Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8587
Peer-review started: September 10, 2021
First decision: January 18, 2022
Revised: January 25, 2022
Accepted: July 19, 2022
Article in press: July 19, 2022
Published online: August 26, 2022
Processing time: 339 Days and 11.4 Hours
The value of conventional magnetic resonance imaging in the differential diagnosis of thyroid nodules is limited; however, the value of multi-parameter diffusion-weighted imaging (DWI) in the quantitative evaluation of thyroid nodules has not been well determined.
To determine the utility of multi-parametric DWI including mono-exponential, bi-exponential, stretched exponential, and kurtosis models for the differentiation of thyroid lesions.
Seventy-nine patients (62 with benign and 17 with malignant nodules) underwent multi-b value diffusion-weighted imaging of the thyroid. Multiple DWI para
Good agreement was found for diffusion parameters of thyroid nodules. Malignant lesions displayed lower diffusion parameters including apparent diffusion coefficient (ADC), the true diffusion coefficient (D), the perfusion fraction (f), the distributed diffusion coefficient (DDC), the intravoxel water diffusion heterogeneity (α) and kurtosis model-derived ADC (Dapp), and higher apparent diffusional kurtosis (Kapp) than benign entities (all P < 0.01), except for the pseudodiffusion coefficient (D*) (P > 0.05). The area under the ROC curve (AUC) of the ADC(0 and 1000) was not significantly different from that of the ADC(0 and 2000), ADC(0 to 2000), ADC(0 to 1000), D, DDC, Dapp and Kapp (all P > 0.05), but was significantly higher than the AUC of D*, f and α (all P < 0.05) for differentiating benign from malignant lesions.
Multiple DWI parameters including ADC, D, f, DDC, α, Dapp and Kapp could discriminate benign and malignant thyroid nodules. The metrics including D, DDC, Dapp and Kapp provide additional information with similar diagnostic performance of ADC, combination of these metrics may contribute to differentiate benign and malignant thyroid nodules. The ADC calculated with higher b values may not lead to improved diagnostic performance.
Core Tip: Multiple diffusion coefficient parameters obtained by fitting with mono-exponential, bi-exponential, stretched exponential, and kurtosis diffusion-weighted imaging models are feasible techniques for investigating thyroid nodules; The metrics including D, distributed diffusion coefficient, Dapp and Kapp provide additional information with similar diagnostic performance of ADC, and combination of these metrics may contribute to differentiate benign and malignant thyroid nodules; The apparent diffusion coefficient calculated with a mono-exponential model using a single pair of conventional b values (b = 1000 s/mm2) have similar diagnostic performance to those calculated with higher b values (b value > 1000 s/mm2).
- Citation: Zhu X, Wang J, Wang YC, Zhu ZF, Tang J, Wen XW, Fang Y, Han J. Quantitative differentiation of malignant and benign thyroid nodules with multi-parameter diffusion-weighted imaging. World J Clin Cases 2022; 10(24): 8587-8598
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8587.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8587
Thyroid nodules are common lesions that occur thyroid cells growth into an abnormal lump within the thyroid gland; these nodules are often palpable and radiologically distinct from the surrounding parenchyma[1,2]. As with most common endocrine tumors, thyroid nodules have a reported prevalence of 4%-7% in the adult population on physical examination (identified by palpation), and 10 times more nodules are identified with imaging studies[1]. Approximately 5% of detected thyroid nodules are diagnosed as malignancies[3]. The incidence of thyroid cancer has continuously and sharply increased worldwide at a rate higher than any other cancer and has tripled over the past three decades[4-6].
The treatment options recommended for benign and malignant thyroid lesions are completely different; surgical treatment is recommended for the vast majority of thyroid cancer, while most benign nodules can be safely followed with an active surveillance management approach[2]. Therefore, initial management recommendations are very dependent on preoperative studies designed to distinguish between malignant and benign thyroid nodules[1,2,7]. Ultrasonography (US) has been used as the first-line imaging modality for patients with known or suspected thyroid cancer[8]. US also provides guidance for fine-needle aspiration biopsy (FNAB). However, evaluation by US is operator-dependent, and no single sonographic feature is of sufficient diagnostic value to accurately differentiate malignant nodules from benign nodules[9]. Although FNAB is the most accurate tool for evaluating thyroid nodules, it is an invasive procedure associated with possible complications, and its accuracy may vary depending on many factors, such as the skills of the operator and the location of aspiration[7,9].
Currently, diffusion-weighted imaging (DWI) is becoming a very useful tool for the detection and characterization of head and neck cancer. Restricted and hindered diffusion of water molecules within malignant tumors lead to high contrast on diffusion-weighted (DW) images compared with images of normal tissues thus facilitating the diagnosis. The acquired DW images are often postprocessed using standard mono-exponential fitting, which applies a linear shape to obtain apparent diffusion coefficient (ADC) maps. More restricted diffusion is observed in malignant tumors than in benign lesions, which is indicated by a reduction in the ADC[10]. Previous studies of thyroid cancer have indicated that ADC parameters can distinguish malignant and benign thyroid nodules. In routine practice, however, the b values applied in thyroid DWI studies are usually lower than 1000 s/mm2. Moreover, the diagnostic performance of the ADC calculated with higher b values (b value > 1000 s/mm2) has not been well investigated in patients with thyroid cancer.
The microscopic motion of water molecules detected by DWI is influenced by diffusion of water molecules and by microcirculation of blood in capillary networks[10]. A specialized bi-exponential model known as intravoxel incoherent motion (IVIM) can separate the incoherent motion of water molecules in the randomly oriented capillaries from molecular diffusion in the extravascular space[10,11]. In addition, these two components can be quantitatively expressed by three parameters: The true diffusion coefficient (D), the pseudodiffusion coefficient (D*), and the perfusion fraction (f)[10,11]. Recently, only one study used IVIM to evaluate thyroid cancer and reported that IVIM data might be helpful for differentiating malignant thyroid nodules from benign nodules[10].
Different from the bi-exponential model, the stretched exponential DWI model has been recently proposed by Bennett et al[12] and provides a measure of signal deviation from the mono-exponential behavior caused by pseudoperfusion effects; thus, this model provides information on tissue heterogeneity and diffusion simultaneously with two parameters: the distributed diffusion coefficient (DDC) and the intravoxel water diffusion heterogeneity (α)[11,13,14]. In addition, diffusion kurtosis models yield an estimate of the excess kurtosis of the diffusion displacement probability distribution with two parameters, Dapp and Kapp, which are produced from the DKI model and represent the diffusion coefficient and the diffusional kurtosis, respectively. To the best of our knowledge, this is the first study to evaluate thyroid nodules with ADCs calculated with higher b values (b value > 1000 s/mm2) and to compare the diagnostic performance of diffusion parameters fitted by mono-exponential, bi-exponential, stretched exponential, and kurtosis DWI models to quantitatively differentiate thyroid cancer.
The aim of this study was to prospectively evaluate the following: (1) To determine whether ADCs calculated with higher b values (b value > 1000 s/mm2) had benefits over ADCs calculated with conventional b values (b = 1000 s/mm2) to quantitatively differentiate thyroid cancer; and (2) to evaluate the feasibility and diagnostic performance of using multiple diffusion coefficient parameters by fitting with mono-exponential (ADCs fitted with conventional and higher b values), bi-exponential (D, D* and f), stretched exponential (DDC and α), and kurtosis DWI (Dapp and Kapp) models to quantitatively differentiate thyroid cancer.
This study was approved by the First Hospital of Jiaxing Research and Ethics Committee. Written informed consent was obtained from all participants, and the hard copy was archived in the Department of Radiology at the First Hospital of Jiaxing. From January 2017 to July 2019, 79 consecutive patients with thyroid nodules determined by US who were scheduled to undergo thyroidectomy were prospectively enrolled. Multiparameter magnetic resonance imaging (MRI) examinations (including multiple b value DWI) were performed for each patient. The mean age of the included patients was 53 years (range, 20-72 years). Fifty-four of the 78 patients were female. All the patients enrolled in this study had been scheduled to undergo thyroidectomy within 2 wk. Histopathology revealed that 62 patients had benign nodules, including nodular goiter (n = 48) and thyroid adenoma (n = 14), while 17 patients had malignant nodules, including papillary thyroid carcinoma (n = 12), medullary thyroid carcinoma (n = 4), and follicular thyroid carcinoma (n = 1).
All MRI examinations were performed in the prone position using a 3.0 Tesla (T) MRI scanner (Discovery MR750; GE Healthcare, United States) with an eight-channel neurovascular phased-array coil. Patients were instructed to breathe calmly and avoid swallowing as much as possible and practice this. The MRI examination consisted of conventional multiplanar (sagittal, axial, and coronal planar) T1- and T2-weighted imaging scans followed by multiple b value DWI scans. The duration of the whole examination was approximately 30 min. Multiple b value DWI was performed by using a standard, bipolar, single-shot echo-planar sequences in the axial plane. The DWI images were obtained with a repetition time/echo time (TR/TE) of 3000 ms/79.3 ms, section thickness of 4 mm, gap of 1.5 mm, field of view of 200 mm × 200 mm, and matrix of 128 mm× 128 mm. Parallel imaging was used with an acceleration factor of 2. A local shim box covering the thyroid was applied to minimize susceptibility artifacts. Thirteen b values from 0 to 2000 s/mm2 (0, 30, 50, 80, 100, 150, 200, 400, 600, 800, 1000, 1500, and 2000 s/mm2) were used in three orthogonal diffusion directions. The acquisition time for multiple b value DWI sequences was 1 min 57 s.
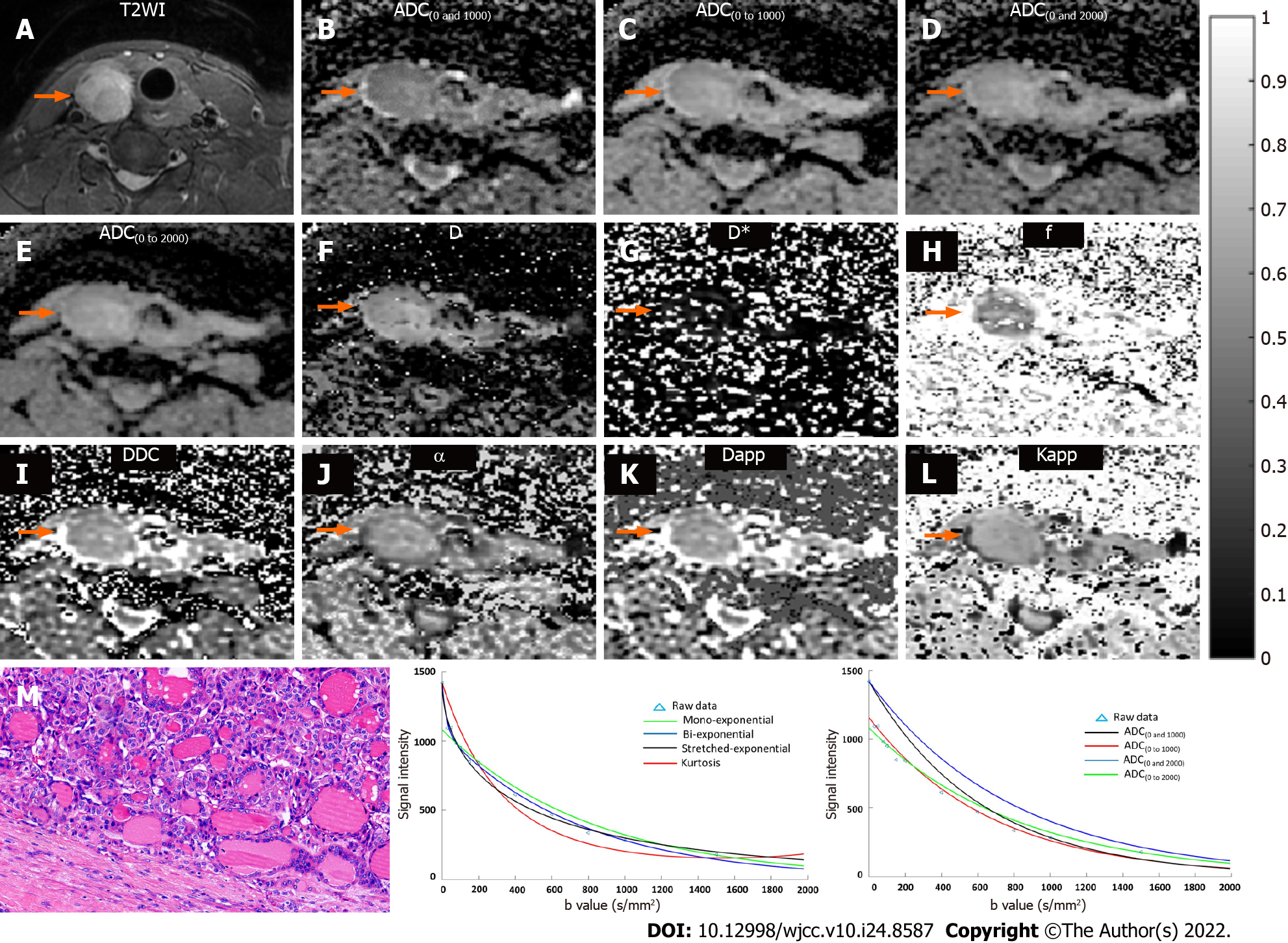
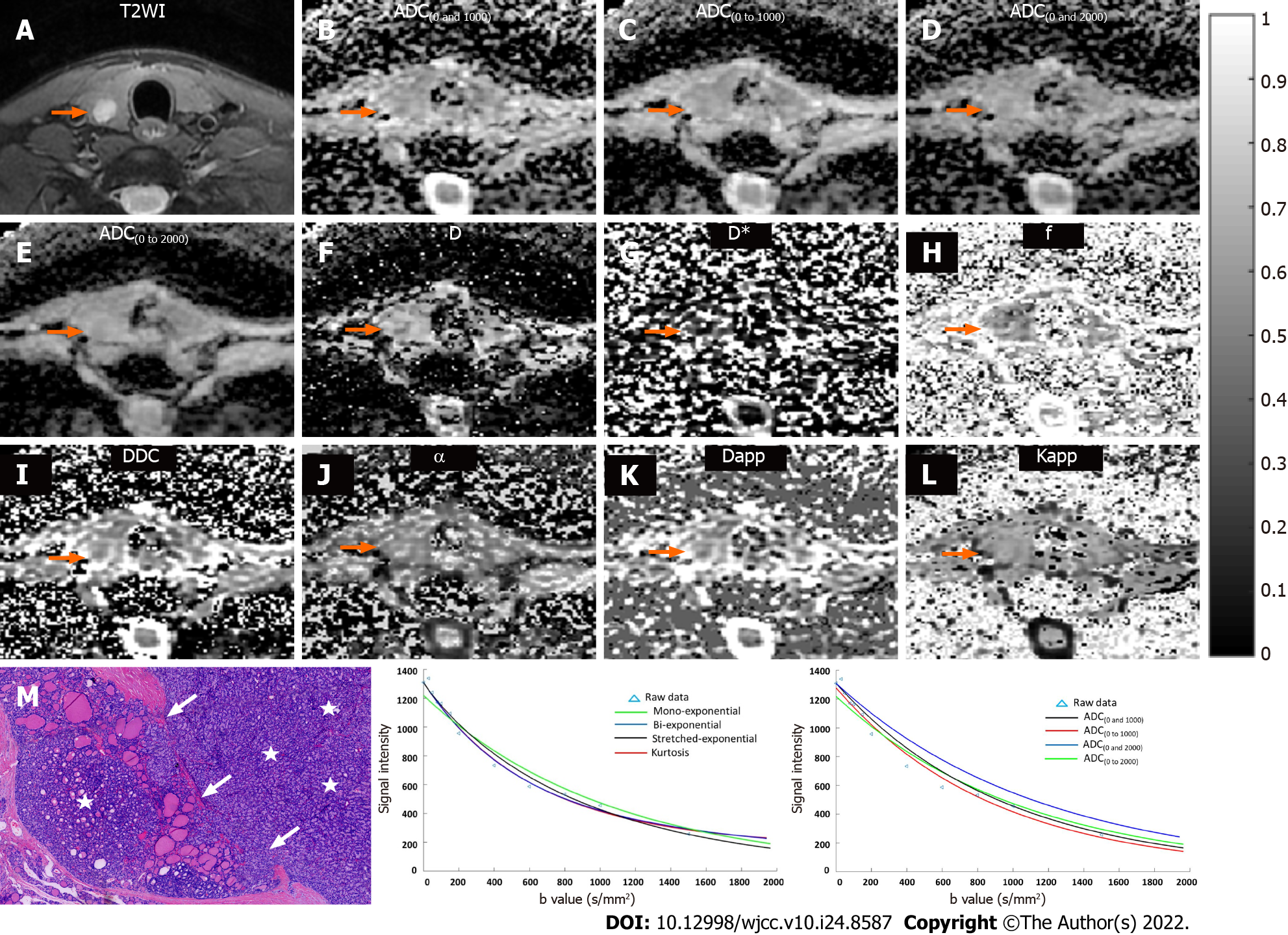
DWI images were transferred to a PC and postprocessed with mono-exponential, bi-exponential, stretched exponential, and kurtosis models using in-house software written in Matlab (version R2014b; MathWorks, Natick, MA, United States). Parameter maps were generated and included the mean ADC, D, D*, f, DDC, α, kurtosis model-derived ADC (Dapp), and apparent diffusional kurtosis (Kapp) values. Two independent thyroid radiologists (J.W. and X.Z. with 15 and 18 years of head and neck MR diagnostic experience, respectively) who were blinded to the histopathological results performed the postprocessing. The regions of interest were as large as possible to encompass the whole solid portion of the thyroid nodule while carefully avoiding areas of obvious necrosis, cystic degeneration, hemorrhagic, or calcified portions, according to T1-, T2-, and contrast-enhanced T1-weighted images for reference. The corresponding mathematical expressions are as follows.
Mono-exponential: ADC was automatically calculated by using the mono-exponential model with b values: S(b) = S0 ∙ exp (-b ∙ ADC) where S(b) is the diffusion sensitizing factor; S0 is the signal intensity without a diffusion gradient; and S(b) is the signal intensity at a particular b value. In our study, ADC(0 and 1000) was calculated with b values of 0 s/mm2 and 1000 s/mm2, ADC(0 to 1000) was calculated with b values from 0 s/mm2 to 1000 s/mm2 (0, 30, 50, 80, 100, 150, 200, 400, 600, 800, and 1000 s/mm2), ADC(0 and 2000) was calculated with b values of 0 s/mm2 and 2000 s/mm2, ADC(0 to 1000) was calculated with b values from 0 s/mm2 to 2000 s/mm2 (0, 30, 50, 80, 100, 150, 200, 400, 600, 800, 1000, 1500, and 2000 s/mm2).
Bi-exponential: According to the bi-exponential model, the mathematical relationship between b values and signal intensities was described using the following formula: S(b) = S0 ∙ [(1-f) ∙ exp (-b ∙ D) + f ∙ exp
Stretched exponential: By fitting the stretched exponential model, DDC and α were calculated as follows: S(b) = S0 ∙ exp [-(b ∙ DDC)α] where DDC represents the distributed diffusion coefficient, and α represents the anomalous exponent term that parameterizes “tissue heterogeneity” (0 ≤ α ≤ 1).
Kurtosis-exponential: The multi-b value DWI images fitted the following diffusion kurtosis imaging (DKI) signal decay equation:
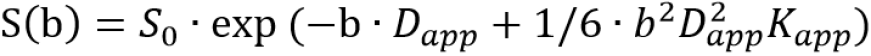
where S(b) is the signal intensity at a particular b value; Dapp is the kurtosis model-derived ADC (in mm2/s); and Kapp is the apparent diffusional kurtosis (dimensionless parameter), which suggest that the status of water molecular motion deviates from a Gaussian distribution. A minimum Kapp value showed that the curve fit closely to a Gaussian distribution. However, increased Kapp indicated increased contributions of the lesion area attributable to kurtosis behavior.
Histopathological examination findings from surgical specimens obtained after radical thyroidectomy or lobectomy were obtained from pathology reports, which were reviewed by two pathologists (X.W.W. and Y.F. with 25 and 18 years of experience with pathological diagnosis, respectively) specializes in the pathological diagnosis of thyroid diseases. According to the characteristics of the thyroid nodules, patients were divided into two groups. The benign tumor group included those with nodular goiter and thyroid adenoma. The malignant tumor group included those with papillary thyroid carcinoma, medullary thyroid carcinoma, and follicular thyroid carcinoma. Histological subtype was determined for all lesions by immunohistochemical analysis.
Data are presented as the mean ± standard deviation (SD). All statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL, United States). The Mann-Whitney U test was used to compare the diffusion parametric differences in the subgroups. A P value < 0.05 was deemed statistically significant. Interreader reliability between the two radiologists was assessed by using the intraclass correlation coefficient (ICC). According to Fleiss[15], an ICC of 0.4 represents poor agreement, a value of 0.75 represents good agreement, and a value between 0.4 and 0.75 represents fair to moderate agreement. Moreover, receiver operating characteristic (ROC) curves were generated to evaluate the diagnostic performance of diffusion parameters for detecting malignant tumors. Sensitivity and specificity were computed at the optimal cutoff value for each diffusion parameter that maximized the Youden index. The area under the ROC curve (AUC) was compared between the ADC and other diffusion parameters by using Z test.
T2-weighted images, ADC maps, D map, D* map, f map, DDC map, α map, Dapp map, Kapp map, and histopathological maps of representative cases of the benign and malignant patient groups are shown in Figures 1 and 2. In addition, the plot of the decay in diffusion-weighted signal intensity fitted by mono-exponential, bi-exponential, stretched-exponential, and kurtosis models, and fitted by a mono-exponential model with different b values for the benign and malignant thyroid nodules are shown in Figures 1 and 2.
The interobserver reproducibility between the two radiologists for determining thyroid nodule diffusion parameter measurements was calculated by using the ICC (Table 1). Good agreement was found for all metrics including ADC, D, D*, f, DDC, α, Dapp, and Kapp, indicating good reproducibility between the two readers. Therefore, the diffusion parameters measured by the first radiologist (Z.X.) were included for comparison. Table 2 shows the quantitative comparison of diffusion parameters between benign and malignant thyroid nodules. The diffusion parameters including ADC, D, f, DDC, α, and Dapp were significantly lower in malignant thyroid nodules than in benign nodules (all P < 0.01). Kapp was significantly higher in malignant thyroid nodules than in benign nodules (P < 0.01). D* was not significantly different between the two groups (P > 0.05).
| Diffusion parameter | ICC | 95%CI |
| Mono-exponential | ||
| ADC(0 and 1000) | 0.944 | (0.852, 0.979) |
| ADC(0 to 1000) | 0.938 | (0.898, 0.962) |
| ADC(0 and 2000) | 0.904 | (0.844, 0.941) |
| ADC(0 to 2000) | 0.942 | (0.905, 0.962) |
| Biexponential | ||
| D | 0.949 | (0.921, 0.967) |
| D* | 0.935 | (0.900, 0.958) |
| f | 0.897 | (0.844, 0.933) |
| stretched exponential | ||
| DDC | 0.994 | (0.990, 0.996) |
| α | 0.973 | (0.958, 0.983) |
| Kurtosis DWI | ||
| Dapp | 0.787 | (0.685, 0.859) |
| Kapp | 0.855 | (0.782, 0.905) |
| Diffusion parameter | Benign nodules | Malignant nodules | P value |
| Mono-exponential | |||
| ADC(0 and 1000) | 1.85 ± 0.24 | 1.29 ± 0.27 | < 0.01 |
| ADC(0 to 1000) | 1.79 ± 0.021 | 1.19 ± 0.23 | < 0.01 |
| ADC(0 and 2000) | 1.35 ± 0.15 | 0.95 ± 0.15 | < 0.01 |
| ADC(0 to 2000) | 1.41 ± 0.15 | 0.95 ± 0.15 | < 0.01 |
| Biexponential | |||
| D | 1.34 ± 0.41 | 0.89 ± 0.26 | < 0.01 |
| D* | 22.08 ± 18.53 | 26.09 ± 20.87 | > 0.05 |
| f | 0.38 ± 0.19 | 0.30 ± 0.14 | < 0.01 |
| Stretched exponential | |||
| DDC | 2.15 ± 0.62 | 1.45 ± 0.51 | < 0.01 |
| α | 0.78 ± 0.19 | 0.67 ± 0.19 | < 0.01 |
| Kurtosis DWI | |||
| Dapp | 2.88 ± 0.73 | 2.11 ± 0.67 | < 0.01 |
| Kapp | 0.57 ± 0.12 | 0.85 ± 0.19 | < 0.01 |
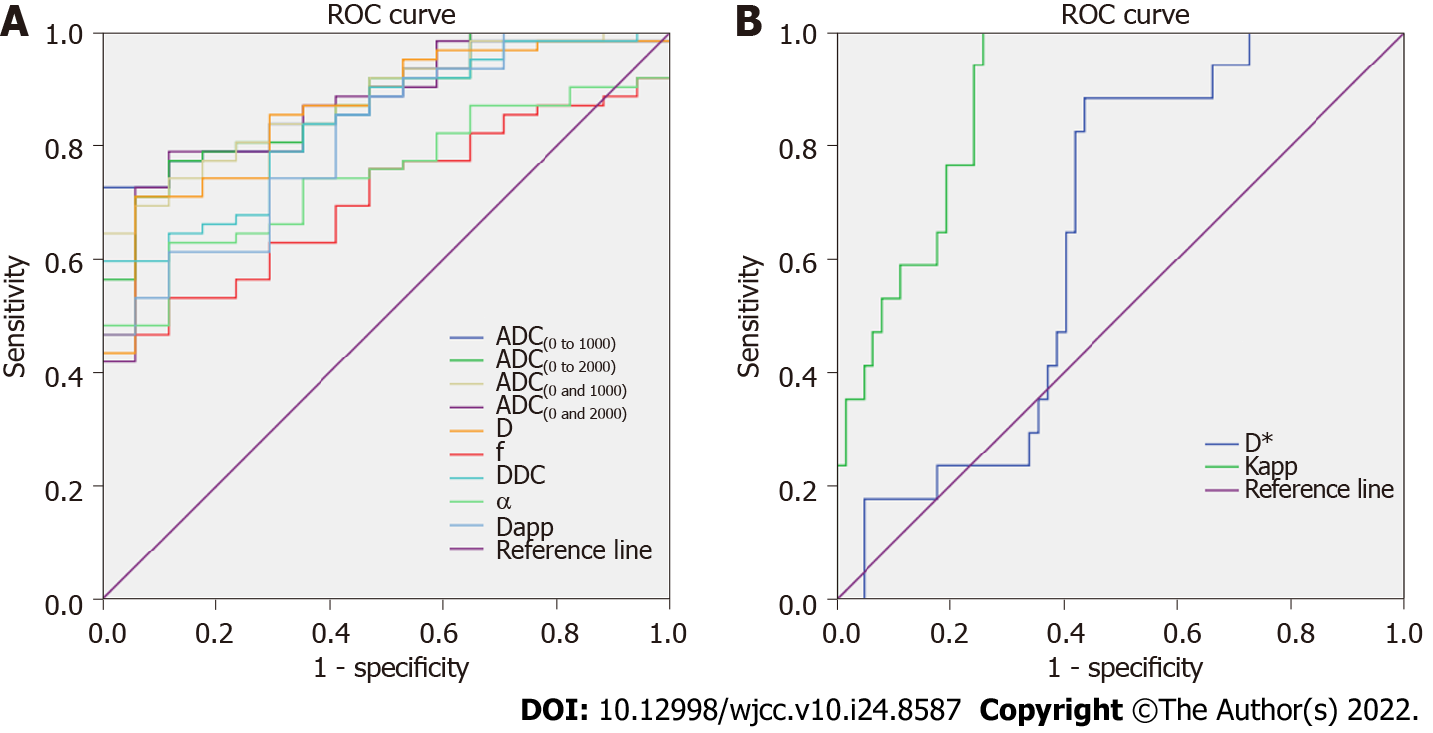
The results of the ROC analyses of diffusion parameters for differentiating malignant nodules from benign nodules are summarized in Table 3. ROC curves of the ADC(0 and 1000), ADC(0 and 2000), ADC(0 to 1000), ADC(0 to 2000), D, D*, f, DDC, α, Dapp and Kapp are presented in Figure 3. ROC analysis demonstrated that ADC(0 to 1000) metrics showed trends toward higher AUC values than the ADC(0 and 1000), ADC(0 and 2000), and ADC(0 to 2000), using an ADC(0 to 1000) cutoff value of 1.5 × 10-3 mm2/s, and malignant nodules could be diagnosed with a sensitivity of 72.6% and a specificity of 100%, but none of these differences reached statistical significance (all P > 0.05). The ADC(0 and 1000), ADC(0 to 1000), Kapp metrics had significantly higher AUC values than D*, f, and α (all P < 0.05). The ADC(0 and 2000), ADC(0 to 2000), and D metrics had significantly higher AUC values than D*, and f (all P < 0.05). The DDC, and Dapp metrics had significantly higher AUC values than D* (all P < 0.05).
| Diffusion parameter | AUC | Cut-off value | Sensitivity, % | Specificity, % | Youden index | P value |
| Mono-exponential | ||||||
| ADC(0 and 1000) | 0.879 | 1.6 | 64.5 | 100.0 | 0.645 | Ref. |
| ADC(0 and 2000) | 0.874 | 1.1 | 79.0 | 88.2 | 0.672 | 0.94 |
| ADC(0 to 1000) | 0.882 | 1.5 | 72.6 | 100.0 | 0.726 | 0.94 |
| ADC(0 to 2000) | 0.878 | 1.1 | 77.4 | 88.2 | 0.656 | 0.99 |
| Biexponential | ||||||
| D | 0.861 | 1.1 | 71.0 | 94.1 | 0.651 | 0.76 |
| D* | 0.643 | 22.2 | 88.2 | 56.5 | 0.447 | < 0.01 |
| f | 0.709 | 0.37 | 46.8 | 100.0 | 0.468 | < 0.05 |
| Stretched exponential | ||||||
| DDC | 0.841 | 2.0 | 59.7 | 100.0 | 0.597 | 0.53 |
| α | 0.748 | 0.73 | 62.9 | 88.2 | 0.511 | < 0.05 |
| Kurtosis DWI | ||||||
| Dapp | 0.815 | 2.6 | 61.3 | 88.2 | 0.495 | 0.33 |
| Kapp | 0.889 | 0.65 | 100.0 | 74.2 | 0.742 | 0.84 |
To our best of knowledge, this work is the first study to investigate the feasibility of using multiple diffusion coefficient parameters by fitting with mono-exponential, bi-exponential, stretched exponential, and kurtosis DWI models to quantitatively differentiate malignant thyroid nodules from benign thyroid nodules. All parameters of the four models showed high interobserver agreement, suggesting that measurement of these parameters had good reproducibility and reliability.
Previous studies on DWI in thyroid cancer have mostly focused on mono-exponential models with conventional b values (b value ≤ 1000 s/mm2), and most studies have suggested that ADCs of malignant lesions were significantly lower than those of benign lesions[9,16-19]. Similarly, in our study, the ADC(0 and 1000) and ADC(0 to 1000) showed that malignant thyroid lesions had significantly lower ADCs than benign lesions, with AUC values of 0.879 and 0.882, respectively. Generally, although higher b values might produce more susceptibility to distortions and could increase the noise in DWI images, higher b values produced more diffusion weighting and therefore higher contrast between lesions and normal tissue[16]. Recent reports have shown advantages of using a higher b value (b value > 1000 s/mm2) for DWI and ADC calculations for the diagnosis of acute stroke[20], malignant lymphoma[21], and prostate cancer[22]. In our study, although the ADC(0 and 2000) and ADC(0 to 2000) showed relatively higher sensitivities of 79.0% and 77.4%, respectively, than ADC(0 and 1000) and ADC(0 to 1000) sensitivities of 64.5% and 72.6%, respectively, the ADC(0 and 2000) and ADC(0 to 2000) showed similar diagnostic performance to the ADC(0 and 1000) and ADC(0 to 1000), with AUC values of 0.874 and 0.878, respectively. Thus, our study indicates that DWI acquired with regular b values (b = 1000 s/mm2) can be used to calculate DWI parameters similar to those obtained with DWI acquired with higher b values in patients with thyroid nodules. This phenomenon has also been reported by Eghtedari, M. on quantitative breast DWI for breast cancer[23]. Additionally, the ADC derived from a mono-exponential model with more than two b values is theoretically good for ADC fitting. In our study, the ADC(0 to 1000) showed a relatively higher sensitivity of 72.6% than the that of the ADC(0 and 1000) of 64.5%; the ADC(0 to 2000) showed similar sensitivity of 77.4% to that of the ADC(0 and 2000) of 79.0%. In addition, the diagnostic performance was similar between the ADC(0 and 1000) and ADC(0 to 1000) and between the ADC(0 and 2000) and ADC(0 to 2000). Therefore, if the ADC fitted with the mono-exponential model alone was used to differentiate malignant thyroid nodules from benign thyroid nodules, using a single pair of b values (usually 0 s/mm2 and 1000 s/mm2) was acceptable for ADC calculation.
Bi-exponential IVIM DWI is a method initially proposed by Le Bihan et al[24] to quantitatively assess the microscopic motion that occurs at the subvoxel scale on MRI. D values mainly reflect the molecular diffusion of water protons. The D* value and f value mainly reflect perfusion status. In our study, the D values of malignant thyroid nodules were significantly lower than those of benign thyroid nodules. This finding was similar to the results of previous studies of thyroid cancer[10], breast cancer[11], na
The stretched exponential diffusion model is an alternate method that can simultaneously assess both tissue heterogeneity and diffusion. Prior studies have reported that the stretched exponential diffusion model has better diagnostic performance for differentiating malignant from benign lesions than ADC parameters derived from a mono-exponential model in breast cancer and prostate cancer[31,32]. The DDC can be considered a weighted sum over a distribution of ADCs that represent multiexponential decay properties[31]. In our study, the DDC of the malignant thyroid nodule was significantly lower than that of benign thyroid nodules, but the results of the ROC curve analysis indicate that the diagnostic value of the DDC did not achieve a significant difference compared with ADC. The diffusion parameter α is supposed to represent intravoxel water diffusion heterogeneity. Values of α near 0 indicate high intravoxel diffusion heterogeneity. In our study, α was significantly lower in malignant thyroid nodules than in benign thyroid nodules, suggesting that malignant thyroid nodules exhibited higher intravoxel diffusion heterogeneity than the benign thyroid nodules. However, the diagnostic performance of α was lower than that of the ADC.
Recently, the potential utility of DKI using higher b values rather than conventional DWI has been reported for detecting pathological alterations in tissue diffusion properties in neural diseases and oncologic applications[33]. Our study showed that malignant thyroid lesions had significantly lower Dapp and higher Kapp values than benign lesions, similar to the results reported by Shi et al[34]. This finding might be because the malignant group had lesions with higher cell density than those in the benign group, which contributed to restriction of water diffusion in the extracellular space. Moreover, Shi et al[34] found that quantitative DKI was superior to conventional DWI because the Dapp value corrected by the DKI model showed a greater AUC than the ADC, and Kapp showed a higher sensitivity than the ADC. However, in our study, Kapp achieved the highest AUC of 0.889 with a higher sensitivity of 100% than ADC, but the diagnostic performance of those two parameters was not significantly different. Kapp may be a promising indicator with good diagnostic performance.
This study has several limitations. First, the study sample included was relatively small, especially the sample of patients with thyroid cancer, which is a possible reason that may account for the 100% sensitivity or specificity in the ROC curve analysis results. Our findings need to be validated in a larger and more heterogeneous cohort that includes a wider spectrum of thyroid tumor types. Second, regarding the patients in our study, the benign group included only patients with solid nodular goiter and thyroid adenoma, rather than those with all types of benign nodules. In addition, the malignant group did not include all types of thyroid cancer; thus, this may have resulted in some selection bias because of histopathological heterogeneity of thyroid nodules. Third, we selected only the solid regions of the lesion instead of the entire lesion, which may have resulted in some selection bias because of histopathological heterogeneity.
In conclusion, our study demonstrates the following: (1) Multiple diffusion coefficient parameters obtained by fitting with mono-exponential, biexponential, stretched exponential, and kurtosis DWI models are feasible techniques for investigating thyroid nodules; (2) The metrics including D, DDC, Dapp and Kapp provide additional information with similar diagnostic performance of ADC, and combination of these metrics may contribute to differentiate benign and malignant thyroid nodules; and (3) the ADC calculated with a mono-exponential model using a single pair of conventional b values (b = 1000 s/mm2) have similar diagnostic performance to those calculated with higher b values (b value > 1000 s/mm2). Clinically, therefore, for the institution has ability to generate higher b values for DWI, the metrics of D, DDC, Dapp and Kapp could be evaluated, which might provide additional information; otherwise, using a single pair of conventional b values (b = 1000 s/mm2) still remained a valuable diffusion parameter for differentiating malignant thyroid nodules from benign thyroid nodules.
The value of multiparameter diffusion-weighted imaging (DWI) in quantitative evaluation of thyroid nodule has not been clarified.
It provides a new idea for differentiating benign and malignant thyroid results by using multiparametric diffusion-weighted imaging.
To provide a non-invasive diagnostic means for differentiating benign and malignant thyroid nodules by multiparametric DWI, furthermore, we elucidated which parameters have diagnostic function in differentiating the nature of thyroid nodule
We obtained Multiple DWI parameters by patients who underwent multi-b value diffusion-weighted imaging of the thyroid,then the data of benign and malignant nodules were obtained and analyzed.
Malignant lesions displayed lower diffusion parameters including apparent diffusion coefficient (ADC), the true diffusion coefficient (D), the perfusion fraction (f), the distributed diffusion coefficient (DDC), the intravoxel water diffusion heterogeneity (α) and kurtosis model-derived ADC (Dapp), and higher apparent diffusional kurtosis (Kapp) than benign entities (all P < 0.01). The area under the ROC curve (AUC) of the ADC(0 and 1000) was significantly higher than the AUC of D*, f and α (all P < 0.05) for differentiating benign from malignant lesions.
The metrics including D, DDC, Dapp and Kapp provide additional information with similar diagnostic performance of ADC, combination of these metrics may contribute to differentiate benign and malignant thyroid nodules.
In the future, multiple parameters of magnetic resonance diffusion can be used to accurately distinguish benign and malignant thyroid nodules.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chisthi MM, India; Zahran M, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Fisher SB, Perrier ND. The incidental thyroid nodule. CA Cancer J Clin. 2018;68:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Paschke R, Hegedüs L, Alexander E, Valcavi R, Papini E, Gharib H. Thyroid nodule guidelines: agreement, disagreement and need for future research. Nat Rev Endocrinol. 2011;7:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Schob S, Meyer HJ, Dieckow J, Pervinder B, Pazaitis N, Höhn AK, Garnov N, Horvath-Rizea D, Hoffmann KT, Surov A. Histogram Analysis of Diffusion Weighted Imaging at 3T is Useful for Prediction of Lymphatic Metastatic Spread, Proliferative Activity, and Cellularity in Thyroid Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic Review of Trends in the Incidence Rates of Thyroid Cancer. Thyroid. 2016;26:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 5. | Lubitz CC, Sosa JA. The changing landscape of papillary thyroid cancer: Epidemiology, management, and the implications for patients. Cancer. 2016;122:3754-3759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid. 2014;24:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Haugen BR. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer. 2017;123:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 413] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 8. | American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5299] [Cited by in RCA: 4746] [Article Influence: 296.6] [Reference Citation Analysis (0)] |
| 9. | Hao Y, Pan C, Chen W, Li T, Zhu W, Qi J. Differentiation between malignant and benign thyroid nodules and stratification of papillary thyroid cancer with aggressive histological features: Whole-lesion diffusion-weighted imaging histogram analysis. J Magn Reson Imaging. 2016;44:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Tan H, Chen J, Zhao YL, Liu JH, Zhang L, Liu CS, Huang D. Feasibility of Intravoxel Incoherent Motion for Differentiating Benign and Malignant Thyroid Nodules. Acad Radiol. 2019;26:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Suo S, Cheng F, Cao M, Kang J, Wang M, Hua J, Hua X, Li L, Lu Q, Liu J, Xu J. Multiparametric diffusion-weighted imaging in breast lesions: Association with pathologic diagnosis and prognostic factors. J Magn Reson Imaging. 2017;46:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Bennett KM, Schmainda KM, Bennett RT, Rowe DB, Lu H, Hyde JS. Characterization of continuously distributed cortical water diffusion rates with a stretched-exponential model. Magn Reson Med. 2003;50:727-34.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 340] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Ertas G, Onaygil C, Akin Y, Kaya H, Aribal E. Quantitative differentiation of breast lesions at 3T diffusion-weighted imaging (DWI) using the ratio of distributed diffusion coefficient (DDC). J Magn Reson Imaging. 2016;44:1633-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Wang F, Wang Y, Zhou Y, Liu C, Xie L, Zhou Z, Liang D, Shen Y, Yao Z, Liu J. Comparison between types I and II epithelial ovarian cancer using histogram analysis of monoexponential, biexponential, and stretched-exponential diffusion models. J Magn Reson Imaging. 2017;46:1797-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Senn S. Review of Fleiss, statistical methods for rates and proportions. Res Synth Methods. 2011;2:221-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Wu Y, Yue X, Shen W, Du Y, Yuan Y, Tao X, Tang CY. Diagnostic value of diffusion-weighted MR imaging in thyroid disease: application in differentiating benign from malignant disease. BMC Med Imaging. 2013;13:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Dilli A, Ayaz UY, Cakir E, Cakal E, Gultekin SS, Hekimoglu B. The efficacy of apparent diffusion coefficient value calculation in differentiation between malignant and benign thyroid nodules. Clin Imaging. 2012;36:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Shi HF, Feng Q, Qiang JW, Li RK, Wang L, Yu JP. Utility of diffusion-weighted imaging in differentiating malignant from benign thyroid nodules with magnetic resonance imaging and pathologic correlation. J Comput Assist Tomogr. 2013;37:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Lu Y, Hatzoglou V, Banerjee S, Stambuk HE, Gonen M, Shankaranarayanan A, Mazaheri Y, Deasy JO, Shaha AR, Tuttle RM, Shukla-Dave A. Repeatability Investigation of Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging on Thyroid Glands. J Comput Assist Tomogr. 2015;39:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Purroy F, Begue R, Quílez A, Sanahuja J, Gil MI. Contribution of high-b-value diffusion-weighted imaging in determination of brain ischemia in transient ischemic attack patients. J Neuroimaging. 2013;23:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Doskaliyev A, Yamasaki F, Ohtaki M, Kajiwara Y, Takeshima Y, Watanabe Y, Takayasu T, Amatya VJ, Akiyama Y, Sugiyama K, Kurisu K. Lymphomas and glioblastomas: differences in the apparent diffusion coefficient evaluated with high b-value diffusion-weighted magnetic resonance imaging at 3T. Eur J Radiol. 2012;81:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Ning P, Shi D, Sonn GA, Vasanawala SS, Loening AM, Ghanouni P, Obara P, Shin LK, Fan RE, Hargreaves BA, Daniel BL. The impact of computed high b-value images on the diagnostic accuracy of DWI for prostate cancer: A receiver operating characteristics analysis. Sci Rep. 2018;8:3409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Eghtedari M, Ma J, Fox P, Guvenc I, Yang WT, Dogan BE. Effects of magnetic field strength and b value on the sensitivity and specificity of quantitative breast diffusion-weighted MRI. Quant Imaging Med Surg. 2016;6:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Le Bihan D, Turner R. The capillary network: a link between IVIM and classical perfusion. Magn Reson Med. 1992;27:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 340] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Zhang SX, Jia QJ, Zhang ZP, Liang CH, Chen WB, Qiu QH, Li H. Intravoxel incoherent motion MRI: emerging applications for nasopharyngeal carcinoma at the primary site. Eur Radiol. 2014;24:1998-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Pesapane F, Patella F, Fumarola EM, Panella S, Ierardi AM, Pompili GG, Franceschelli G, Angileri SA, Magenta Biasina A, Carrafiello G. Intravoxel Incoherent Motion (IVIM) Diffusion Weighted Imaging (DWI) in the Periferic Prostate Cancer Detection and Stratification. Med Oncol. 2017;34:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Liang L, Luo X, Lian Z, Chen W, Zhang B, Dong Y, Liang C, Zhang S. Lymph node metastasis in head and neck squamous carcinoma: Efficacy of intravoxel incoherent motion magnetic resonance imaging for the differential diagnosis. Eur J Radiol. 2017;90:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Tezelman S, Giles Y, Tunca F, Gok K, Poyanli A, Salmaslioglu A, Terzioglu T. Diagnostic value of dynamic contrast medium enhanced magnetic resonance imaging in preoperative detection of thyroid carcinoma. Arch Surg. 2007;142:1036-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. J Magn Reson Imaging. 2010;31:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 30. | Lemke A, Laun FB, Simon D, Stieltjes B, Schad LR. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med. 2010;64:1580-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 31. | Liu X, Zhou L, Peng W, Wang H, Zhang Y. Comparison of stretched-Exponential and monoexponential model diffusion-Weighted imaging in prostate cancer and normal tissues. J Magn Reson Imaging. 2015;42:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Liu C, Wang K, Li X, Zhang J, Ding J, Spuhler K, Duong T, Liang C, Huang C. Breast lesion characterization using whole-lesion histogram analysis with stretched-exponential diffusion model. J Magn Reson Imaging. 2018;47:1701-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Minosse S, Marzi S, Piludu F, Vidiri A. Correlation study between DKI and conventional DWI in brain and head and neck tumors. Magn Reson Imaging. 2017;42:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Shi RY, Yao QY, Zhou QY, Lu Q, Suo ST, Chen J, Zheng WJ, Dai YM, Wu LM, Xu JR. Preliminary study of diffusion kurtosis imaging in thyroid nodules and its histopathologic correlation. Eur Radiol. 2017;27:4710-4720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |