Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8797
Peer-review started: April 29, 2022
First decision: May 12, 2022
Revised: May 24, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 26, 2022
Processing time: 108 Days and 16.9 Hours
Diffuse uterine leiomyomatosis (DUL) is a benign uterine smooth muscle neoplasm with unknown etiology. Since DUL is rarely reported, knowledge regarding it is limited. The rate of early diagnosis is low, and DUL is often misdiagnosed as common multiple uterine leiomyomas before surgery.
A 27-year-old patient with no sexual activity presented to the Emergency Department of our hospital complaining of heavy vaginal bleeding. She had a history of uterine fibroids and menorrhagia. Pelvic examination showed a regularly enlarged uterus, similar in size to that associated with a 4-mo preg
Individuals with DUL are easily misdiagnosed due to the lack of specific manifestations of this disease. MRI is helpful for early identification and preoperative evaluation. There is currently no unified method of diagnosis. For women who want to preserve fertility, conservative surgery should be made an option. When TM is chosen, a modified new myomectomy should be considered to avoid the drawbacks of traditional TM.
Core Tip: Diffuse uterine leiomyomatosis (DUL) is a benign uterine smooth muscle neoplasm with unknown etiology. Hysterectomy is the only curative therapy. We report a 27-year-old female with no sexual activity who was diagnosed with DUL. The patient was misdiagnosed with leiomyoma preoperatively, followed by treatment with transabdominal myomectomy (TM), a gonadotropin-releasing hormone analog, and hysteroscopic myomectomy plus hymen repair. This case highlights the importance of pelvic magnetic resonance imaging as a diagnostic tool. For women who want to preserve fertility, conservative surgery should be an option. When TM is chosen, a modified new myomectomy should be considered to avoid the drawbacks of traditional TM.
- Citation: Ren HM, Wang QZ, Wang JN, Hong GJ, Zhou S, Zhu JY, Li SJ. Diffuse uterine leiomyomatosis: A case report and review of literature. World J Clin Cases 2022; 10(24): 8797-8804
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8797.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8797
Diffuse uterine leiomyomatosis (DUL) is a benign and rare uterine smooth muscle neoplasm. It was first reported by Murray and Glynn[1] in 1924 and described as “complete uterine fibromyomatosis”. It was then named DUL by Lapan and Solomon[2] in 1979. It presents mainly in young women of childbearing age. Since DUL is rare and has not been widely reported, knowledge regarding it is limited. The rate of early diagnosis is low, and DUL is often misdiagnosed as common multiple uterine leiomyomas before surgery. Total abdominal hysterectomy is the main treatment for DUL[3,4]. However, considering the early age of onset, preserving reproductive function has become the focus of treatment. At present, there is no unified standard treatment at home or abroad. We report a case of DUL in which the uterus was successfully retained during treatment and review the literature.
A 27-year-old patient presented to the Emergency Department of our hospital complaining of heavy vaginal bleeding.
The patient’s symptoms started half a month prior and were accompanied by dizziness.
The patient was diagnosed with multiple uterine myomas via an ultrasound scan in 2015. Given the age and normal menses amounts, her family chose a follow-up observation at that time. Her usual menstrual cycle was 30-45 d with moderate bleeding lasting 7 d with clots and dysmenorrhea. However, since 2017, her menstrual period has changed to 10-15 d of heavy bleeding. She also sometimes felt dizzy and fatigued. Her lowest hemoglobin concentration was 32 g/L. She was not sexually active.
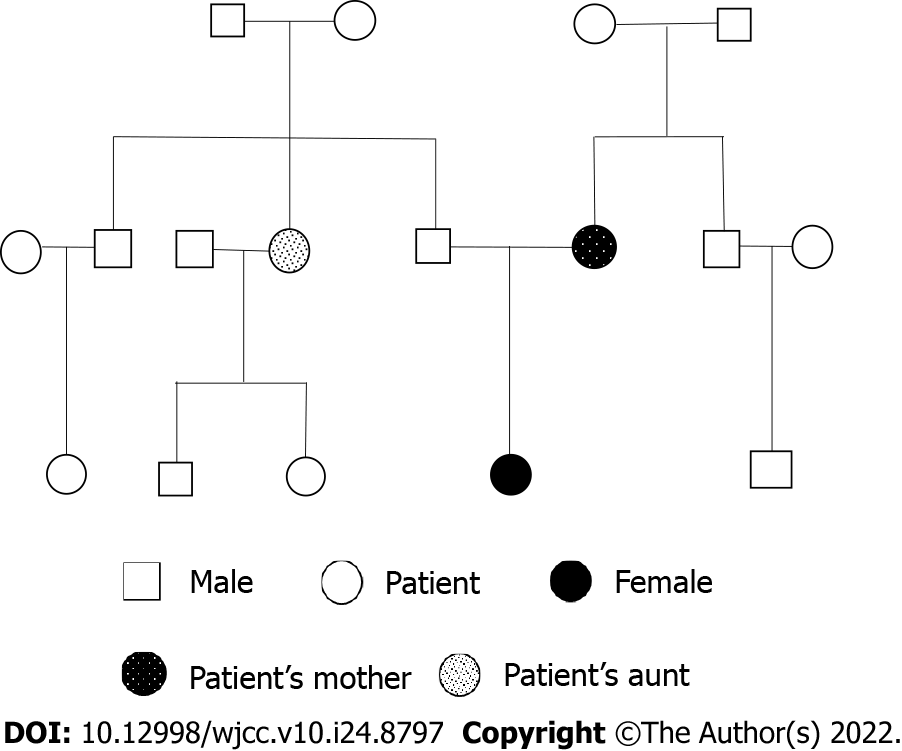
The patient’s mother underwent an abdominal hysterectomy for uterine fibroids at the age of 40. The patient’s aunt (her father’s sister) had a history of uterine fibroids without surgery. The pedigree of this family is shown in Figure 1.
Pelvic examination showed a regularly enlarged uterus, similar to the size at 4 mo of pregnancy, with a hard texture.
Blood analysis revealed moderate anemia, with a hemoglobin level of 52 g/L. Serum tumor markers (CA125, CA199, CEA, AFP, SCC) were normal.
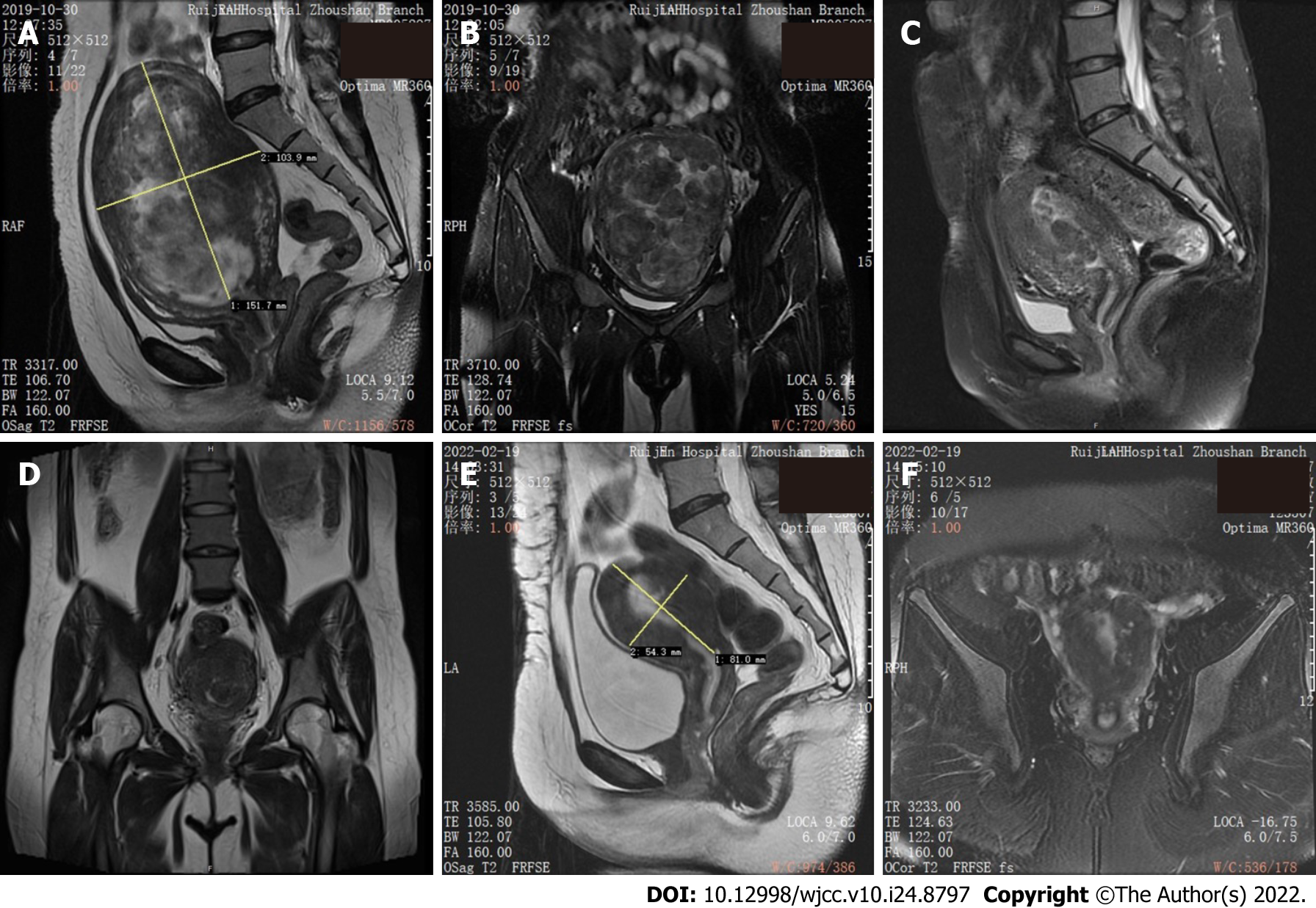
Ultrasound showed that the volume of the uterus was significantly increased. The size of the uterus was approximately 13 cm × 9.2 cm × 11 cm, and the myometrium and uterine cavity were full of numerous hypoechoic masses of variable sizes. Pelvic magnetic resonance imaging (MRI) was performed one month later and revealed numerous multiple uterine fibroids (Figure 2A and B).
The final diagnosis was DUL, which was made after the operation.
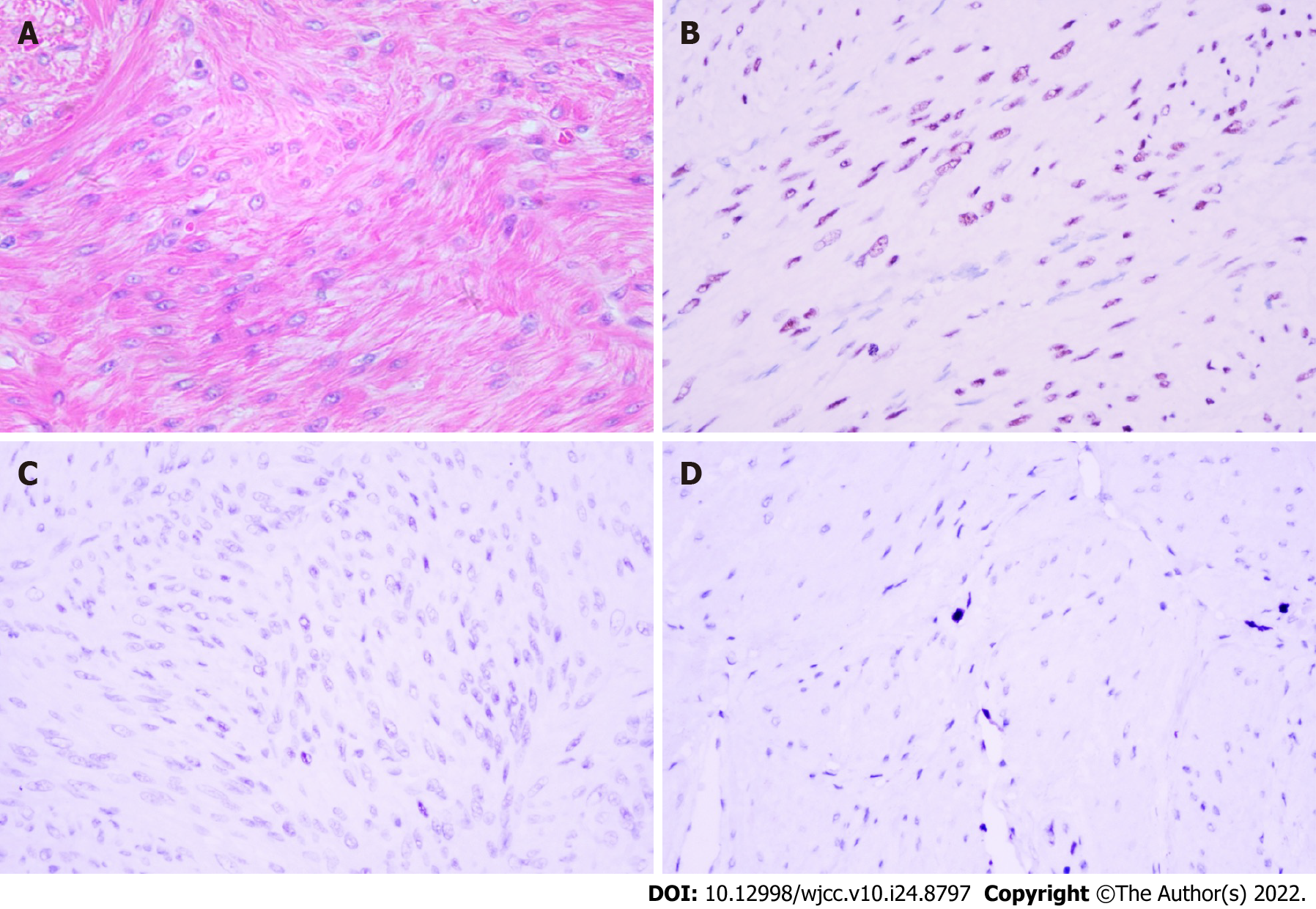
In the emergency room, the patient received ethinylestradiol and cyproterone acetate to stop bleeding and iron supplements to improve anemia. The patient was admitted two months later and underwent transabdominal myomectomy (TM). Intraoperative exploration revealed an enlarged uterus similar to that at 5 mo of pregnancy and normal adnexa. The myometrium was full of myoma nodules of different sizes. The largest nodule was 5 cm in diameter, and the smallest was 0.8 cm in diameter. An abundant blood supply was noted. To reduce intraoperative bleeding, a soft catheter was used as a uterine artery tourniquet at the uterine isthmus level (a tourniquet hole was made in the transparent area of the anterior and posterior lobe of the broad ligament, and a pea clip was inserted). Six units of vasopressin were injected into the uterine body, and 20 units of oxytocin were administered continuously via an intravenous drip during the operation. Over 50 leiomyomas were removed. The total operative time was 220 min. Despite the measures for bleeding prevention, intraoperative bleeding was up to 1800 mL, and 4 units of red blood cells and 400 mL plasma were transfused. The pathology report revealed leiomyoma (Figure 3). The patient was discharged and received 3.75 mg of gonadotropin-releasing hormone analog (GnRHa) for 6 mo. Ultrasonography at the follow-up visit revealed that the size of the uterus had decreased to 8.4 cm × 7.4 cm × 7.1 cm after GnRHa treatment.
The patient presented to the hospital for abnormal uterine bleeding 10 mo following surgery. MRI showed an irregular mass with a diameter of 5.2 cm without sharp demarcation in the uterine cavity. Submucosal leiomyoma was considered first (Figure 2C and D). Hysteroscopic myomectomy (HM) plus hymen repair was performed 11 mo after the first surgery. Intraoperative exploration showed that the depth of the cavity was 9 cm. There were several leiomyomatosis masses that measured from 1 to 2.6 cm in diameter in the cavity and a yellowish-brown abnormal growth 2 cm in diameter in the uterine fundus. The ostia of both fallopian tubes were clear. Postoperative pathological examination confirmed submucosal leiomyoma and necrotic and generative tissue.
Although the menstrual cycle was still irregular (7-8/30-60 d), the patient had a 28-mo postoperative follow-up, and her symptoms of menorrhagia were resolved. Pelvic MRI at the last follow-up showed that the shape of the uterus was irregular, the myometrium was uneven, and the endometrium was slightly thickened (Figure 2E and F).
We report a 27-year-old female with no sexual activity who was diagnosed with DUL. The patient was misdiagnosed with leiomyoma preoperatively, followed by treatment with TM, GnRHa, and HM plus hymen repair.
The etiology of DUL is unclear. Currently, it is considered multiple leiomyomata of a hyperactive state. Baschinsky et al[5] performed a clonality analysis of several lesions in one patient. All foci showed nonrandom X-chromosome inactivation, while different foci had different inactivated X-chromosomes, suggesting that the cells in one focus were from a monoclonal origin and those from different foci were polyclonal. The gross appearance of DUL is a symmetrically enlarged uterus. The diffusely thickened myometrium was almost completely crowded with innumerous solid, weakly defined confluent nodules ranging from 2 to 30 mm in diameter[2,6]. However, in the presented case, the maximum diameter of the nodule was up to 50 mm. To the best of our knowledge, the presented case is the first to describe a nodule with a diameter up to 50 mm. This indicates that DUL lesions should not be limited to 30 mm. Histopathologic examination of DUL lesions reveals hypercellular and shorter smooth muscle cells arranged irregularly and/or compactly. Cellular pleomorphism and abnormal mitotic figures are absent. Vascular invasion is also negative[7]. Immunohistochemical staining of the progesterone receptor (PR) and the estrogen receptor (ER) is usually higher in a myoma than in adjacent myometrium, while the expression of Ki-67 is not different. The expression in DUL shows that PR in nodules is significantly higher than that in the surrounding normal myometrium, and ER and Ki-67 do not differ between these two tissues[8]. The rapid growth of DUL lesions and intralesional bleeding after the use of clomiphene and norethisterone have been reported[9]. The immunohistochemical results of our patient were entirely consistent with those of DUL, and the enlargement of the lesions in a short time may correlate with ethinylestradiol and cyproterone acetate treatment.
Clinical symptoms and ultrasound findings associated with DUL lesions are similar to those of uterine leiomyoma and lack specificity[10]. MRI, due to its high resolution of soft tissue, is helpful for early identification and preoperative evaluation. It reveals symmetrical and uniform enlargement of the uterus with innumerable and ill-defined myomas of different sizes in the myometrium and uterine cavity[10]. The differential diagnosis of a diffusely enlarged uterus with countless fibroid nodules includes hereditary leiomyomatosis and renal cell carcinoma (HLRCC) and Alport syndrome with diffuse leiomyomatosis (ASDL). HLRCC is an autosomal dominant disorder caused by mutations of the FH gene. Cutaneous leiomyoma is usually the first manifestation, concomitant with multiple uterine leiomyomas and renal cell carcinoma[11]. ASDL is an X-linked inherited disorder that results from distinct mutations in the COL4A5 gene. Myoma lesions are widely spread over the esophagus, trachea, bronchi, and genitalia[12].
Due to its rarity, preoperative misdiagnosis of DUL is common, and the diagnosis is largely dependent on preoperative MRI and postoperative pathology, which means that initially, the patients are treated inappropriately. The mother of the patient in this case underwent a hysterectomy at 40 years of age for multiple myomas. If the diagnosis of DUL is confirmed, the occurrence of DUL may result from complex genetic factors. Thus, the genetic mechanism of DUL should be further studied.
To date, the most effective treatment for DUL is total hysterectomy. However, considering that it is common in young women of reproductive age, gynecologists are working to identify appropriate methods to preserve reproductive function for those who desire fertility[6]. Medical and conservative surgeries include the use of GnRHa[13], HM[14-17], uterine artery embolization (UAE)[18,19], high-intensity focused ultrasound (HIFU) ablation[20], and TM. Successful pregnancies after GnRHa, HM, UAE, and combined treatment have been reported[21]. HIFU ablation has been demonstrated to be effective in controlling the symptoms of DUL[22]. However, TM is the most commonly used therapy. Nevertheless, there are many disadvantages to traditional TM. Traditional TM can lead to massive intraoperative bleeding, intrauterine adhesions, incomplete clearance, and recurrence after surgery. Therefore, several new types of myomectomy have been attempted over the last decade. Generally, these strategies share the same critical steps: (1) Longitudinal midline incisions penetrating through both the anterior and posterior full uterine walls are made; (2) As many leiomyomas as possible are removed from the incision; and (3) The incision is closed in three layers with absorbable sutures. In addition, hormonal treatment before surgery is recommended. The average amount of blood lost is 1437 g (range, 428-4421 g), and the average operation time is 271 min (range 180-407 min). In studies of the new myomectomy technique, five patients became pregnant, with four undergoing cesarean section. Regrettably, the fifth pregnancy ended in miscarriage. No uterine rupture was reported[23-25].
For patients with fertility requirements, GnRHa should be considered as the initial therapy. When it fails, HM could be an option to restore as much morphology of the uterine cavity as possible. Repeated surgery with damage to the endometrium should be avoided. To avoid recurrence, patients should try to have children as early as possible postoperatively, and assisted conception techniques can be used when necessary[26].
Our patient was misdiagnosed with multiple uterine fibroids before the operation, and because of her asexual life history, traditional TM was performed. Despite the steps taken to reduce bleeding, intraoperative bleeding reached 1800 mL. Weak uterine contractions, extensive uterine incisions secondary to misdiagnosis and incomplete preoperative preparation were the main reasons for bleeding. In a Cochrane meta-analysis, Thubert et al[27] found that GnRHa therapy before myomectomy significantly reduced fibroid volume and improved postoperative hemoglobin levels. Taken together with the 5 cases of patients treated with the new technique and pretreatment, GnRHa therapy prior to myomectomy is effective in reducing hemorrhage in patients with uterine fibroids and those with DUL. The administration of GnRHa before myomectomy in patients who have numerous lesions and a massive uterus might minimize the complications of misdiagnosis.
Although a total of 6 cycles of GnRHa were administered postsurgery, menorrhagia recurred approximately 10 mo after TM. HM and hymen repair were performed after receiving informed consent. To date, the patient has recovered well with no return of her symptoms. The cause of her recurrent heavy bleeding was attributed to residual disease. The anterior and posterior full uterine walls were not cut open, and the whole uterine cavity was not exposed completely during the operation. The disadvantage of TM was then partly compensated by HM.
Although DUL is a benign condition, it may exhibit parametrial, ovarian, mesenteric, and bone metastasis in rare cases[28-30]. After hysterectomy and removal of metastatic lesions, no recurrence has been reported[22]. Even without the treatment of multiple metastatic lesions in bones, they completely regressed after hysterectomy[22].
DUL is easily misdiagnosed as multiple uterine leiomyomas. Thus, it is necessary to consider DUL in this situation. Improvement in ultrasound technology and the popularization of preoperative MRI for multiple uterine fibroids are beneficial to the early diagnosis of DUL. Hysterectomy is the only cure for DUL. For women who want to preserve fertility, a deliberate choice of conservative surgery should be made. When TM is chosen, a new modified myomectomy should be considered to avoid the drawbacks of traditional TM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Beji H, Tunisia; Singh R, India S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Murray HL, Glynn E. A Case of Complete Fibromyomatosis of the Corpus Uteri. BJOG. 1924;31:398-401. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Lapan B, Solomon L. Diffuse leiomyomatosis of the uterus precluding myomectomy. Obstet Gynecol. 1979;53:82S-84S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Grignon DJ, Carey MR, Kirk ME, Robinson ML. Diffuse uterine leiomyomatosis: a case study with pregnancy complicated by intrapartum hemorrhage. Obstet Gynecol. 1987;69:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Clement PB, Young RH. Diffuse leiomyomatosis of the uterus: a report of four cases. Int J Gynecol Pathol. 1987;6:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Baschinsky DY, Isa A, Niemann TH, Prior TW, Lucas JG, Frankel WL. Diffuse leiomyomatosis of the uterus: a case report with clonality analysis. Hum Pathol. 2000;31:1429-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Fedele L, Bianchi S, Zanconato G, Carinelli S, Berlanda N. Conservative treatment of diffuse uterine leiomyomatosis. Fertil Steril. 2004;82:450-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Mulvany NJ, Ostör AG, Ross I. Diffuse leiomyomatosis of the uterus. Histopathology. 1995;27:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Kim SJ, Roh MS. Diffuse Leiomyomatosis of the Uterus-A Brief Case Report. Korean J Pathol. 2005;39:63-65. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Lai FM, Wong FW, Allen PW. Diffuse uterine leiomyomatosis with hemorrhage. Arch Pathol Lab Med. 1991;115:834-837. [PubMed] [DOI] [Full Text] |
| 10. | Dai YX, Feng FZ, Leng JH, Shi HH, Cheng NH, Wan XR, Zhu L. [Imaging features and clinical analysis of diffuse uterine leiomyomatosis cases]. Zhonghua Yi Xue Za Zhi. 2020;100:2263-2267. [PubMed] |
| 11. | Chayed Z, Kristensen LK, Ousager LB, Rønlund K, Bygum A. Hereditary leiomyomatosis and renal cell carcinoma: a case series and literature review. Orphanet J Rare Dis. 2021;16:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Burgos R, Muñiz E, Rosa ER, Olivares CJ, Romaguera J. Comprehensive management of diffuse leiomyomatosis in a patient with Alport syndrome. P R Health Sci J. 2013;32:200-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Purohit R, Sharma JG, Singh S. A case of diffuse uterine leiomyomatosis who had two successful pregnancies after medical management. Fertil Steril. 2011;95:2434.e5-2434.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Zhao H, Yang B, Li H, Xu Y, Feng L. Successful Pregnancies in Women with Diffuse Uterine Leiomyomatosis after Hysteroscopic Management Using the Hysteroscopy Endo Operative System. J Minim Invasive Gynecol. 2019;26:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Yen CF, Lee CL, Wang CJ, Soong YK, Arici A. Successful pregnancies in women with diffuse uterine leiomyomatosis after hysteroscopic management. Fertil Steril. 2007;88:1667-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Shimizu Y, Yomo H, Kita N, Takahashi K. Successful pregnancy after gonadotropin-releasing hormone analogue and hysteroscopic myomectomy in a woman with diffuse uterine leiomyomatosis. Arch Gynecol Obstet. 2009;280:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Mazzon I, Favilli A, Grasso M, Morricone D, Di Renzo GC, Gerli S. Is 'cold loop' hysteroscopic myomectomy a better option for reproduction in women with diffuse uterine leiomyomatosis? J Obstet Gynaecol Res. 2015;41:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kido A, Monma C, Togashi K, Ueda H, Itoh K, Fujii S, Konishi J. Uterine arterial embolization for the treatment of diffuse leiomyomatosis. J Vasc Interv Radiol. 2003;14:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Scheurig C, Islam T, Zimmermann E, Hamm B, Kroencke TJ. Uterine artery embolization in patients with symptomatic diffuse leiomyomatosis of the uterus. J Vasc Interv Radiol. 2008;19:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Chen L, Xiao X, Wang Q, Wu C, Zou M, Xiong Y. High-intensity focused ultrasound ablation for diffuse uterine leiomyomatosis: A case report. Ultrason Sonochem. 2015;27:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Koh J, Kim MD, Jung DC, Lee M, Lee MS, Won JY, Lee DY, Park SI, Lee KH. Uterine artery embolization (UAE) for diffuse leiomyomatosis of the uterus: clinical and imaging results. Eur J Radiol. 2012;81:2726-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Zhang X, Tian T, Zhu J, Lin B, Feng X, Zhang L, Yang X, Aili A. High intensity focused ultrasound treatment for diffuse uterine leiomyomatosis: a feasibility study. Int J Hyperthermia. 2020;37:1060-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Konishi I. Diffuse Leiomyomatosis: Complete Myomectomy for Innumerable Small Nodules to Achieve Fertility Sparing and Childbearing. Surg J (N Y). 2020;6:S50-S57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Nishida M, Ichikawa R, Arai Y, Sakanaka M, Otsubo Y. New myomectomy technique for diffuse uterine leiomyomatosis. J Obstet Gynaecol Res. 2014;40:1689-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Otsubo Y, Nishida M, Arai Y, Ichikawa R, Sakanaka M. Diffuse uterine leiomyomatosis in patient with successful pregnancy following new surgical management. Arch Gynecol Obstet. 2014;290:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Kitaya K, Yasuo T, Nakamura Y. Recovery from endometrial thinning and successful pregnancy following vitamin E and C supplementation in infertile woman undergoing myomectomy for diffuse leiomyomatosis of the uterus: a case report. Clin Exp Obstet Gynecol. 2014;41:357-359. [PubMed] |
| 27. | Thubert T, Foulot H, Vinchant M, Santulli P, Marzouk P, Borghese B, Chapron C. Surgical treatment: Myomectomy and hysterectomy; Endoscopy: A major advancement. Best Pract Res Clin Obstet Gynaecol. 2016;34:104-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Beattie GJ, Williams AR, Duncan A, Smart GE. Diffuse leiomyomatosis of the uterus with local pelvic spread. Acta Obstet Gynecol Scand. 1993;72:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Robles-Frías A, Severín CE, Robles-Frías MJ, Garrido JL. Diffuse uterine leiomyomatosis with ovarian and parametrial involvement. Obstet Gynecol. 2001;97:834-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Thomas EO, Gordon J, Smith-Thomas S, Cramer SF. Diffuse uterine leiomyomatosis with uterine rupture and benign metastatic lesions of the bone. Obstet Gynecol. 2007;109:528-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |