safety and tolerability of pegvorhyaluronidase alfa (PEGPH 20) in combination with gemcitabine for advanced pancreatic cancer. PEGPH 20 was well tolerated, especially in patients with high hyaluronic acid[12]. It has also been found that adding istiratumab to GA improves chemotherapeutic activity[13]. Several studies have demonstrated the high activity of hydroxychloroquine (HCQ) in pancreatic cancer models, and HCQ can improve the efficacy of chemotherapy especially for pancreatic cancer[14,15]. As a heat shock protein antagonist, only by binding to heat shock protein (Hsp) 27 RNA, apatorsen could function and will not be transformed into a functional protein, which can provide a new therapeutic idea for treating pancreatic cancer[16]. Low-molecular-weight heparin reduces the degradation of heparan sulfate proteoglycans by downregulating the expression of heparinase[17,18]. In preliminary experiments, necuparanib has been found to inhibit tumor cell proliferation and invasion[19]. As a multitargeted heparan sulfate mimetic, it effectively avoids the drawbacks of heparin analogs that are prone to bleeding while retaining antiangiogenic effects[20,21], and it effectively participates in the antitumor immunomodulatory process[22]. In this study, we conducted a systematic collection and screening to evaluate the evidence and results of studies in relevant randomized controlled trials (RCTs).
We performed a combined computerized and manual search using key words “pancreatic cancer”, “gemcitabine”, “nab-paclitaxel” and “metastatic”. We finished the final search on December 1, 2021. We only included papers written in English.
Inclusion criteria: (1) The study met the requirements of the RCTs’ experimental design; (2) The trial group was GA + a targeted drug and the control group was GA regimen or GA + placebo; (3) The study subjects were patients with advanced or metastatic pancreatic cancer diagnosed by pathology; and (4) Observed indicators included OS, PFS, objective response rate (ORR) and toxicity (fatigue, anemia, diarrhea, vomiting, neutropenia). Exclusion criteria: (1) Studies in patients with pancreatic cancer with significant comorbidities; (2) No complete observational index or single-arm pilot study; (3) Non-RCTs such as observational studies, reviews, case reports, and duplicate studies; and (4) Non-English papers.
The titles and abstracts of all papers were evaluated by two investigators independently. If one of the investigators thought that the title and abstract of a particular paper had met the inclusion requirements, the final decision was made by the two investigators after reading the full text together. Any conflicts during the screening process would be resolved through discussion or a third party. The methodological quality of the included studies was determined using the Jadad scale[23]. We only included high-quality studies. All data including first author, year of publication, number of patients in the trial and control groups, treatment regimen observations (OS, PFS, ORR, and toxic response), and HR and 95%CI in the survival curves should be extracted from the included studies.
Statistical analysis of all data in this study was performed by Stata 15.1[24] with OS as the primary analysis; with PFS, ORR and adverse events as secondary analysis. Heterogeneity analysis before each trial was evaluated by Cochrane’s Q test and I2 statistics, and a random-effects model was used if P > 0.1 or I2 > 50% indicated heterogeneity between studies[25]. In contrast, we used a fixed-effects model. The studies were evaluated for publication bias by funnel plot and Egger test. P < 0.05 indicated a statistical difference.
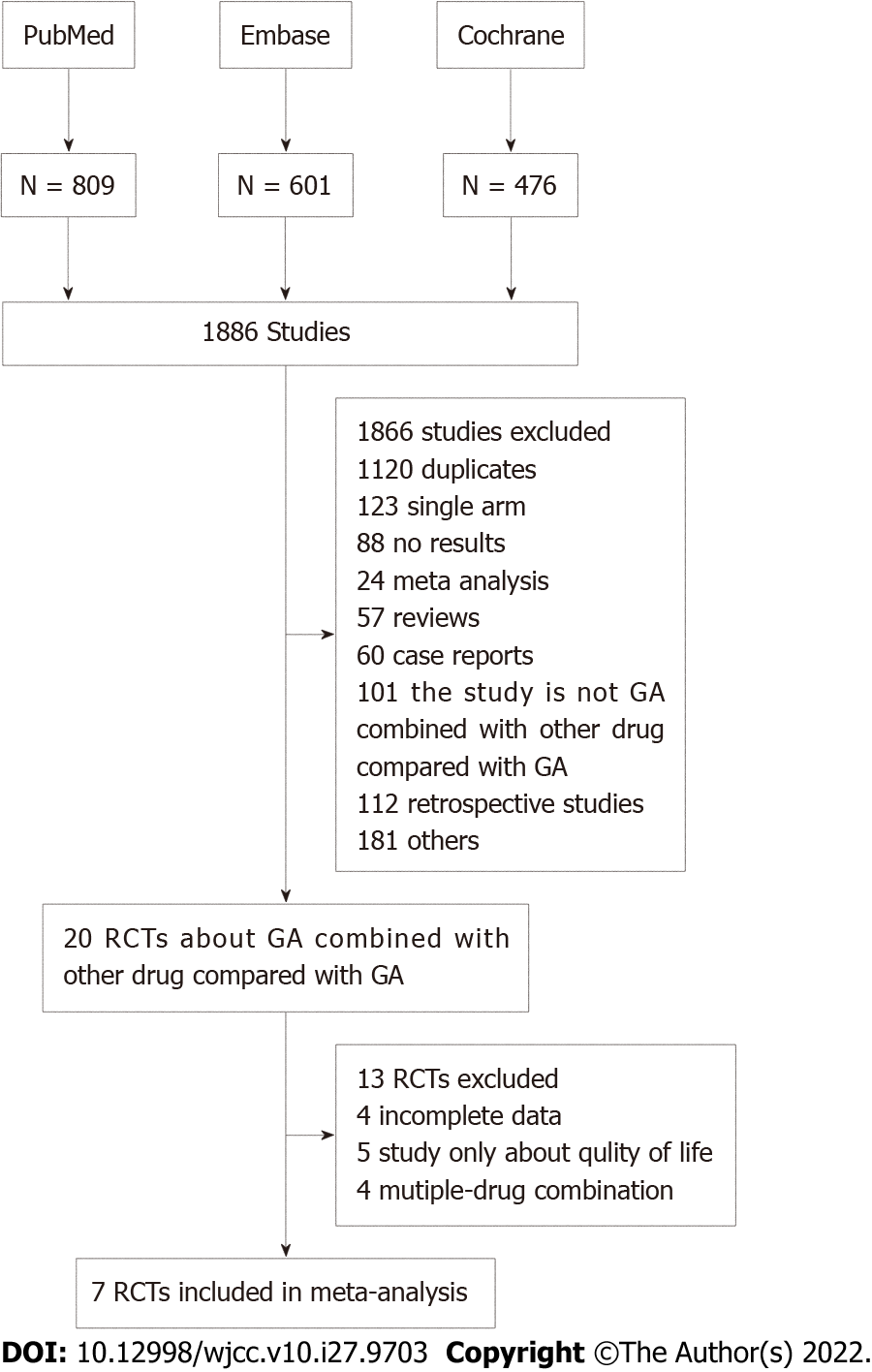
The steps for evaluating the inclusion and exclusion of studies in this system are shown in Figure 1. A total of 1886 relevant studies were screened out as ineligible after the titles and abstracts (included duplicates, single-arm studies, meta-analyses, reviews, case reports, retrospective studies, control groups and experimental groups) were read and did not meet the requirements. After reading the full text, we excluded 13 RCTs due to incomplete data and noncompliance of observation indicators, and we only included seven RCTs[26-32] in the final meta-analysis. The main characteristics of the seven included RCTs are listed in Table 1, with a total of 1544 patients, including 853 in the GA + a targeted drug group (tarextumab, ibrutinib, PEGPH 20, istiratumab, apatorsen, HCQ and necuparanib) and 691 in the GA + placebo group. All included trials met strict RCT trial design requirements and were of high quality (Jadad score > 3.) Seven studies provided data on OS, ORR, and grade 3/4 toxicity (fatigue, anemia, diarrhea, vomiting and neutropenia).
| Ref. | Median age | Gender (f/m) | Treatment | Media OS (mo) | Median PFS (mo) | ORR [n (%)] |
| Kundranda et al[26] | 60 | 16/27 | GA+ istiratumab | 8.7 | 3.6 | 17 (39.5%) |
| 60 | 26/19 | GA+ Placebo | 11.7 | 7.1 | 22 (51.2%) | |
| Ko et al[29] | 66.5 | 29/37 | GA+ apatorsen | 5.3 | 2.7 | 12 (18%) |
| 65.5 | 28/38 | GA+ Placebo | 6.9 | 3.8 | 12 (18%) | |
| O’Reilly et al[30] | 65 | 33/29 | GA+ necuparanib | 10.71 | 5.52 | 14 (23%) |
| 61 | 25/33 | GA+ placebo | 9.99 | 6.93 | 8 (14%) | |
| Karasic et al[28] | 65 | 45/67 | GA+ HCQ | 11.1 | 5.7 | 21 (38.2%) |
| GA+ Placebo | 12.1 | 6.4 | 12 (22.1%) | |||
| Tempero et al[32] | 64 | 97/114 | GA+ Ibrutinib | 9.69 | 5.32 | 62 (29%) |
| 64 | 92/120 | GA+ Placebo | 10.78 | 6.03 | 90 (42%) | |
| Hu et al[31] | 66 | 39/50 | GA+ Tarextumab | 6.4 | 3.7 | 18 (20%) |
| 66 | 34/54 | GA+ Placebo | 7.9 | 5.5 | 28 (32%) | |
| Van Cutsem et al[27] | 63.8 | 147/180 | GA+PEGPH20 | 11.2 | 7.1 | 153 (47%) |
| 62.3 | 85/80 | GA+Placebo | 11.5 | 7.1 | 59 (36%) |
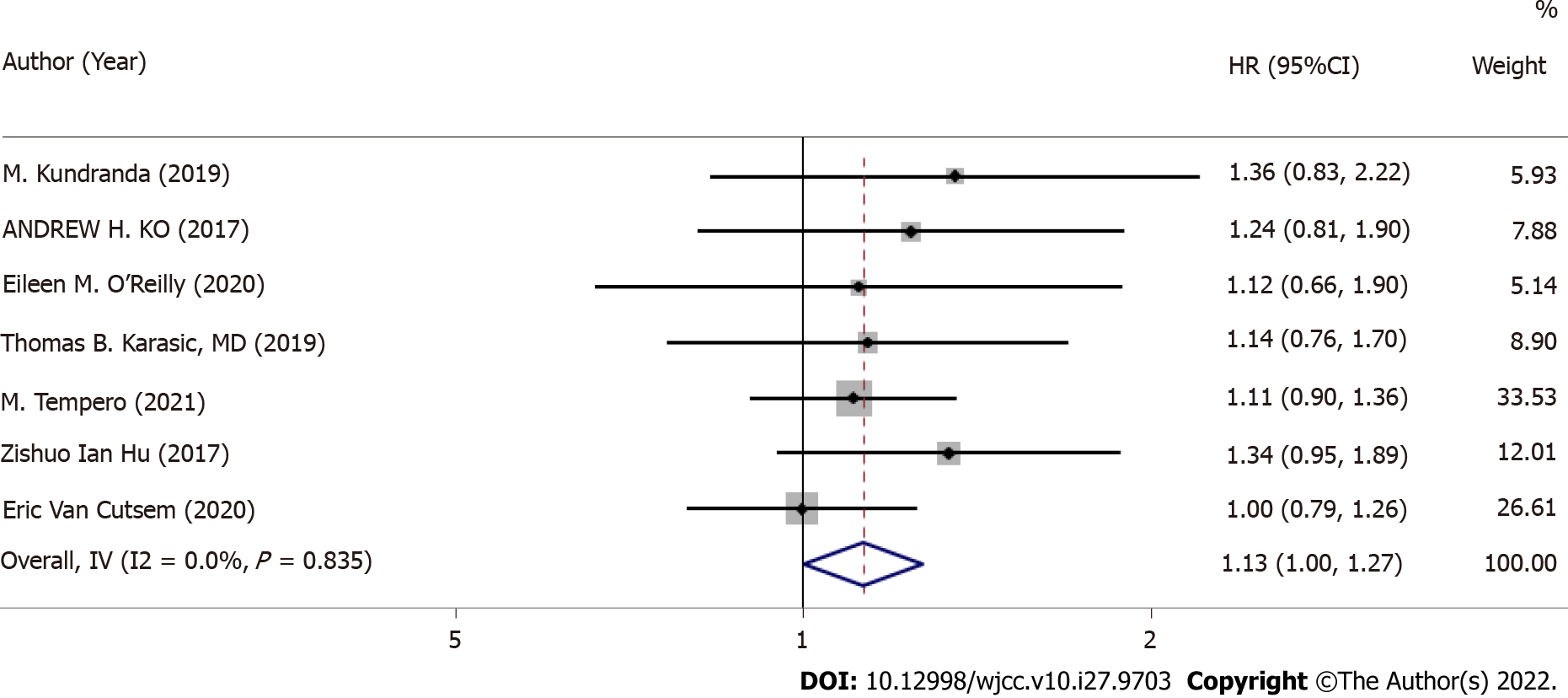
Median OS was provided for all seven RCTs, and HRs and 95%CIs were also available (Figure 2). OS I2 = 0.0%, P = 0.083 was not heterogeneous; therefore, we conducted a meta-analysis using a fixed-effects model. In accordance with the meta-analysis, there was no marked difference between GA + a targeted drug and GA + placebo for improving OS (HR: 1.13, 95%CI: 1.00-1.27, P = 0.044).
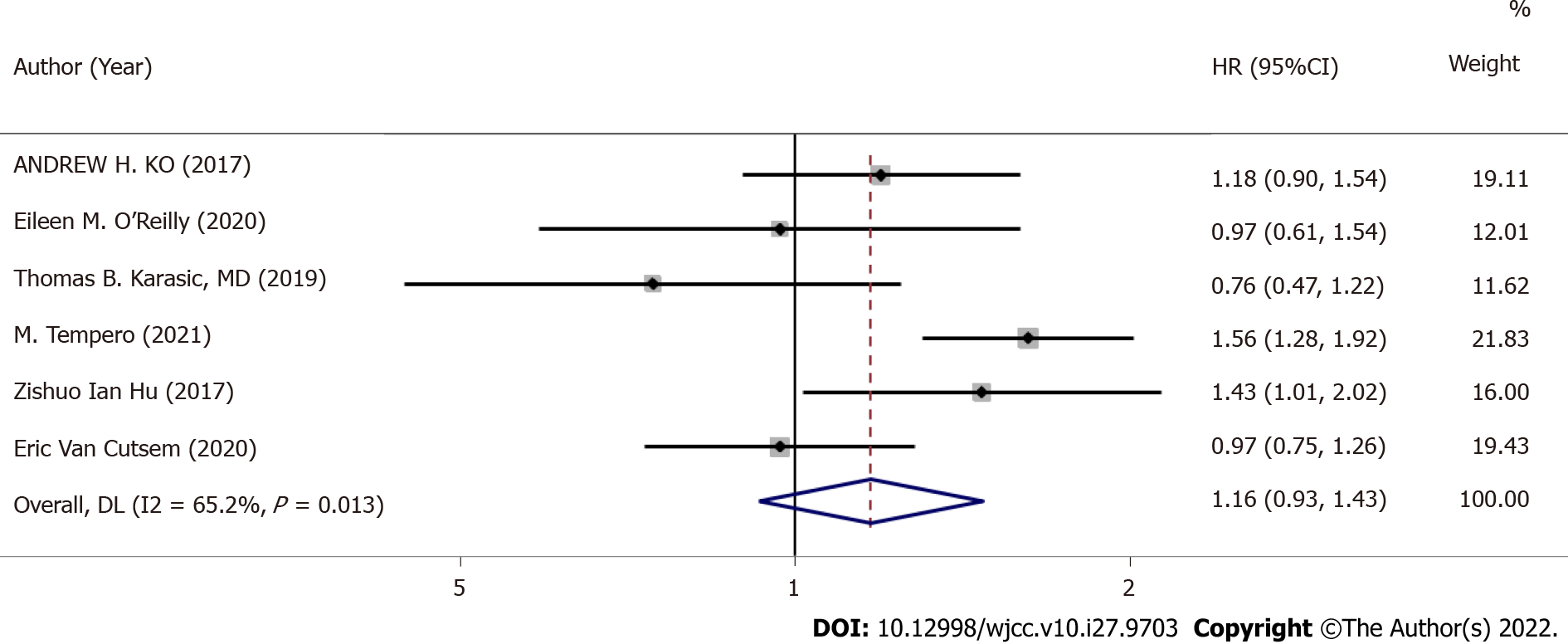
Six RCTs provided median PFS (I2 = 65.2%, P = 0.013), so we adopted a random-effects model (Figure 3). There was no marked difference between GA + a targeted drug and GA + placebo for improving PFS (HR: 1.16, 95%CI: 0.93-1.43, P = 0.187).
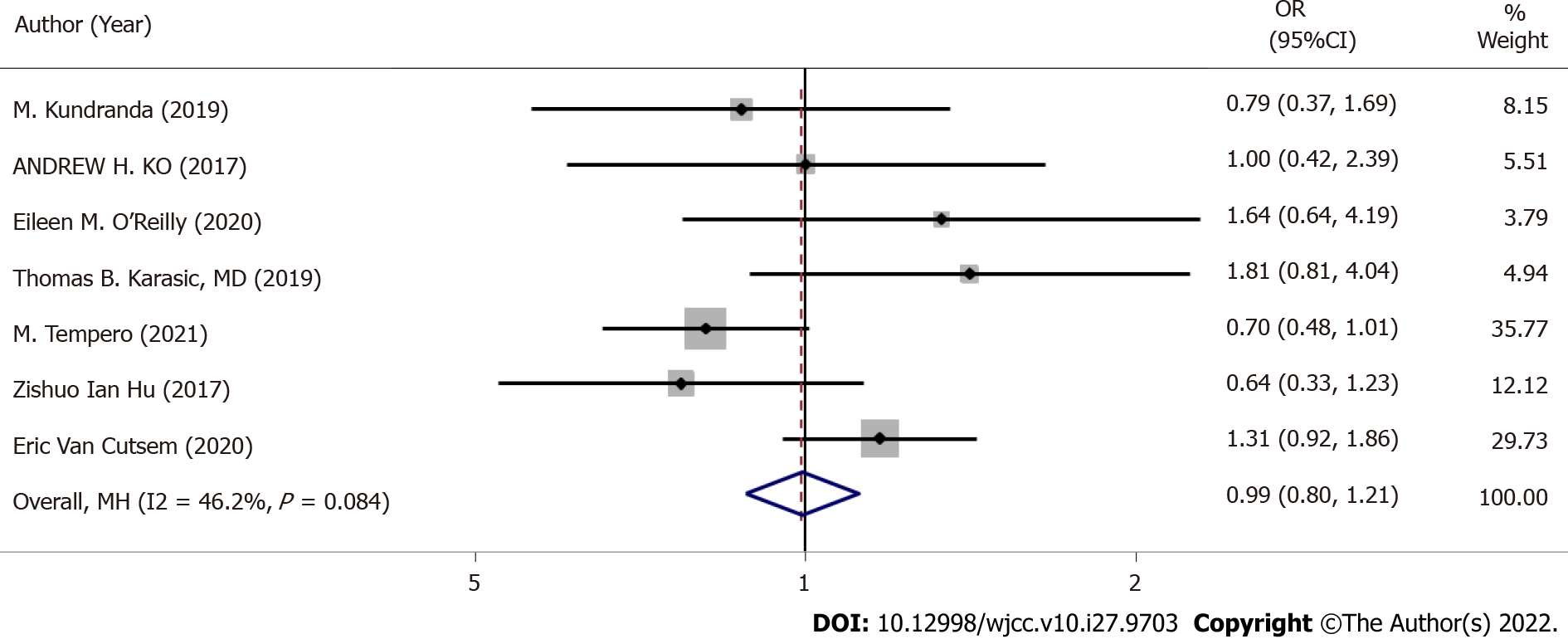
Seven RCTs provided ORRs (I2 = 46.2%, P = 0.084), thus we used a dichotomous fixed-effects model for analysis (Figure 4). There was no significant difference in ORR between GA + a targeted drug and GA + placebo (OR: 0.99, 95%CI: 0.80-1.21, P = 0.892).
Grade 3/4 toxicity, including fatigue, anemia, diarrhea, vomiting and neutropenia, was analyzed using a fixed-effects model (Table 2). There was no significant difference between the two groups in fatigue (OR: 1.07, 95%CI: 0.88-1.29, P = 0.522), anemia (OR: 0.97, 95%CI: 0.75-1.25, P = 0.822), vomiting (OR: 1.07, 95%CI: 0.84-1.36, P = 0.595), neutropenia (OR: 0.94, 95%CI: 0.73-1.22, P = 0.657), while there was a significant difference between the two groups for diarrhea (OR: 1.46, 95%CI: 1.17-1.83, P = 0.001).
| Grade 3/4 toxicity | GA+ a targeted drug (n/N) | GA+ placebo (n/N) | Analysis model | OR (95%CI) | Z | P |
| Fatigue | 367/777 | 264/607 | Fixed effect | 1.07 (0.88,1.29) | 0.642 | 0.522 |
| Anemia | 166/452 | 169/451 | Fixed effect | 0.97 (0.75,1.25) | 0.225 | 0.822 |
| Vomiting | 221/663 | 160/497 | Fixed effect | 1.07 (0.84,1.36) | 0.532 | 0.595 |
| Neutropenia | 150/452 | 158/451 | Fixed effect | 0.94 (0.73,1.22) | 0.444 | 0.657 |
| Diarrhea | 281/452 | 194/451 | Fixed effect | 1.46 (1.17,1.83) | 3.309 | < 0.01 |
Although GA regimen and modified FOLFIRINOX are the core first-line regimens for treating pancreatic cancer in clinical practice, we have to face the problem that the current regimens have limited effect in prolonging survival of pancreatic cancer patients, and we need to keep finding new therapeutic approaches for pancreatic cancer. Having demonstrated the effectiveness of some new targeted drugs for antitumor therapy, clinical trials have started to use GA in combination with targeted drugs for pancreatic cancer. However, GA regimens combined with targeted drugs did not bring better efficacy and did not significantly improve OS, PFS and ORR. Moreover, even combination regimens were less effective than GA regimens. There was no difference in grade 3/4 toxicity, including fatigue, anemia, vomiting and neutropenia, except for a significant increase in the incidence of diarrhea. Our results have important clinical implications, and more caution is needed for combining targeted agents on top of GA regimens in patients with metastatic pancreatic cancer.
The Notch pathway plays an important role in cancer treatment as anti-Notch 2/3 can reduce the incidence of tumors by downregulating Notch target genes. It has been found that anti-Notch 2/3 combined with chemotherapy is effective in a variety of cancers including pancreatic cancer, and it has been demonstrated that gemcitabine combined with anti-Notch 2/3 is more sensitive in the treatment of pancreatic cancer patients with a higher expression level of Notch3 gene. It has also been shown that GA in combination with anti-Notch 2/3 drugs has stronger antitumor effects than gemcitabine alone[9]. Although tarextumab showed high potential in preclinical studies, the three low, medium and high Notch subgroups did not show any discrepancies in PFS and OS in a randomized phase II study[31]. The above results may be due to patient variation in clinical studies, but also suggest that the specific role of the Notch pathway in pancreatic cancer is still controversial and needs further study. BTK may be involved in a variety of immune-related signaling pathways, and it may be a new antitumor target[33]. Studies have shown that the combination of ibrutinib and chemotherapy in the treatment of other cancers is beneficial in improving the effectiveness[34,35]. However, ibrutinib did not improve PFS and OS in patients with metastatic pancreatic cancer in the phase III RESOLVE study[32], which was considered to be related to the addition of ibrutinib shortening the treatment duration of the original GA regimen. The main component of the extracellular matrix is hyaluronic acid (HA), increasing the interstitial gel fluid pressure within the tumor and reducing drug delivery to malignant cells. PEGPH 20 is a new drug that degrades HA to increase cytotoxic release, and PEGPH 20 inhibits tumor growth by degrading the HA-assembled extracellular skeleton to disintegrate this matrix and thereby inhibit tumor growth[36]. Nevertheless, in a randomized trial of PEGPH 20 and modified FOLFIRINOX regimens for metastatic pancreatic cancer, the primary endpoint of early termination of the PEGPH 20 and modified FOLFIRINOX regimens (median OS 7 mo vs 14.4 mo) has not been met[37]. Furthermore, the same occurred in an RCT of PEGPH 20 and GA regimens, with a median OS of 11.2 mo for GA + PEGPH 20 and median OS of 11.6 mo for GA + placebo (HR: 1.0, 95%CI: 0.80-1.27)[27]. Insulin-like growth factor receptor 1 (IGF-1R) is involved in tumor progression of pancreatic cancer and promotes cancer cell growth. Istiratumab, a novel bispecific antibody, enhances drug sensitivity by blocking inhibition of AKT phosphorylation and promoting degradation of IGF-1R and receptor tyrosine protein kinase B3, thereby restoring paclitaxel and gemcitabine activity. In an RCT, ganitumab, an IGF-1R antibody, was added to gemcitabine, resulting in a significant improvement in OS for pancreatic cancer patients[38]. Besides, the trial was terminated early in a subsequent phase III clinical study. In the included trial, GA combined with istiratumab did not show an improvement in OS or shorter PFS in the GA combined with istiratumab group, even in the subgroup with high IGF-1R levels. There are many reasons for this result, one of which may be related to the fact that blocking IGF-1R leads to a negative impact on the disease by compensatory signals from other pathways[39]. Cellular autophagy is closely related to the growth of cancer cells, and HCQ plays an effective role in inhibiting autophagy by inhibiting the binding of autophagosomes and lysosomes[40]. In a randomized study of colorectal cancer, the addition of HCQ to a regimen of FOLFOX (oxaliplatin, calcium folinate and fluorouracil) combined with bevacizumab did not significantly improve OS[28]. For this reason, it was expected that the addition of HCQ to GA would not improve OS. A retrospective analysis of the study revealed that the reason for this result may be due to genetic grouping imbalance but in-depth validation of the antitumor effect of HCQ is still needed. Hsp27 inhibits apoptosis by inhibiting caspase protein activity, and several malignancies, including pancreatic cancer, are highly expressed for Hsp27[41]. The activity of apatorsen alone has been demonstrated[42], and preclinical studies have demonstrated the role of Hsp27 in the treatment of pancreatic cancer[43]. However, in a study that added apatorsen to GA regimen, the apatorsen group had even worse performance than the GA group. The above results indicated that any new targeted drug entering the clinic needs more rigorous trials and evaluation.
The mechanism of action of different targeted drugs for cancer treatment has its own complex characteristics. If the GA regimen is combined with drugs with the same target, the results of the systematic analysis will definitely be more objective. However, there are insufficient data from studies on combination of GA with drugs with the same target.
The GA regimen combined with targeted agents did not have promising results as in preclinical studies. Addition of novel targeted agents did not result in a survival benefit for patients with metastatic pancreatic cancer, and the targeted agents may cause more severe diarrhea. Although the results are not optimistic, we expect more high-level clinical studies to be conducted to improve evaluation of this study.
Although the results of this study were not promising, it is undeniable that for first-line treatment of metastatic pancreatic cancer, the GA regimen has significant antitumor effects and tolerable side effects[44], and is also a safe and effective neoadjuvant treatment option against potentially resectable pancreatic cancer[45]. In the second-line treatment of metastatic pancreatic cancer, the results of a meta-analysis suggested that the use of irinotecan-fluorouracil-folic acid regimen may be beneficial in terms of OS and PFS in patients not previously treated with these agents[46]. In gemcitabine-refractory metastatic pancreatic cancer with an extremely poor prognosis, the combination of nanoliposomal irinotecan with folinic acid is an important option in the second-line treatment of patients with metastatic pancreatic cancer[47]. With more positive results from phase III studies, there are more treatment options available for metastatic pancreatic cancer, and these treatments continue to bring incremental improvements and slowly prolong patient survival[48]. In this challenging field, we have reasons to be optimistic and believe that one day modern medicine can overcome the challenge of metastatic pancreatic cancer.
Pancreatic cancer is a highly malignant disease. Gemcitabine plus albumin combined with nab-paclitaxel (GA) is a common first-line treatment regimen for metastatic pancreatic cancer, and there is currently much clinical controversy about the effectiveness of adding a novel targeted agent to this regimen.
An analysis of studies using GA in combination with targeted drug regimens for the treatment of metastatic pancreatic cancer is presented to discuss its efficacy and safety.
Analysis comparing the effectiveness and safety of GA combined with targeted drug regimens and GA regimens.
Eligible randomized controlled trials related to GA and GA + targeted agents were searched in PubMed, EMBASE and Cochrane Library, and overall survival (OS), progression-free survival (PFS), objective response rate (ORR) and toxicity were pooled and finally analyzed by Stata version 15.1. In addition, use Reference Citation Analysis (https://www.referencecitationanalysis.com/) to collect the latest related literature to improve the latest cutting-edge research results.
There were no significant differences in PFS, OS,and ORR between GA + targeted drugs and GA. Grade 3/4 toxicity, such as fatigue, anemia, vomiting and neutropenia, was not significantly different between the two groups, and the incidence of grade 3/4 diarrhea was significantly increased by GA + targeted agents.
Adding a novel targeted agent to the GA regimen did not improve survival in patients with metastatic pancreatic cancer.
There is a gap between clinical and theoretical. Theoretically, new targeted drugs will improve the therapeutic effect but not the same result in the clinic.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reshkin SJ, Italy; Sperti C, Italy S-Editor: Wang DM L-Editor: Kerr C P-Editor: Wang DM
| 1. | Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326:851-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 1062] [Article Influence: 265.5] [Reference Citation Analysis (0)] |
| 2. | Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw Open. 2021;4:e214708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 776] [Article Influence: 194.0] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15290] [Article Influence: 3058.0] [Reference Citation Analysis (4)] |
| 4. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5123] [Article Influence: 465.7] [Reference Citation Analysis (0)] |
| 5. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4865] [Article Influence: 405.4] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Xu J, Hua J, Liu J, Liang C, Meng Q, Ni Q, Shi S, Yu X. Nab-paclitaxel plus gemcitabine as first-line treatment for advanced pancreatic cancer: a systematic review and meta-analysis. J Cancer. 2019;10:4420-4429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Pusceddu S, Ghidini M, Torchio M, Corti F, Tomasello G, Niger M, Prinzi N, Nichetti F, Coinu A, Di Bartolomeo M, Cabiddu M, Passalacqua R, de Braud F, Petrelli F. Comparative Effectiveness of Gemcitabine plus Nab-Paclitaxel and FOLFIRINOX in the First-Line Setting of Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 8. | Li Q, Yuan Z, Yan H, Wen Z, Zhang R, Cao B. Comparison of gemcitabine combined with targeted agent therapy versus gemcitabine monotherapy in the management of advanced pancreatic cancer. Clin Ther. 2014;36:1054-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B, Henner WR, Meisner R, Sato A, Shah J, Tang T, Wallace B, Wang M, Zhang C, Kapoun AM, Lewicki J, Gurney A, Hoey T. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin Cancer Res. 2015;21:2084-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 10. | Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B, Chang BY, Varner JA, Coussens LM. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016;6:270-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 398] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 11. | Massó-Vallés D, Jauset T, Serrano E, Sodir NM, Pedersen K, Affara NI, Whitfield JR, Beaulieu ME, Evan GI, Elias L, Arribas J, Soucek L. Ibrutinib exerts potent antifibrotic and antitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res. 2015;75:1675-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R, Raghunand N, Dychter S, Jiang P, Shepard HM, Devoe CE. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res. 2016;22:2848-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 13. | Camblin AJ, Pace EA, Adams S, Curley MD, Rimkunas V, Nie L, Tan G, Bloom T, Iadevaia S, Baum J, Minx C, Czibere A, Louis CU, Drummond DC, Nielsen UB, Schoeberl B, Pipas JM, Straubinger RM, Askoxylakis V, Lugovskoy AA. Dual Inhibition of IGF-1R and ErbB3 Enhances the Activity of Gemcitabine and Nab-Paclitaxel in Preclinical Models of Pancreatic Cancer. Clin Cancer Res. 2018;24:2873-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, Lengrand J, Deshpande V, Selig MK, Ferrone CR, Settleman J, Stephanopoulos G, Dyson NJ, Zoncu R, Ramaswamy S, Haas W, Bardeesy N. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524:361-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 15. | Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, Von Hoff DD, Maitra A, Kimmelman AC. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 397] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 16. | Kamada M, So A, Muramaki M, Rocchi P, Beraldi E, Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol Cancer Ther. 2007;6:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer. 2002;2:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 507] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 18. | Franchini M, Mannucci PM. Low-molecular-weight heparins and cancer: focus on antitumoral effect. Ann Med. 2015;47:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | MacDonald A, Priess M, Curran J, Guess J, Farutin V, Oosterom I, Chu CL, Cochran E, Zhang L, Getchell K, Lolkema M, Schultes BC, Krause S. Necuparanib, A Multitargeting Heparan Sulfate Mimetic, Targets Tumor and Stromal Compartments in Pancreatic Cancer. Mol Cancer Ther. 2019;18:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Abu Arab W, Kotb R, Sirois M, Rousseau E. Concentration- and time-dependent effects of enoxaparin on human adenocarcinomic epithelial cell line A549 proliferation in vitro. Can J Physiol Pharmacol. 2011;89:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Fluhr H, Seitz T, Zygmunt M. Heparins modulate the IFN-γ-induced production of chemokines in human breast cancer cells. Breast Cancer Res Treat. 2013;137:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Zhou H, Roy S, Cochran E, Zouaoui R, Chu CL, Duffner J, Zhao G, Smith S, Galcheva-Gargova Z, Karlgren J, Dussault N, Kwan RY, Moy E, Barnes M, Long A, Honan C, Qi YW, Shriver Z, Ganguly T, Schultes B, Venkataraman G, Kishimoto TK. M402, a novel heparan sulfate mimetic, targets multiple pathways implicated in tumor progression and metastasis. PLoS One. 2011;6:e21106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12870] [Article Influence: 443.8] [Reference Citation Analysis (1)] |
| 24. | Boston RC, Sumner AE. STATA: a statistical analysis system for examining biomedical data. Adv Exp Med Biol. 2003;537:353-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 25. | DerSimonian R, Levine RJ. Resolving discrepancies between a meta-analysis and a subsequent large controlled trial. JAMA. 1999;282:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Kundranda M, Gracian AC, Zafar SF, Meiri E, Bendell J, Algül H, Rivera F, Ahn ER, Watkins D, Pelzer U, Charu V, Zalutskaya A, Kuesters G, Pipas JM, Santillana S, Askoxylakis V, Ko AH. Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Ann Oncol. 2020;31:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Van Cutsem E, Tempero MA, Sigal D, Oh DY, Fazio N, Macarulla T, Hitre E, Hammel P, Hendifar AE, Bates SE, Li CP, Hingorani SR, de la Fouchardiere C, Kasi A, Heinemann V, Maraveyas A, Bahary N, Layos L, Sahai V, Zheng L, Lacy J, Park JO, Portales F, Oberstein P, Wu W, Chondros D, Bullock AJ; HALO 109-301 Investigators. Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J Clin Oncol. 2020;38:3185-3194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 28. | Karasic TB, O'Hara MH, Loaiza-Bonilla A, Reiss KA, Teitelbaum UR, Borazanci E, De Jesus-Acosta A, Redlinger C, Burrell JA, Laheru DA, Von Hoff DD, Amaravadi RK, Drebin JA, O'Dwyer PJ. Effect of Gemcitabine and nab-Paclitaxel With or Without Hydroxychloroquine on Patients With Advanced Pancreatic Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:993-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 29. | Ko AH, Murphy PB, Peyton JD, Shipley DL, Al-Hazzouri A, Rodriguez FA, Womack MS 4th, Xiong HQ, Waterhouse DM, Tempero MA, Guo S, Lane CM, Earwood C, DeBusk LM, Bendell JC. A Randomized, Double-Blinded, Phase II Trial of Gemcitabine and Nab-Paclitaxel Plus Apatorsen or Placebo in Patients with Metastatic Pancreatic Cancer: The RAINIER Trial. Oncologist. 2017;22:1427-e129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | O'Reilly EM, Barone D, Mahalingam D, Bekaii-Saab T, Shao SH, Wolf J, Rosano M, Krause S, Richards DA, Yu KH, Roach JM, Flaherty KT, Ryan DP. Randomised phase II trial of gemcitabine and nab-paclitaxel with necuparanib or placebo in untreated metastatic pancreas ductal adenocarcinoma. Eur J Cancer. 2020;132:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Hu ZI, Bendell JC, Bullock A, LoConte NK, Hatoum H, Ritch P, Hool H, Leach JW, Sanchez J, Sohal DPS, Strickler J, Patel R, Wang-Gillam A, Firdaus I, Yu KH, Kapoun AM, Holmgren E, Zhou L, Dupont J, Picozzi V, Sahai V, O'Reilly EM. A randomized phase II trial of nab-paclitaxel and gemcitabine with tarextumab or placebo in patients with untreated metastatic pancreatic cancer. Cancer Med. 2019;8:5148-5157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Tempero M, Oh DY, Tabernero J, Reni M, Van Cutsem E, Hendifar A, Waldschmidt DT, Starling N, Bachet JB, Chang HM, Maurel J, Garcia-Carbonero R, Lonardi S, Coussens LM, Fong L, Tsao LC, Cole G Jr, James D, Macarulla T. Ibrutinib in combination with nab-paclitaxel and gemcitabine for first-line treatment of patients with metastatic pancreatic adenocarcinoma: phase III RESOLVE study. Ann Oncol. 2021;32:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 33. | Schwarzbich MA, Witzens-Harig M. Ibrutinib. Recent Results Cancer Res. 2014;201:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J, Bartlett NL, Dilhuydy MS, Pylypenko H, Loscertales J, Avigdor A, Rule S, Villa D, Samoilova O, Panagiotidis P, Goy A, Mato A, Pavlovsky MA, Karlsson C, Mahler M, Salman M, Sun S, Phelps C, Balasubramanian S, Howes A, Hallek M; HELIOS investigators. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 35. | Brown JR, Barrientos JC, Barr PM, Flinn IW, Burger JA, Tran A, Clow F, James DF, Graef T, Friedberg JW, Rai K, O'Brien S. The Bruton tyrosine kinase inhibitor ibrutinib with chemoimmunotherapy in patients with chronic lymphocytic leukemia. Blood. 2015;125:2915-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Wong KM, Horton KJ, Coveler AL, Hingorani SR, Harris WP. Targeting the Tumor Stroma: the Biology and Clinical Development of Pegylated Recombinant Human Hyaluronidase (PEGPH20). Curr Oncol Rep. 2017;19:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 37. | Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 343] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 38. | McCaffery I, Tudor Y, Deng H, Tang R, Suzuki S, Badola S, Kindler HL, Fuchs CS, Loh E, Patterson SD, Chen L, Gansert JL. Putative predictive biomarkers of survival in patients with metastatic pancreatic adenocarcinoma treated with gemcitabine and ganitumab, an IGF1R inhibitor. Clin Cancer Res. 2013;19:4282-4289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Beckwith H, Yee D. Minireview: Were the IGF Signaling Inhibitors All Bad? Mol Endocrinol. 2015;29:1549-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1943] [Article Influence: 242.9] [Reference Citation Analysis (0)] |
| 41. | Melle C, Ernst G, Escher N, Hartmann D, Schimmel B, Bleul A, Thieme H, Kaufmann R, Felix K, Friess HM, Settmacher U, Hommann M, Richter KK, Daffner W, Täubig H, Manger T, Claussen U, von Eggeling F. Protein profiling of microdissected pancreas carcinoma and identification of HSP27 as a potential serum marker. Clin Chem. 2007;53:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Chi KN, Yu EY, Jacobs C, Bazov J, Kollmannsberger C, Higano CS, Mukherjee SD, Gleave ME, Stewart PS, Hotte SJ. A phase I dose-escalation study of apatorsen (OGX-427), an antisense inhibitor targeting heat shock protein 27 (Hsp27), in patients with castration-resistant prostate cancer and other advanced cancers. Ann Oncol. 2016;27:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Guo Y, Ziesch A, Hocke S, Kampmann E, Ochs S, De Toni EN, Göke B, Gallmeier E. Overexpression of heat shock protein 27 (HSP27) increases gemcitabine sensitivity in pancreatic cancer cells through S-phase arrest and apoptosis. J Cell Mol Med. 2015;19:340-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548-4554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 870] [Article Influence: 62.1] [Reference Citation Analysis (2)] |
| 45. | Ielpo B, Duran H, Diaz E, Fabra I, Caruso R, Ferri V, Malavé L, Hidalgo M, Alvarez R, Plaza C, Quijano Y, Vicente E. Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur J Surg Oncol. 2016;42:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 46. | Citterio C, Baccini M, Orlandi E, Di Nunzio C, Cavanna L. Second-line chemotherapy for the treatment of metastatic pancreatic cancer after first-line gemcitabine-based chemotherapy: a network meta-analysis. Oncotarget. 2018;9:29801-29809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 47. | Passero FC Jr, Grapsa D, Syrigos KN, Saif MW. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev Anticancer Ther. 2016;16:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (2)] |
| 48. | Ko AH. Progress in the treatment of metastatic pancreatic cancer and the search for next opportunities. J Clin Oncol. 2015;33:1779-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (2)] |