Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8703
Peer-review started: March 4, 2022
First decision: June 7, 2022
Revised: June 16, 2022
Accepted: July 21, 2022
Article in press: July 21, 2022
Published online: August 26, 2022
Processing time: 164 Days and 17.3 Hours
Tofacitinib is an oral Janus kinase (JAK) inhibitor that is currently approved by the United States Food and Drug Administration for the treatment of rheumatoid arthritis (RA). Varicella zoster virus reactivation leading to herpes zoster (HZ) is an adverse effect of this drug; however, recurrent HZ at the same site is a rare clinical condition.
A 70-year-old female RA patient had undergone 1-year of tofacitinib treatment (10 mg daily). About 1 mo after initiation of oral tofacitinib, she developed blisters on the left flank and abdomen and was diagnosed with HZ; antiviral therapy with acyclovir was resolutory. However, 5 d prior to presentation at our hospital, erythema and blisters with severe pain recurred at the same site. Small clustered blisters and bullous were visible on the left lumbar abdomen and perineum, with a pain score of 8 (visual analogue scale). Antiviral, nutritional supplement, analgesic and other treatments led to healing but over an atypically long period (approximately 26 d, vs approximately 1 wk). HZ is a common and serious adverse reaction of JAK inhibitors, but it rarely recurs. Our patient’s experience of HZ recurrence at the same site, with a wider affected area, more severe pain and longer healing period, is inconsistent with previous reports.
Same-anatomical site HZ recurrence may occur during oral tofacitinib treatment, with more severe clinical manifestations than in the initial occurrence.
Core Tip: Herpes zoster (HZ) is caused by the reactivation of varicella zoster virus. We report a case of recurrent HZ in a patient with rheumatoid arthritis treated with oral tofacitinib. This patient’s HZ recurred at the same site, with a wider affected area and more significant pain than the first occurrence. After antiviral therapy, the rash slowly resolved. The characteristics of this case are different to those of HZ induced by tofacitinib summarized and analyzed in the literature. We hope that our report will prompt clinicians to standardize the diagnosis and treatment of HZ during tofacitinib therapy.
- Citation: Lin QX, Meng HJ, Pang YY, Qu Y. Recurrent herpes zoster in a rheumatoid arthritis patient treated with tofacitinib: A case report and review of the literature. World J Clin Cases 2022; 10(24): 8703-8708
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8703.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8703
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized pathologically by synovitis involving multiple joints, often accompanied by persistent joint pain, swelling, and stiffness. This disease occurs at all ages and can cause cartilage destruction and bone erosion, leading to destruction of the joint structure and loss of joint function, which seriously affects the patient's quality of life[1,2]. The purpose of treatment is to improve joint function and the quality of life. Commonly used drugs mainly include non-steroidal anti-inflammatory drugs, glucocorticoids (GCs), and disease-modifying anti-rheumatic drugs[3]; however, the therapeutic effects are limited and there are many associated adverse reactions.
In recent years, Janus kinase (JAK) inhibitors have been approved for the treatment of a variety of immune-mediated inflammatory diseases, including RA and inflammatory bowel disease[4-6]. Tofacitinib was approved in 2012 for the treatment of adult patients with moderate-to-severe active RA and an inadequate response or intolerance to methotrexate[7]. It blocks the signal transduction of various proinflammatory cytokines in RA by inhibiting the activity of JAK1 and JAK3[8]. It also controls the activity of RA by regulating immune and inflammatory responses, thus reducing synovial inflammation and cartilage damage[9]. As the JAK/STAT signaling pathway is important in cells and is widely involved in cell proliferation, differentiation, and immune regulation[10], severe infections including herpes zoster (HZ) are common adverse reactions of JAK inhibitors such as tofacitinib[11].
HZ is a common skin disease that results from the reactivation of varicella zoster virus (VZV). It is common in middle-aged and elderly people and is characterized by erythema and clustered blisters distributed in bands along one peripheral nerve, accompanied by severe pain. Due to differences in nerve invasion by the virus and different immune status of the body, HZ may appear as bleeding, gangrenous or visceral lesions, among other presentations. However, recurrent HZ is rare and often occurs in immunocompromised people, such as those with human immunodeficiency virus (HIV) infection or organ transplantation[12,13]. HZ often occurs during the use of tofacitinib in patients with diseases such as RA, but recurrent HZ has rarely been reported in the literature[14].
Here, we report a case of recurrent HZ in a patient with RA who developed HZ twice at the same site during treatment with tofacitinib. The second occurrence affected a wider area, caused more severe pain, and followed a longer disease course than the first rash; these many clinical characteristics differed from those described in previous clinical studies.
A 70-year-old female patient presented with erythema and blistering on her left waist and abdomen, which were accompanied by pain.
Five days prior to presentation at our clinic, the patient had developed a small swath of red bullous eruption on the left side of her waist and abdomen, with mild pain (pain score of 3 on the visual analogue scale [VAS 3]). Three days previously, the blisters had rapidly increased, involving the lower back, waist and abdomen, and perineum, accompanied by severe pain (VAS 5). The patient was diagnosed with HZ in the outpatient department and treated with oral acyclovir; however, the area of the lesion continued to expand, blood blisters and bullae appeared, and the pain continued to worsen (VAS 8), which seriously affected the patient’s sleep and daily life.
The patient was diagnosed with RA 3 years ago, and had been treated with prednisone, leflunomide, hydroxychloroquine, adalimumab, and other drugs. One year ago, for economic reasons, adalimumab was discontinued and changed to oral tofacitinib (10 mg daily). Approximately 1 mo after initiation of oral tofacitinib, painful blisters appeared on the left flank and abdomen, which were diagnosed as HZ; the blisters healed 2 wk after treatment with the antiviral drug acyclovir. Following her recovery from HZ, the patient continues to receive tofacitinib. At present, her RA is stable.
The patient’s personal and family histories were unremarkable.
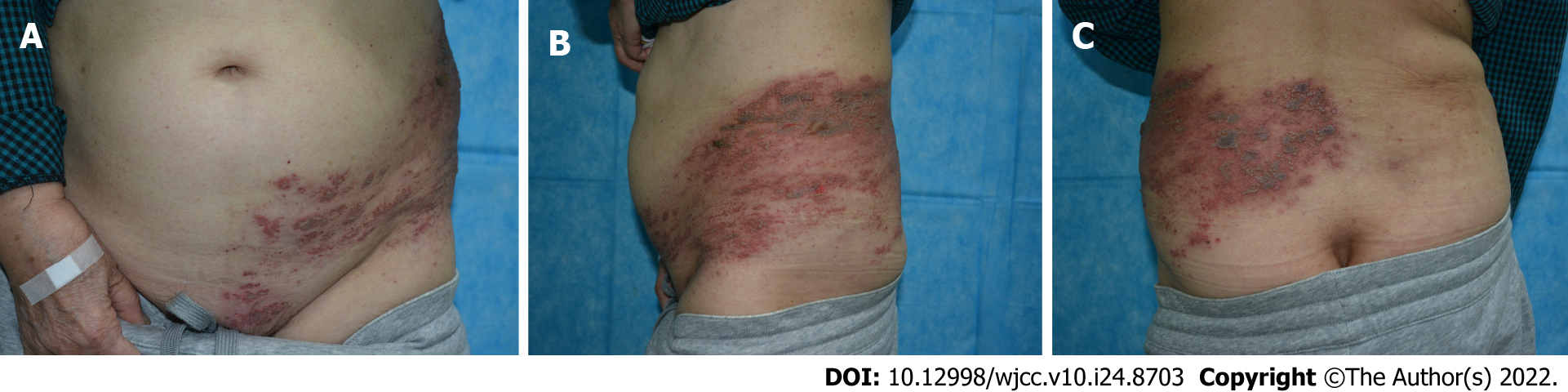
On physical examination, the patient’s temperature was 36.4°C, pulse rate was 72 bpm, respiratory rate was 17 breaths/min, and blood pressure was 145/85 mmHg. Both knees and wrists were swollen without significant deformity or tenderness. Large areas of edematous erythema could be seen on the left abdomen, left perineal area, and left side of the back, with clustered blisters, bullae, and blood blisters. The herpes rash was distributed in bands and did not exceed the midline of the body surface (Figure 1A-C).
Routine blood tests showed the following: white blood cell count of 3.67 × 109/L (normal: 3.5-9.5 × 109/L); lymphocyte percentage of 45.2% (normal: 20%-50%); monocyte percentage of 18.7% (normal: 3%-10%); neutrophil percentage of 35.1% (normal: 40%-75%); C-reactive protein of 7.98 mg/L (normal: 0-10 mg/L); and serum amyloid A level of 19.36 mg/L (normal: 0-10 mg/L). Routine urine tests were negative for protein, glucose, red blood cells and white blood cells. There were no abnormal findings following tests for liver function, renal function, blood lipid level, blood glucose level, electrolytes, rheumatoid factor, immunoglobulin (Ig) A, IgM and IgG levels, complement 3 and 5, lymphocytes (CD3, CD4, and CD8; immunoassay), antistreptolysin-O, and anti-HIV.
Chest and abdominal computed tomography scans were normal.
Recurrent HZ due to tofacitinib.
Tofacitinib was discontinued. Foscarnet sodium 2 g (40 mg/kg) was infused intravenously every 8 h and mecobalamin 1500 mg/d was administered for 7 d. The patient had severe pain, and was successively treated with ibuprofen 150 mg/d, gabapentin 1200 mg/d, and tramadol 100 mg/d. After 7 d of treatment, some blisters healed. Antiviral drugs were discontinued, and nutritional supplement and analgesic therapies were continued.
After 26 d of treatment, the HZ lesions completely healed, but the patient still had some pain (VAS 3). At the 3-mo follow-up, the pain had disappeared, but the patient was readmitted due to recurrent abdominal pain and diarrhea, and the possibility of gastrointestinal tumors was not excluded.
We report herein a case of recurrent HZ in an RA patient treated with tofacitinib. HZ is an infectious skin disease caused by VZV reactivation. VZV infection in humans is often latent in nerve cells and is protected from attack by the body’s lymphocytes[15]. When the body's immunity is decreased, VZV reactivation can occur, leading to HZ. In most cases, HZ occurs only once; however, recurrent HZ is sometimes seen in immunocompromised or immunosuppressed people, such as those undergoing chemotherapy for malignant tumors or with HIV infection[13]. The incidence of recurrent HZ has been reported to be 6.41%, with a predominance in women[16]. The specific mechanism of HZ recurrence is not clear. Current studies suggest that it may be caused by weakening of the body's immune response to VZV after the occurrence of HZ, or by a decrease in the body's specific immunity to VZV with increasing age[17].
The risk of HZ is nearly 2-fold higher in RA patients than in those without RA[18]. The use of immunosuppressive medications, especially GCs, can increase the occurrence of HZ in patients with RA, whereas patients treated with biological therapy may develop severe HZ, with the greatest risk in the initial stage[19]. Tofacitinib can also significantly increase the incidence of HZ in patients[20]. The specific mechanism by which tofacitinib increases the risk of infection is not clear and may be related to its inhibition of the JAK/STAT signaling pathway, resulting in reduced downstream signaling and cytokine immune responses to viral infections[21]. Patients treated with tofacitinib have decreased neutrophils, lymphocytes, and natural killer cells[22,23], which may predispose them to VZV activation. The cellular immunity and humoral immunity tests provided values in the normal range for our patient, suggesting that the cause of VZV reactivation was not the persistent immunosuppression due to her RA but, instead, to a tofacitinib-induced abnormal immune reaction leading to HZ; this hypothesis has also been cited in the literature[24]. Patients treated with 10 mg tofacitinib twice daily have a 2.5-fold higher risk of developing HZ compared with 5 mg twice daily in the first 3 mo, whereas the risk is comparable after 6 mo[25]. The risk of HZ is doubled when tofacitinib is used in combination with GC therapy[26], and the incidence of HZ is higher in Japan and South Korea than in Europe[25,27]. Therefore, it is recommended that tofacitinib be used while avoiding concomitant use of GCs. Female sex, increasing age, prior biological exposure, prednisone > 7.5 mg/d, prior outpatient infection, and a greater number of hospitalizations are associated with an increased risk of HZ, and tofacitinib is associated with a nearly 2-fold higher risk of HZ compared with other biological therapies[28,29]. Although the risk of HZ is increased with tofacitinib, there have been few cases of severe, disseminated, and multiganglionic zoster, which recover quickly after antiviral therapy[30]; however, the occurrence of recurrent HZ has not been reported.
Previous studies have shown that recurrent HZ has a shorter course, more localized skin lesions, less pain, and a lower risk of postherpetic neuralgia than primary HZ[17]. However, our patient had many different characteristics than the other clinical cases in the literature, including more extensive skin lesions, more severe pain, longer course of disease, and recurrence at the same site. We speculate that these characteristics may be related to oral tofacitinib. Genetic analysis of RA or psoriatic arthritis treated with tofacitinib identified multiple genetic loci associated with an increased risk of HZ. Variants near the immune-relevant gene cluster of differentiation 83 in European populations and near interleukin 17 receptor B in East Asian populations may contribute to the increased risk of HZ, demonstrating that genetic factors play a role in causing VZV reactivation during tofacitinib treatment[31]. To address the problem of VZV reactivation during treatment with tofacitinib, it is recommended that patients be given a HZ vaccine when tofacitinib or combination systemic treatment is used[32]. It is recommended that live HZ vaccine be injected in patients with RA 2-3 wk before the use of tofacitinib, which can produce significant cellular and humoral immunity, and its safety and efficacy have been confirmed[33].
Our case is a reminder that HZ vaccine should be administered promptly, and clinicians should always be alert to the possible reactivation of VZV before using tofacitinib in elderly patients with previous immunosuppression. The specific genetic and immune mechanisms of HZ recurrence caused by tofacitinib need to be studied further.
Tofacitinib can cause recurrent HZ, which may be associated with an immunosuppressive state due to JAK/STAT signaling. We present a rare case of recurrent HZ at the same site, with a more severe and extensive rash, more significant pain, and a longer course than the primary outbreak. The characteristics of this case are inconsistent with previous literature reports. Clinicians should be aware of this condition in patients receiving tofacitinib treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bhargava S, India; Munasinghe B, Sri Lanka S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Lin YJ, Anzaghe M, Schülke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 494] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 2. | Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110:102400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 462] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 3. | Drosos AA, Pelechas E, Kaltsonoudis E, Voulgari PV. Therapeutic Options and Cost-Effectiveness for Rheumatoid Arthritis Treatment. Curr Rheumatol Rep. 2020;22:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Food and Drug Administration. Xeljanz full prescribing information [Accessed 18 March 2019]. Available from: https://www.accessdata.fda.gov/drugsatfdadocs/Label/2018/203214s018 Lbl.pdf. |
| 5. | Taylor PC. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford). 2019;58:i17-i26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 6. | Drugs.com.Pfizer announces U.S. FDA approves Xeljanz (tofacitinib) for the treatment of moderately to severely active ulcerative colitis. Drugs.com https://www.drugs.com/newdrugs/pfizer-announces-u-s-fdaapproves-xeljanz-tofacitinib-moderately-severelyactive-ulcerative-colitis-4756.html (2018). |
| 7. | Traynor K. FDA approves tofacitinib for rheumatoid arthritis. Am J Health Syst Pharm. 2012;69:2120. [PubMed] |
| 8. | Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs. 2017;77:521-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 821] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 9. | Dhillon S. Tofacitinib: A Review in Rheumatoid Arthritis. Drugs. 2017;77:1987-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 10. | Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou X, Ma H, Wei D, Sun S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. 2020;80:106210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 610] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 11. | Choy EH. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology (Oxford). 2019;58:953-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 12. | Dayan RR, Peleg R. Herpes zoster - typical and atypical presentations. Postgrad Med. 2017;129:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186 Suppl 1:S91-S98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Yamaoka K. Benefit and Risk of Tofacitinib in the Treatment of Rheumatoid Arthritis: A Focus on Herpes Zoster. Drug Saf. 2016;39:823-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Burkhart CN. Recurrent herpes zoster revisited. Int J Dermatol. 2002;41:528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Shiraki K, Toyama N, Daikoku T, Yajima M; Miyazaki Dermatologist Society. Herpes Zoster and Recurrent Herpes Zoster. Open Forum Infect Dis. 2017;4:ofx007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Nakamura Y, Miyagawa F, Okazaki A, Okuno Y, Mori Y, Iso H, Yamanishi K, Asada H; Shozu Herpes Zoster Study Group. Clinical and immunologic features of recurrent herpes zoster (HZ). J Am Acad Dermatol. 2016;75:950-956.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, Chan KA. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Liao TL, Chen YM, Liu HJ, Chen DY. Risk and severity of herpes zoster in patients with rheumatoid arthritis receiving different immunosuppressive medications: a case-control study in Asia. BMJ Open. 2017;7:e014032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Zhang Z, Deng W, Wu Q, Sun L. Tuberculosis, hepatitis B and herpes zoster in tofacitinib-treated patients with rheumatoid arthritis. Immunotherapy. 2019;11:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Telliez JB, Dowty ME, Menon S, Lamba M, Zwillich S. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:318-328. [PubMed] |
| 22. | Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology (Oxford). 2019;58:i34-i42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 23. | Wollenhaupt J, Lee EB, Curtis JR, Silverfield J, Terry K, Soma K, Mojcik C, DeMasi R, Strengholt S, Kwok K, Lazariciu I, Wang L, Cohen S. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 24. | Munasinghe BM, Fernando U, Mathurageethan M, Sritharan D. Reactivation of varicella-zoster virus following mRNA COVID-19 vaccination in a patient with moderately differentiated adenocarcinoma of rectum: A case report. SAGE Open Med Case Rep. 2022;10:2050313X221077737. [PubMed] |
| 25. | Winthrop KL, Yamanaka H, Valdez H, Mortensen E, Chew R, Krishnaswami S, Kawabata T, Riese R. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2675-2684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 26. | Curtis JR, Xie F, Yang S, Bernatsky S, Chen L, Yun H, Winthrop K. Risk for Herpes Zoster in Tofacitinib-Treated Rheumatoid Arthritis Patients With and Without Concomitant Methotrexate and Glucocorticoids. Arthritis Care Res (Hoboken). 2019;71:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Winthrop KL, Curtis JR, Lindsey S, Tanaka Y, Yamaoka K, Valdez H, Hirose T, Nduaka CI, Wang L, Mendelsohn AM, Fan H, Chen C, Bananis E. Herpes Zoster and Tofacitinib: Clinical Outcomes and the Risk of Concomitant Therapy. Arthritis Rheumatol. 2017;69:1960-1968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 28. | Colombel JF. Herpes Zoster in Patients Receiving JAK Inhibitors For Ulcerative Colitis: Mechanism, Epidemiology, Management, and Prevention. Inflamm Bowel Dis. 2018;24:2173-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 29. | Curtis JR, Xie F, Yun H, Bernatsky S, Winthrop KL. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1843-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 30. | Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, Winthrop KL, Charles-Schoeman C, Thirunavukkarasu K, DeMasi R, Geier J, Kwok K, Wang L, Riese R, Wollenhaupt J. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 300] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 31. | Bing N, Zhou H, Chen X, Hirose T, Kochi Y, Tsuchida Y, Ishigaki K, Sumitomo S, Fujio K, Zhang B, Valdez H, Vincent MS, Martin D, Clark JD. Contribution of a European-Prevalent Variant near CD83 and an East Asian-Prevalent Variant near IL17RB to Herpes Zoster Risk in Tofacitinib Treatment: Results of Genome-Wide Association Study Meta-Analyses. Arthritis Rheumatol. 2021;73:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF. A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2019;81:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Winthrop KL, Wouters AG, Choy EH, Soma K, Hodge JA, Nduaka CI, Biswas P, Needle E, Passador S, Mojcik CF, Rigby WF. The Safety and Immunogenicity of Live Zoster Vaccination in Patients With Rheumatoid Arthritis Before Starting Tofacitinib: A Randomized Phase II Trial. Arthritis Rheumatol. 2017;69:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |