Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8656
Peer-review started: January 20, 2022
First decision: May 11, 2022
Revised: May 23, 2022
Accepted: July 22, 2022
Article in press: July 22, 2022
Published online: August 26, 2022
Processing time: 207 Days and 21.1 Hours
Hypovolemic shock can lead to life-threatening organ dysfunction, and adequate fluid administration is a fundamental therapy. Traditionally, parameters such as vital signs, central venous pressure, and urine output have been used to estimate intravascular volume. Recently, pulse pressure variation (PPV) and non-invasive cardiac monitoring devices have been introduced. In this case report, we introduce a patient with massive active bleeding from giant renal angiomyolipoma (AML). During emergent nephrectomy, we used non-invasive cardiac monitoring with CSN-1901 (Nihon Kohden, Tokyo, Japan) and PPV to evaluate the patient's intravascular volume status to achieve optimal fluid management.
A 30-year-old male patient with giant AML with active bleeding was referred to the emergency room complaining of severe abdominal pain and spontaneous abdominal distension. AML was diagnosed by computed tomography, and emergent nephrectomy was scheduled. Massive bleeding was expected so we decided to use non-invasive cardiac monitoring and PPV to assist fluid therapy because they are relatively easy and fast compared to invasive cardiac monitoring. During the surgery, 6000 mL of estimated blood loss occurred. Along with the patient's vital signs and laboratory results, we monitored cardiac output, cardiac output, stroke volume, stroke volume index with a non-invasive cardiac monitoring device, and PPV using an intra-arterial catheter to evaluate intra
In addition to traditional parameters, non-invasive cardiac monitoring and PPV are useful methods to evaluate patient's intravascular volume status and provide guidance for intraoperative management of hypovolemic shock patients.
Core Tip: We present a giant ruptured renal angiomyolipoma (> 20 cm) with active bleeding. Emergent operation was performed. The successful fluid management was carried with pulse pressure variation and noninvasive cardiac output monitoring.
- Citation: Jeon WJ, Shin WJ, Yoon YJ, Park CW, Shim JH, Cho SY. Anesthetics management of a renal angiomyolipoma using pulse pressure variation and non-invasive cardiac output monitoring: A case report. World J Clin Cases 2022; 10(24): 8656-8661
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8656.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8656
Angiomyolipoma (AML) is a benign tumor of the kidney that accounts for up to 3% of all renal masses[1]. Most of these masses are sporadic and incidentally diagnosed. However, large AMLs (> 4 cm) can present with symptoms such as hemorrhage, pain, a palpable mass and mass-associated symptoms. Giant AMLs > 20 cm in size are rare, and few cases have been reported. Enlarging giant AMLs can produce an aneurysm that can rupture and lead to massive peri-renal bleeding and hypovolemic shock, a condition known as Wunderlich syndrome[2]. The dynamic parameters of fluid responsiveness are related to cardiopulmonary interactions under general anesthesia with mechanical ventilation. Pulse pressure variation (PPV) has shown great advantage to optimize hemodynamic parameters using physiological data from non-invasive means. PPV can assist with fluid administration and hemodynamic stability in patients under general anesthesia receiving mechanical ventilation[3]. We used a CSM-1901 (Nihon Kohden, 15-Tokyo, Japan) to non-invasively monitor the patient’s cardiac output (CO), stroke volume (SV), continuous cardiac index (CCI), and stroke volume index (SVI). Additionally, we monitored PPV using an arterial line to ensure adequate administration of fluid during the surgery.
Old male patient presented to the hospital complaining of severe abdominal pain and a palpable mass in the abdomen.
Abdominal distension started at 2 pm and Lt. abdomen was distended severely on arrival to emergency room at 7:50 pm.
The patient had been in good health without no-known underlying disease.
Abdominal distension began at 2 p.m., and the left side of the abdomen was severely distended upon arrival to the emergency department at 7:50 p.m. His initial vital signs were blood pressure: 142/81 mmHg, heart rate: 112 beats/min, respiratory rate: 20 times/min, and body temperature: 36.3 ºC.
Initial laboratory tests showed hemoglobin 10.3 g/dL and hematocrit 29.8%.
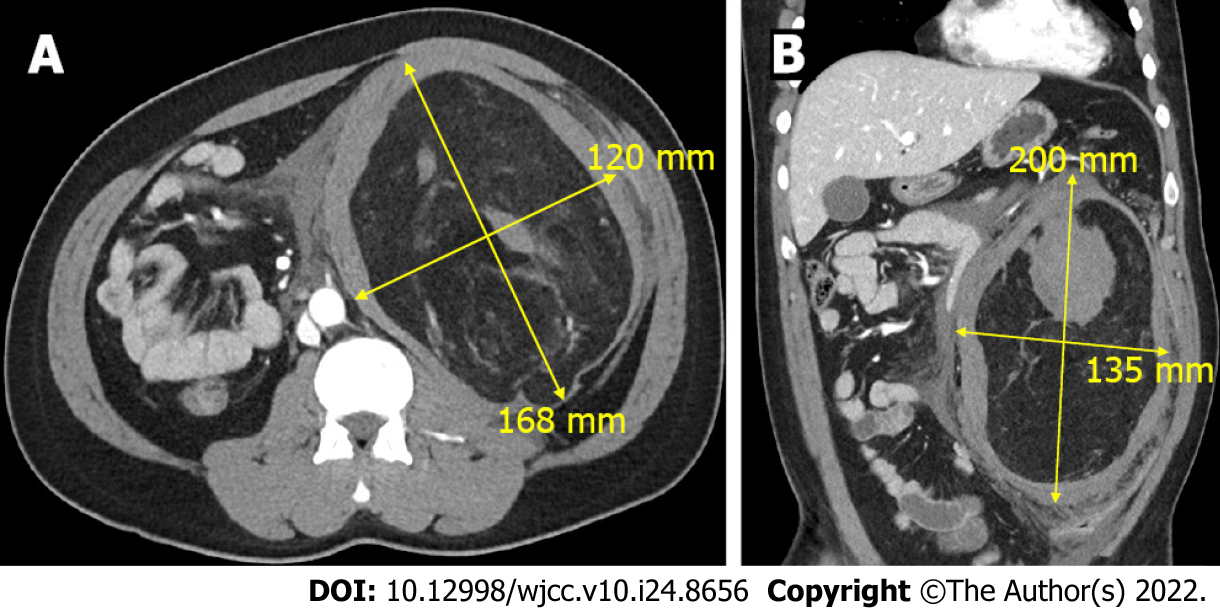
Abdominal computed tomography (CT) revealed a 22-cm AML with active bleeding near the left kidney, with a combined pseudoaneurysm (Figure 1).
Left renal AML.
After considering the patient’s vital signs and laboratory and CT findings, massive active bleeding was suspected, and emergent nephrectomy was scheduled. We expected a large amount of bleeding during surgery and used a non-invasive cardiac monitoring device (CSN-1901, Nihon Kohden, Tokyo, Japan) and PPV to assist evaluation of intravascular volume. These methods are relatively easy and fast to apply compared to invasive cardiac monitoring, which requires placement of a pulmonary catheter.
Upon arrival at the operating room, the patient’s blood pressure was 150/100 mmHg, heart rate was 110 beats/min, and respiratory rate was 20 times/min. Anesthesia was induced through mask ventilation and 100% oxygen with 120 mg intravenous propofol and 50 mg rocuronium. Endotracheal intubation was achieved with a 7.5 endotracheal tube. Anesthesia was maintained with desflurane, nitric oxide, and remifentanil. A right radial arterial cannula was inserted to monitor arterial pressure, and CSM-1901 (Nihon Kohden, Tokyo, Japan) was used to estimate CO, CCI, SV, and SVI. Hemoglobin and hematocrit levels were 10.8 g/dL and 32% at the initial arterial blood gas analysis, respectively.
Laparotomy began with a 15-cm left transverse subcostal incision. Immediately after opening the peritoneum, a huge renal mass with hematoma was observed; the mass was too big to approach all at once. Therefore, part of the mass was dissected, resulting in severe bleeding. A portion of the hematoma was removed, and the surgeon was better able to approach the mass. Nephrectomy was carried out along with removal of the mass.
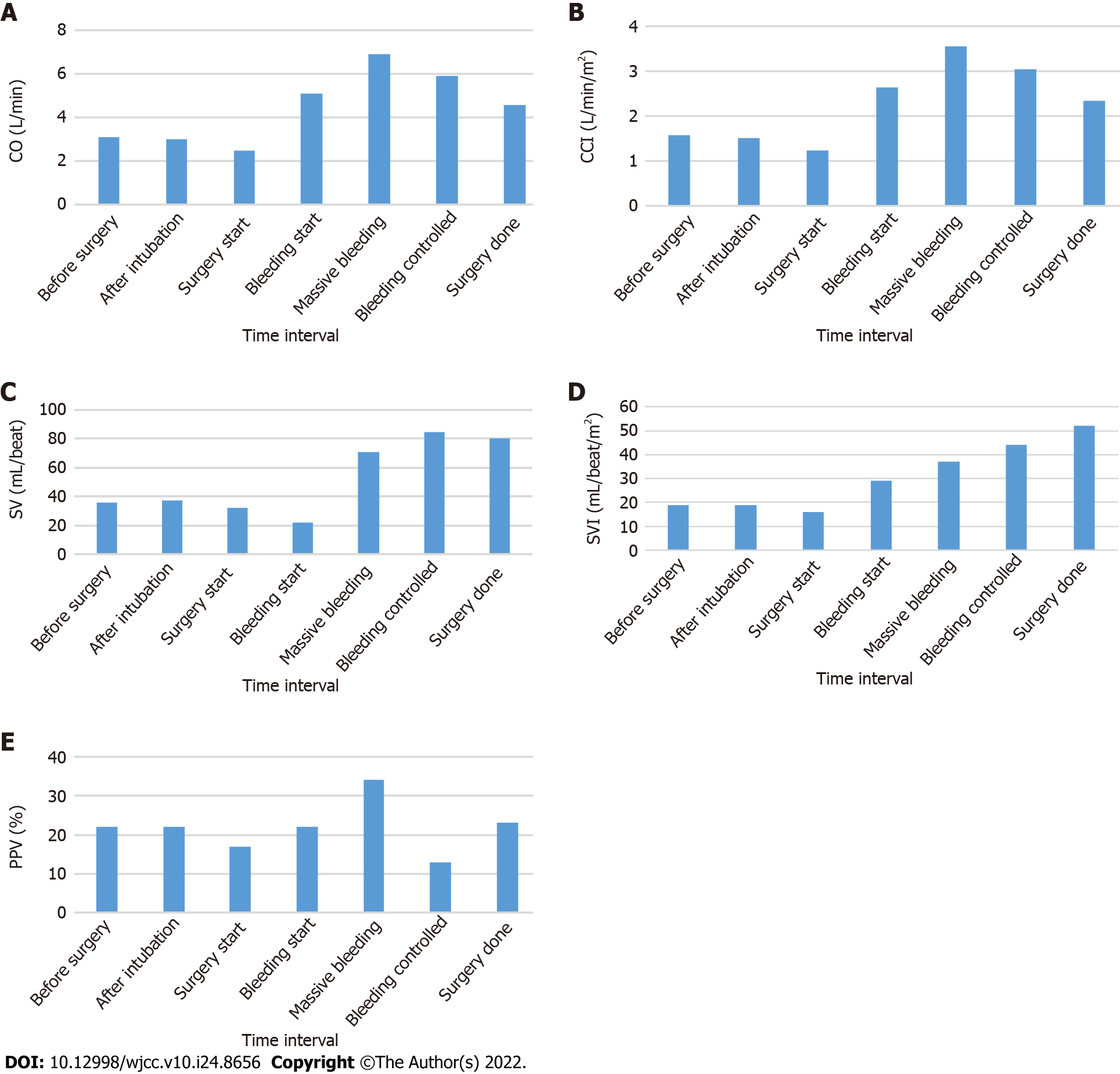
During the surgery, the patient’s CO, CCI, SV, SVI, and PPV were monitored (Figure 2). The patient was estimated to have lost 6000 mL of blood, for which 2950 mL of red blood cells and 953 mL of platelets were transfused. In addition, norepinephrine was administered to maintain proper vital signs. The patient’s final hemoglobin level was 9.7 g/dL, while his hematocrit level was 29%. Prior to extubation, 200 mg of sugammadex was administered for neuromuscular blockade recovery. After extubation, the patient’s vital signs were carefully monitored before transfer to the intensive care unit. Biopsy confirmed the mass to be a ruptured AML that measured 21 cm × 16 cm × 6 cm.
The patient was hospitalized for 9 d and left the hospital without a significant complication.
Renal AML is a benign renal neoplasm. Small AMLs are usually asymptomatic and incidentally diagnosed on imaging. However, as the size increases, vascularity increases, resulting in vulnerability to rupture. In one study, the risk of bleeding was 13% with size < 4 cm and 51% with size > 4 cm[4]. Wunderlich syndrome is a spontaneous, non-traumatic renal hemorrhage that arises in the peri-renal space due to various medical conditions, including renal tumors, vascular disease, coagulation disorders, and idiopathic causes[5]. Urgent contrast-enhanced CT is recommended for diagnosis, and proper management is required because many cases present with hypovolemic shock due to massive hemorrhage. Although proper fluid management is critical, it is extremely difficult to determine the intravascular volume of patients with AML, especially those with active bleeding.
Traditionally, parameters such as blood pressure, heart rate, central venous pressure, and urine output have been used to estimate intravascular volume. However, recent studies support the use of hemodynamic parameters in patients undergoing major invasive surgery because large blood loss and fluid shifting are expected, and this method provides better volume responsiveness compared with traditional parameters[6,7]. The CO is especially important because it is a main determinant of oxygen delivery. CO can be measured invasively (i.e., pulmonary artery and transpulmonary thermodilution), minimally invasively (i.e., esophageal doppler, minimally invasive pulse wave analysis), or non-invasively (i.e., non-invasive pulse wave analysis, pulse wave transit time, thoracic bioimpedance and bioreactance).
PPV was measured using intra-arterial catheter. During controlled mechanical ventilation, the intrathoracic pressure changes between inspiration and expiration, which affects the venous return to the heart and leads to change in SV and blood pressure variations. A PPV > 13% suggests fluid responsiveness and indicates that additional fluid administration might be required[8].
CSM-1901 is a modality based on pulse wave transit time and also utilizes R-waves from the electrocardiogram and pulse waves in the periphery (using a pulse oximeter) along with patient blood pressure and other biometric data to estimate CO and SV. Monitoring traditional parameters using PPV and CO is beneficial to determine intravascular volume[9].
Adequate administration of fluid for patients with hemorrhage is a fundamental treatment. In addition to traditional parameters, non-invasive cardiac monitoring and PPV are useful methods to assist evaluation of patient's intravascular volume status and provide a guidance to anesthesiologist for intraoperative management of hypovolemic shock patients.
The authors thank the Hanyang University E-world center for considerable help during preparation of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bhushan S, China; Wong KL, Taiwan S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Alshehri M, Hakami B, Aljameel N, Alayyaf M, Raheem AA. Sporadic giant renal angiomyolipoma: A case report and literature review of clinical presentation, diagnosis, and treatment options. Urol Ann. 2020;12:167-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Chronopoulos PN, Kaisidis GN, Vaiopoulos CK, Perits DM, Varvarousis MN, Malioris AV, Pazarli E, Skandalos IK. Spontaneous rupture of a giant renal angiomyolipoma-Wunderlich's syndrome: Report of a case. Int J Surg Case Rep. 2016;19:140-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Cannesson M, Aboy M, Hofer CK, Rehman M. Pulse pressure variation: where are we today? J Clin Monit Comput. 2011;25:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol. 2002;168:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 127] [Reference Citation Analysis (0)] |
| 5. | Bhatty T, Zia A, Khan IA, Nawaz G. Wunderlich syndrome with spontaneous renal hemorrhage into renal angiomyolipoma. Urol Ann. 2020;12:392-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Magder S. Flow-directed vs. goal-directed strategy for management of hemodynamics. Curr Opin Crit Care. 2016;22:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108:887-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B, Tavernier B. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a "gray zone" approach. Anesthesiology. 2011;115:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 392] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 9. | Saugel B, Thiele RH, Hapfelmeier A, Cannesson M. Technological Assessment and Objective Evaluation of Minimally Invasive and Noninvasive Cardiac Output Monitoring Systems. Anesthesiology. 2020;133:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |