Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8782
Peer-review started: April 14, 2022
First decision: May 31, 2022
Revised: June 12, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: August 26, 2022
Processing time: 123 Days and 19.8 Hours
Cervical squamous cell carcinoma (SCC) is the most common type of cervical carcinoma and is generally derived from a precancerous stage called cervical high-grade squamous intraepithelial lesion (HSIL). Usually, the cancer metasta
A 57-year-old postmenopausal woman visited our department and requested a routine cervical check-up. Four years ago, she had undergone a cervical loop electrosurgical excision procedure because of HSIL found during the gynecological examination, and she had not been checked again since. This time, a relapse of the cervical HSIL was diagnosed along with uterine pyometra and endometrial polyps. After 2 wk of antibiotic treatment, a laparoscopic hysterectomy was performed, and the final pathological examination revealed that the cervical HSIL had spread directly upward into the uterine cavity, gradually developing into cervical SCC in the endometrium.
Cervical HSIL/SCC can directly spread upward into the uterus with the most common symptoms of pyometra and cervical stenosis. More attention should be given to the early detection and prevention of this disease.
Core Tip: Under unique circumstances, cervical cancer or precancerous lesions can spread directly upward into the uterine cavity, forming endometrial squamous cell carcinoma, which may alter staging and affect prognosis. Emphasis should be placed on prevention as well as early diagnosis, and although a gynecological ultrasound and an endometrial biopsy may help, their impact is still limited. It is imperative to explore the best clinical strategies for treating this disease.
- Citation: Shu XY, Dai Z, Zhang S, Yang HX, Bi H. Endometrial squamous cell carcinoma originating from the cervix: A case report. World J Clin Cases 2022; 10(24): 8782-8787
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8782.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8782
Cervical cancer is the fourth most common cancer among women globally[1]. Among its various classifications, squamous cell carcinoma (SCC) is the most common type and is generally derived from a precancerous stage called cervical high-grade squamous intraepithelial lesion (HSIL). Normally, the tumor extends downward into the vagina or laterally, invading the parametric tissue, and it also metastasizes through the lymphatic glands or by blood dissemination. Rarely does it spread directly upward into the endometrium, forming endometrial SCC, which is called superficial spreading SCC[2]. Here, we report a case of a postmenopausal woman who underwent hysterectomy because of a relapse of cervical HSIL, whose pathology revealed that the cervical HSIL had transformed into endometrial SCC.
A 57-year-old postmenopausal woman came to our department requesting a routine cervical check-up.
Four years ago, the patient was positive for human papillomavirus (HPV) 16, and had a negative Thinprep cytologic test during pelvic examination. The patient did not have any abnormal vaginal bleeding or abdominal pain. A colposcopic examination showed cervical HSIL, and partial invasion could not be excluded. Soon after, she underwent a cervical loop electrosurgical excision procedure (LEEP), and the pathological examination later revealed cervical HSIL, with a positive endocervical margin (also HSIL). The patient refused a hysterectomy at that time and was lost to follow-up after a 3-mo colposcopic examination showed a normal postoperative appearance. This time, she denied having any symptoms and admitted that she had not had regular gynecological examinations during the past 4 years. The patient was still positive for HPV 16 with a negative cytology; however, a subsequent colposcopic examination revealed HSIL within her cervical canal.
The patient had a history of hypertension for > 30 years, diabetes for > 3 years, and had a cardiac stent implanted 3 years earlier; all these diseases were under good control.
The patient had been menopausal for 3 years, gravida 1, para 1, and denied having a family history of malignant tumors.
Her pelvic examination was negative, and both her uterus and adnexa were atrophied.
Her squamous cell carcinoma antigen was 1.8 ng/mL.
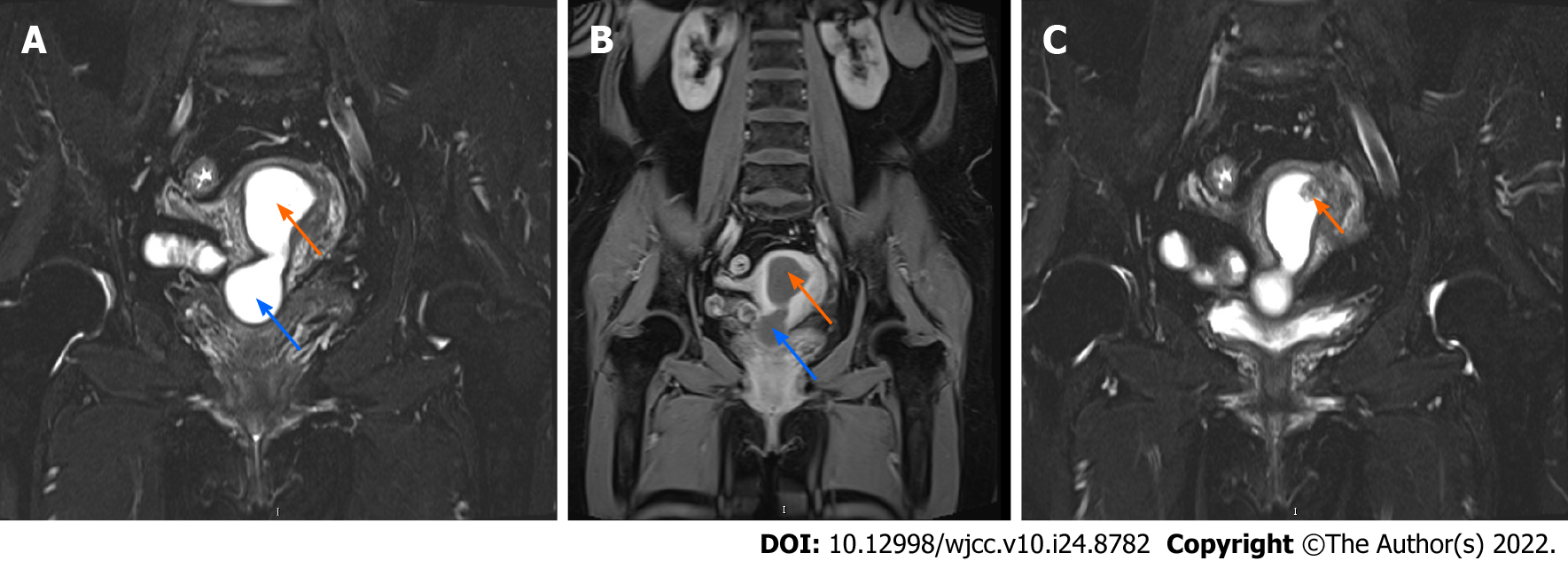
Gynecological ultrasound revealed a fluid-filled uterus and cervix measuring 71 mm × 32 mm × 29 mm, with an unevenly high echogenic mass adhering to the upper endometrium measuring 11 mm × 14 mm × 8 mm. Later, enhanced pelvic magnetic resonance imaging confirmed the findings, suggesting pyometra and possibly endometrial polyps or submucosal myoma (Figure 1).
The patient was finally diagnosed with cervical HSIL, pyometra, and a suspected endometrial polyp.
The patient was then scheduled for LEEP and hysteroscopic examination. During surgery, the cervix was stenotic, and after careful separation, a large amount of brown and odorless pus (approximately 30 mL) came out of the cervical canal. To prevent further infection, the surgery was postponed, and metronidazole hydrochloride was administered for 14 days. During this period, she did not suffer from fever or abdominal pain, and the pus culture was negative. Two weeks later, she underwent the planned surgery, and during hysteroscopy, we found that the endometrium was slightly thicker with two adherent polyp-shaped lumps. One of these was vascular hyperplasia, which we removed; subsequently, we performed curettage of the entire endometrium, along with a second LEEP. The pathology results showed chronic inflammation within the cervix, HSIL in the cervical canal, and endometrial polyps covered by HSIL. This time, after consultation, she decided to undergo a hysterectomy. The patient underwent laparoscopic hysterectomy and bilateral adnexectomy 3 mo later.
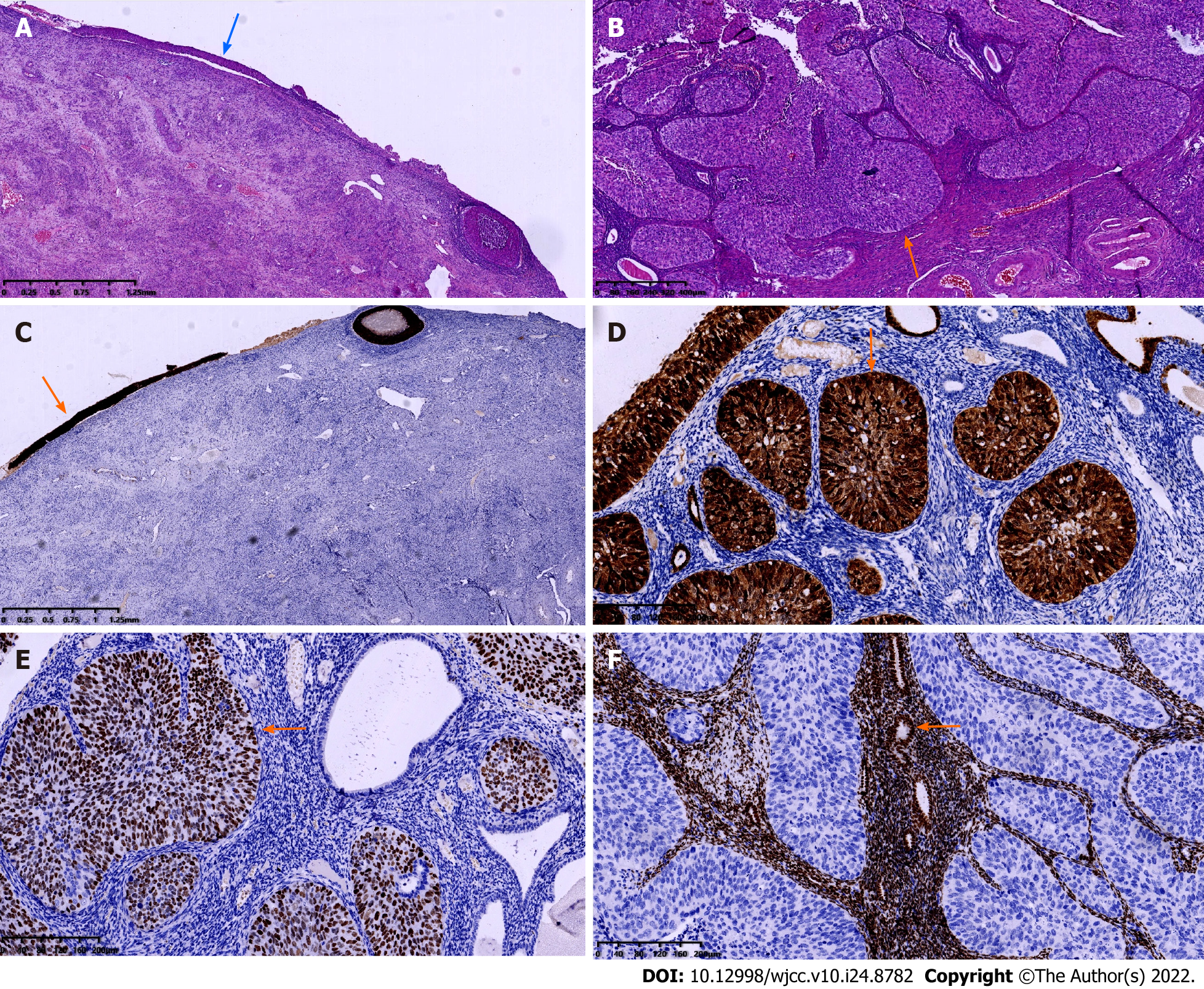
The surgery was successful; the patient recovered well postoperatively and was discharged 4 d after surgery. On macroscopic examination, the uterus was normal sized, weighed 50 g, with 5 mL pus inside (which was culture negative); the endometrium was thin, with a whitish and light brown appearance, and there was polypoid gray tissue extending to the right uterine cornua. Both ovaries were atrophic, along with the fallopian tubes, and the endometrium was unremarkable. Under microscopic examination, the cervix showed HSIL with chronic inflammation (Figure 2A). The endometrium was also replaced by HSIL, which involved the endometrial gland, squeezing into the superficial myometrium and forming SCC (Figure 2B). The bilateral adnexa were unremarkable, and there was no invasion of the nerves, lymph nodes or parametrium. Immunohistochemical staining was positive for p16 and Ki-67 expression in the cervix and endometrium (Figure 2C and D) and strongly positive for p63 expression in the endometrium (Figure 2E) but negative for vimentin and estrogen receptor in the endometrium (Figure 2F). The patient was finally diagnosed with cervical SCC stage IB and received radiotherapy. She was pleased to find that there were no signs of recurrence 1 year after surgery.
In 1900, Cullen first discovered a case of cervical SCC that spread to the entire endometrium; since then, only approximately 50 cases have been reported, with an occurrence of 0.7%[3]. Among these rare cases, although the endometrium is the most common site for metastasis, some cases revealed that the fallopian tubes and the ovaries could also be invaded[4]; the most distant site reported thus far is the omentum and the mucosa of the transverse colon[5]. Since the 1960s, scholars have discussed the potential risk factors that may contribute to upward metastasis, such as long-term estrogen usage, vitamin A deficiency, HPV infection, senile endometrium, pyometra, and radiotherapy[3]; among them, some have persisted until today, namely, advanced age, cervical stenosis and pyometra[6], and these are often the result of previous cervical procedures, such as LEEP or conization[5].
Astonishingly, we have found that some cases even shared the same characteristics as ours, for example, in a very recent case published in Spain, the 66-year-old patient also underwent cervical conization 15 years ago and had a finding of HSIL, was lost to regular follow-up, and underwent laparoscopic hysterectomy for the symptoms of vaginal discharge after discovering cervical stenosis and pyometra. Her final pathology also revealed HSIL within the cervix and SCC in the endometrium with a depth of 2.8 mm[7]. Anne Chao et al[8] also reported a case with fatal pyometra (1200 mL of pus that was cultured Staphylococcus epidermidis) in a 60-year-old patient whose pathology also revealed cervical HSIL that progressed into SCC in the endometrium. Therefore, it is reasonable to infer that these factors often interact with each other simultaneously, contributing to this rare disease. In postmenopausal women, cervical stenosis is easily formed after cervical treatment due to the loss of periodic abscission[9], and it encloses the uterine cavity and accelerates the pyometra (e.g., under inflammation). In time, the occult inflammatory environment within the uterus becomes a hotbed for a number of diseases, namely, if a prior disease existed (a cervical lesion such as in our case), it may trigger the unusual cephalad spreading of the disease, or in other cases, it may be the cause of other diseases, such as the spontaneous rupture of the uterus[10], if there was no prerequisite for any other relevant diseases. Further studies should also be centered on the impact of this occult inflammatory microenvironment.
To date, there are two main theories explaining this unusual phenomenon: Horizontal theory and vertical theory. The horizontal theory proposes that the tumor originates from one stem cell of the cervix spreading upward to replace the benign cells[5]. The vertical theory proposes that the lesion occurs from a group of predetermined cells that are transformed into malignant cells simultaneously in a vertical direction[11]. In 2001, Kushima et al[4] reported 5 similar cases and explained this phenomenon on a genetic level. They found that even if the lesions are discontinuous between the cervix and endometrium, they still shared the same genetic alterations, which is generally the loss of heterozygosity frequently found on the 6p, 6q, 11p and 11q loci of the chromosome, thus supporting the horizontal theory that the SCC originates from one single cell and migrates upward while cloning itself.
Notably, this rare phenomenon should be distinguished from primary endometrial SCC (PESCC). PESCC is exclusively composed of cells with squamous differentiation. The diagnostic criteria for PESCC were established by Fluhmann[12] in 1953 and included the following: (1) No evidence of coexisting endometrial adenocarcinoma or primary cervical SCC; (2) No connection between the endometrial tumor and squamous epithelium of the cervix; or (3) No connection between any existing cervical in situ carcinoma and independent endometrial neoplasm.
As endometrial involvement of cervical SCC may be an indicator of poor prognosis[13] and is usually diagnosed after hysterectomy, early detection is of extreme significance. Many scholars emphasized the importance of evaluating the endometrium before hysterectomy was performed[14]. The key points included the thickness of the endometrium as well as whether there was unusual fluid in the uterine cavity; normally, these can be spotted with a pelvic ultrasound or an magnetic resonance imaging test. Endocervical curettage is also critical since a positive result may be an indication of a deeper lesion[5]. However, as shown in our case, even with all the aforementioned assistance, along with a thorough hysteroscopic exam, there is still an upgrade in the final diagnosis, which reveals the insidiousness of this disease and calls for more detection measurements in the future.
Prevention of this disease should be focused on minimizing recurrence in older patients who have undergone cervical procedures. A wider excision of the cervical lesion is often desired; however, this also increases the likelihood of cervical stenosis, which obscures the true condition of the cervix and thus increases the chances of relapse[9,15]. Although certain precautions, such as hormone therapy and urinary catheter stenting, are suggested[15], the follow-up of these patients is still challenging.
Upward spreading of cervical SCC/HSIL is rare, and advanced age, cervical stenosis, and pyometra are strong indications for this phenomenon. Pelvic ultrasound and hysteroscopy may help in early diagnosis, and better prevention depends on minimizing recurrence in older patients who have undergone prior cervical procedures. Since there are currently no guidelines for this specific entity and this condition may alter staging, more attention should be given to it.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mainenti PP, Italy; Šarenac TM, Serbia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55825] [Article Influence: 7975.0] [Reference Citation Analysis (132)] |
| 2. | Ishida M, Okabe H. Superficial spreading squamous cell carcinoma of the uterine cervix involving the endometrium: Report of two cases with emphasis on the likely molecular mechanism. Oncol Lett. 2013;5:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 3. | Kanbour AI, Stock RJ. Squamous cell carcinoma in situ of the endometrium and fallopian tube as superficial extension of invasive cervical carcinoma. Cancer. 1978;42:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Kushima M, Fujii H, Murakami K, Ota H, Matsumoto T, Motoyama T, Kiyokawa T, Ishikura H. Simultaneous squamous cell carcinomas of the uterine cervix and upper genital tract: loss of heterozygosity analysis demonstrates clonal neoplasms of cervical origin. Int J Gynecol Pathol. 2001;20:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 5. | Nakajima T, Hatta H, Nishida T, Minamisaka T, Miwa S, Terahata S, Imura J. Superficial spread of cervical squamous cell carcinoma to the upper genital tract and dissemination to the omentum. Pathol Int. 2019;69:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Wang W, Zhou F. Cervical HSIL Involving the Endometrium and Adenomyosis: A Case Report and Literature Review. J Coll Physicians Surg Pak. 2021;31:337-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Martín-Vallejo J, Laforga JB, Molina-Bellido P, Clemente-Pérez PA. Superficial spreading cervical squamous cell carcinoma in situ involving the endometrium: a case report and review of the literature. J Med Case Rep. 2022;16:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Chao A, Wang AM, Wang TH, Wu TI, Chao AS. An atypical and fatal case of pyometra accompanied by the superficial spread of squamous cell carcinoma of the endometrium and the fallopian tubes. Taiwan J Obstet Gynecol. 2013;52:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Satturwar S, Zhao C, Austin RM. Cervical Stenosis: Previously Unrecognized Cause of False-Negative Human Papillomavirus Tests in Women Developing Cervical Cancer. J Low Genit Tract Dis. 2020;24:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Uno K, Tano S, Yoshihara M, Mayama M, Ukai M, Kishigami Y, Nishikawa Y, Takeichi Y, Oguchi H. A Case Report and Literature Review of Spontaneous Perforation of Pyometra. J Emerg Med. 2016;50:e231-e236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Sood N, Sinha K S. Superficial spreading squamous cell carcinoma endometrium andicthyosis uteri with CINIII with p16 expression: Report of 2 unusual cases. Journal of Krishna Institute of Medical Sciences University 2017; 6: 126-131. |
| 12. | Fluhmann CF. The histogenesis of squamous cell metaplasia of the cervix and endometrium. Surg Gynecol Obstet. 1953;97:45-58. [PubMed] |
| 13. | Perez CA, Camel HM, Askin F, Breaux S. Endometrial extension of carcinoma of the uterine cervix: a prognostic factor that may modify staging. Cancer. 1981;48:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Bagde MN, Bagde NKD, Hussain N, Thangaraju P. A review and case report of enigmatic superficial endometrial spread of cancer of the uterine cervix: Need for vigilance in the primary care setting. J Family Med Prim Care. 2021;10:3505-3510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Penna C, Fambrini M, Fallani MG, Pieralli A, Scarselli G, Marchionni M. Laser CO2 conization in postmenopausal age: risk of cervical stenosis and unsatisfactory follow-up. Gynecol Oncol. 2005;96:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |