Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.8045
Peer-review started: March 21, 2022
First decision: April 25, 2022
Revised: May 3, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: August 6, 2022
Processing time: 122 Days and 12.9 Hours
The ampulla of Vater is an anatomically and histologically complex region giving rise to a heterogenous group of tumors. This is, to the best of our knowledge, the first case of intra-ampullary papillary-tubular neoplasm combined with ampullary neuroendocrine carcinoma reported in the literature.
A 61-year-old woman presented to the emergency department for evaluation of painless jaundice. Contrast-enhanced computed tomography (CT) of the abdomen and chest showed a periampullary tumor mass measuring 15 mm × 12 mm × 14 mm, with no evidence of locoregional and distant metastases, for which she underwent pancreatoduodenectomy. Histopathologic examination of a resected specimen revealed an intra-ampullary papillary tubular neoplasm with high-grade dysplasia in combination with poorly differentiated grade 3 neuroendocrine carcinoma with a mitotic count of more than 20 mitoses per 10 high power fields and Ki-67 index of 100%. No positive lymph nodes were identified. Her postoperative course was uneventful. Postoperatively, she remained under close surveillance. Multiple liver metastases were observed on follow-up CT 8 mo after the surgery, so systemic therapy with cisplatin and etoposide was initiated.
The simultaneous occurrence of neuroendocrine and non-neuroendocrine tumors in the ampulla of Vater is rare and the pathogenesis of such tumors is largely unknown. Due to unpredictable clinical behavior and lack of solid evidence on optimal treatment strategy, close patient surveillance is advised after radical resection of the primary tumor.
Core Tip: The ampulla of Vater is a transitional region with various distinctive histomorphologic characteristics, although the simultaneous occurrence of neuroendocrine and non-neuroendocrine tumors in this region is rare. When present, problems arise in differentiation between mixed neuroendocrine–non-neuroendocrine neoplasm and the collision of two distinct tumors. Due to the rarity of such tumors, their clinical behavior remains largely unknown, as do appropriate treatment measures. After radical resection, if feasible, the standard of care for the most aggressive and/or predominant component of the tumor from the same site of origin may be adopted. Newly diagnosed cases should be discussed at multidisciplinary team meetings to tailor postoperative treatment and follow-up appropriately.
- Citation: Zavrtanik H, Luzar B, Tomažič A. Intra-ampullary papillary-tubular neoplasm combined with ampullary neuroendocrine carcinoma: A case report. World J Clin Cases 2022; 10(22): 8045-8053
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/8045.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.8045
The ampulla of Vater is an anatomically and histologically complex region constituting the junction of the biliary, pancreatic, and digestive tracts, giving rise to a heterogenous group of tumors with different growth patterns and histologic types[1]. However, the simultaneous occurrence of exocrine and neuroendocrine tumors is very infrequent.
The term intra-ampullary papillary-tubular neoplasm (IAPN) is relatively new, introduced by Ohike et al[1] in 2010 to describe mass-forming preinvasive neoplasms growing predominantly within the ampullary channel, with minimal or no involvement of the bile duct, pancreatic duct, or duodenal papilla. Due to their papillary and/or tubular growth, and variable cell lineage and spectrum of dysplastic changes (adenoma-carcinoma sequence), these tumors are remarkably analogous to pancreatic and biliary intraductal papillary and tubular neoplasms [i.e., intraductal papillary mucinous neoplasms (IPMNs), intraductal tubular papillary neoplasms (ITPNs), and intraductal papillary neoplasms][1]. IAPNs are relatively rare, constituting 33% of primary ampullary tumors and 5.5% of all pancreatoduodenectomy/ampulectomy species[1]. Most cases of IAPN are associated with high-grade dysplasia (94%) or small parts of invasive carcinoma (78%)[1,2]. In their series of 82 IAPN cases, Ohike et al[1] reported four cases of IAPN-associated mixed adenocarcinomas: Two with mucionous, one squamous, and one with a neuroendocrine component.
Neuroendocrine carcinomas (NECs) are poorly differentiated high-grade epithelial neoplasms showing morphological and immunohistochemical features of neuroendocrine differentiation[2]. Although rare, constituting 0.9%-2% of primary ampullary tumors[3-5], NECs in the small intestine are almost exclusive to the ampullary region[2,6]. However, available data is limited to small case series or retrospective reviews[3-8], with only few reports concerning ampullary tumors with neuroendocrine and non-neuroendocrine components[9].
In the present case, we describe an unusual combination of IAPN with high-grade dysplasia and NEC with a Ki-67 proliferation index of 100% arising within the ampulla of Vater. We discuss its clinical and histopathological features, as well as possible pathogenesis.
A 61-year-old woman presented to the emergency department for evaluation of painless jaundice.
A week before presentation, the patient noticed darker urine and pruritus. Her stools became completely pale and yellowing of her skin appeared. She denied abdominal pain, fever, and chills but reported nausea and loss of appetite, with a loss of 4 kg over the last 4 mo.
The patient’s medical history was notable for ankylosing spondylitis and arterial hypertension. She had undergone cholecystectomy due to cholecystolithiasis in the past.
The patient reported an 18 pack-year history of smoking. She had no history of alcohol abuse. Her medications included esomeprazole for ulcer prophylaxis, perindopril/indapamide for arterial hypertension, meloxicam for ankylosing spondylitis, and cholecalciferol for prevention of vitamin D deficiency-related disorders. She had no known allergies. Her family history was unremarkable.
The patient’s vital signs were normal on admission. Physical examination revealed jaundice. Her abdomen was nondistended, soft, and nontender with no palpable mass.
Initial laboratory findings showed elevated levels of bilirubin (total: 91 µmol/L; direct: 65 µmol/L), and pancreatic (amylase: 3.27 µkat/L; lipase: 3.79 µkat/L) and liver enzymes (aspartate aminotransferase: 3.84 µkat/L; alanine aminotransferase: 9.73 µkat/L; gamma-glutamyltransferase: 27.31 µkat/L; alkaline phosphatase: 10.61 µkat/L). Serum levels of the tumor markers carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) were within the normal range (CA 19-9: 20.8 kU/L; CEA: 3.9 µg/L).
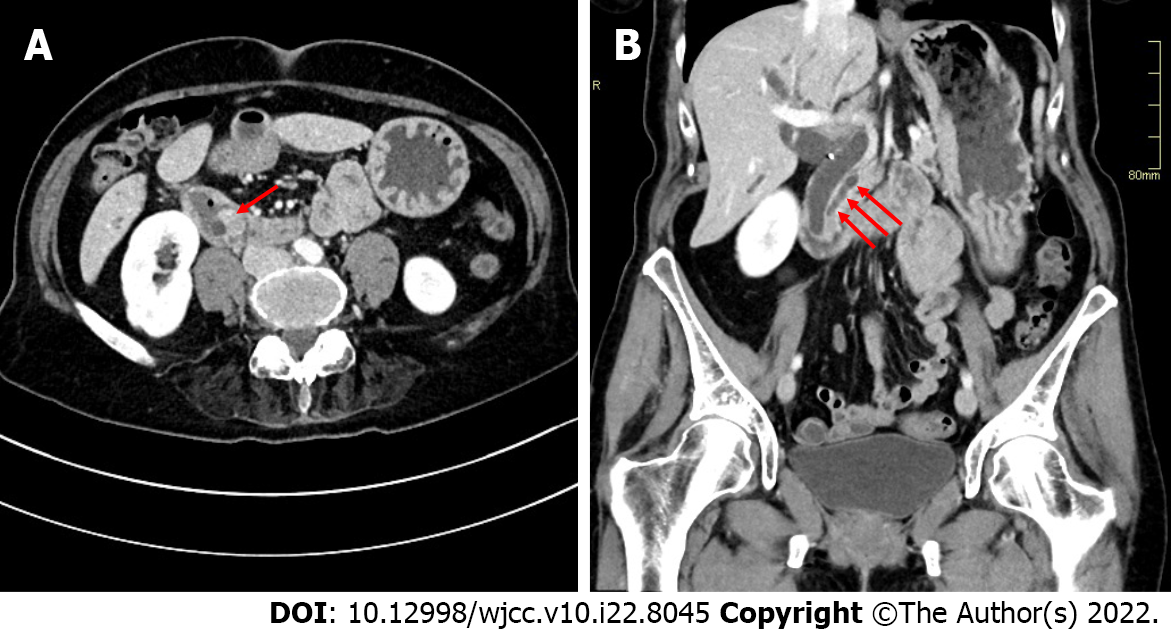
Abdominal ultrasound showed grossly distended intra- and extra-hepatic bile ducts with a probable level of obstruction at the ampulla of Vater. A contrast-enhanced computed tomography (CT) scan of the abdomen and chest revealed a well-defined homogenously enhancing mass measuring 15 mm × 12 mm × 14 mm in the duodenal ampullary region, causing upstream dilatation of intra- and extra-hepatic bile ducts and the main pancreatic duct (Figure 1). An enlarged lymph node measuring 1 cm in the hepatoduodenal ligament and separate lymph nodes measuring 8 mm in the retroperitoneum were observed. In the laterobasal segment of the left lower pulmonary lobe, a small 4 mm soft tissue nodule of uncertain potential was described. There was no convincing evidence of distant metastases. Following discussion of the patient’s case at a multidisciplinary team meeting, the patient underwent pancreatoduodenectomy (Whipple procedure) and was discharged after an uneventful recovery.
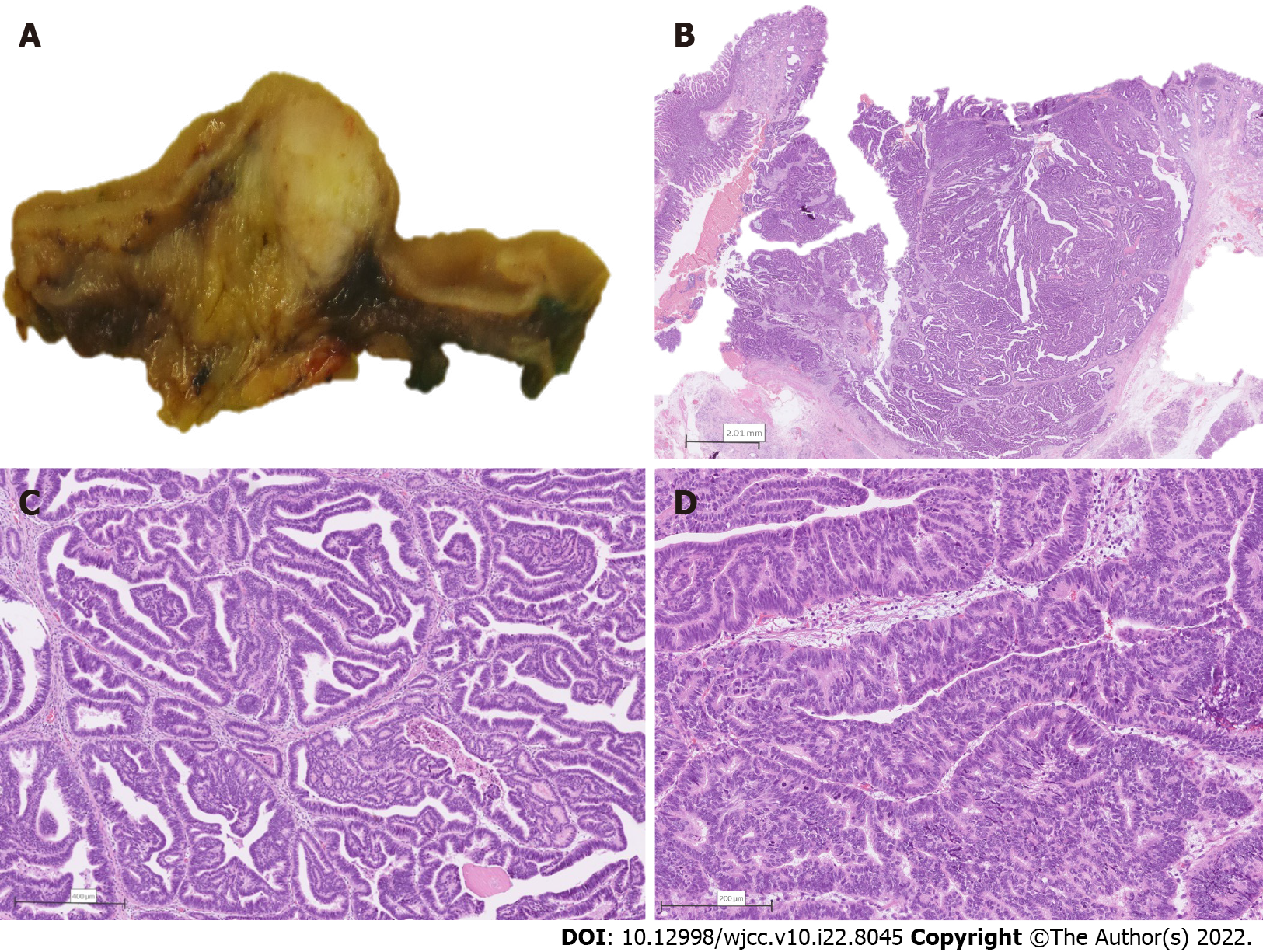
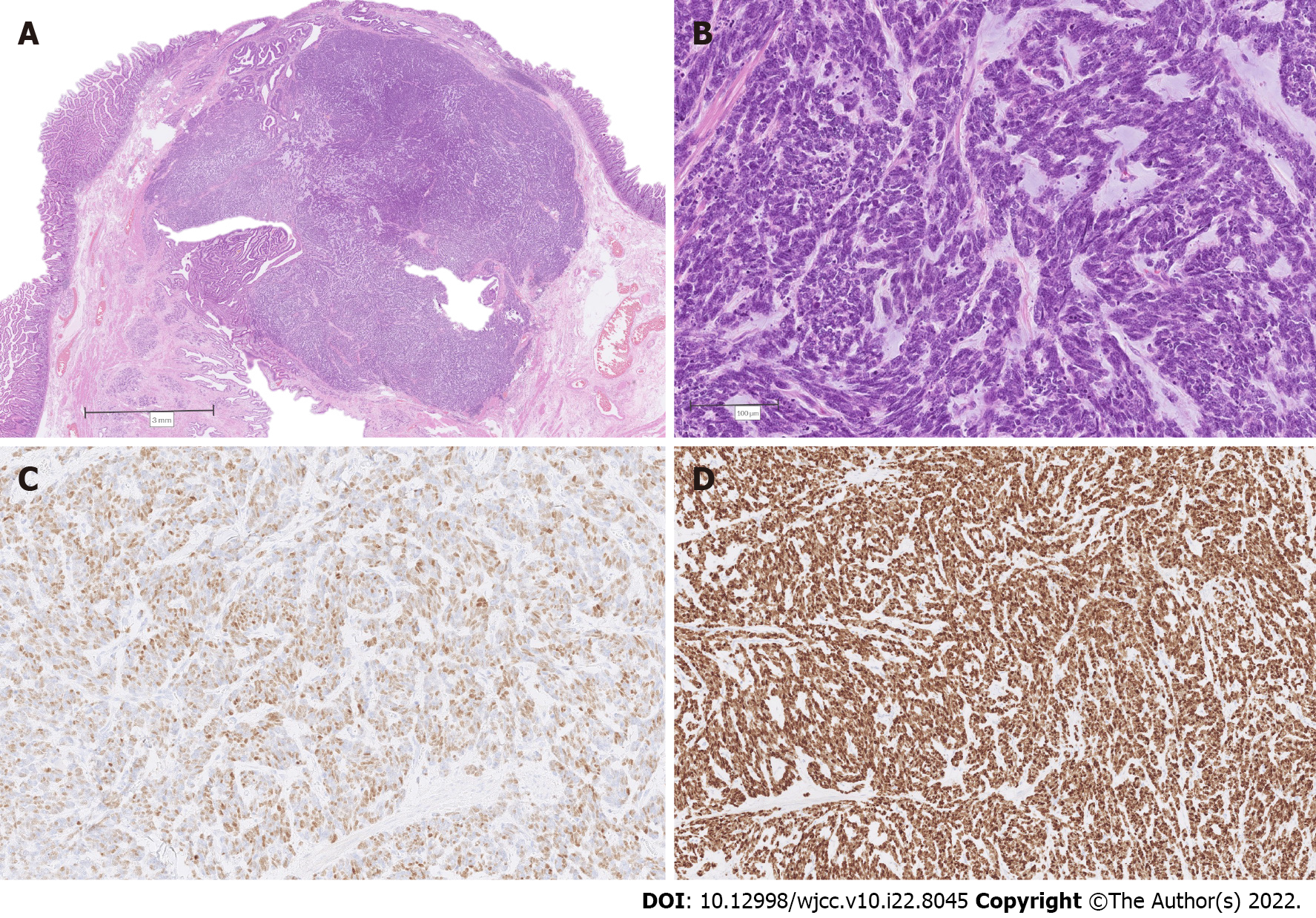
The resected specimen was submitted for histopathological examination. Macroscopic findings revealed a relatively well-delineated greyish-white solid tumor measuring 1.7 cm × 1.4 cm × 1.1 cm obstructing the ampulla of Vater, with no macroscopically apparent infiltration of the pancreatic tissue (Figure 2A). On histology, the tumor was composed predominantly of papillary structures lined by pseudostratified mildly dysplastic epithelium (Figure 2B and C). Focal areas with high-grade dysplasia were also found (Figure 2D), representing less than 25% of the tumor. However, an invasive component was lacking. There was a sharp transition to poorly differentiated grade 3 NEC (Figure 3A), measuring 11 mm in the greatest diameter, with a mitotic count of more than 20 mitoses per 10 high power fields (Figure 3B). Immunohistochemical analysis of the neuroendocrine tumor revealed cells positive for synaptophysin, insulinoma-associated protein 1 (Figure 3C), diffusely positive for cytokeratin (CK) 7, focally positive for CK19, diffusely positive for thyroid transcription factor-1 (TTF1), and negative for chromogranin A, CK20, gastrin, insulin, somatostatin, and glucagon. The proliferative index (Ki-67) was 100% (Figure 3D). The papillary-tubular component lacked immunoreactivity for neuroendocrine markers, CK20, and TTF1, but was diffusely positive for CK7, CK19, and mucin 2. There was no lymphovascular or perineural invasion. Surgical margins were negative. No metastases to 25 examined lymph nodes were found. The histological features were consistent with a combined IAPN with high-grade dysplasia and poorly differentiated NEC.
Pancreatoduodenectomy (Whipple procedure) with lymphadenectomy was performed for tumor removal. Considering the final histopathologic diagnosis, close postoperative surveillance was advised at a multidisciplinary team meeting for neuroendocrine tumors.
The postoperative course was unremarkable and the patient was discharged from the hospital on postoperative day 6.
Further diagnostic work-up of the patient was performed with the aim of excluding the possibility of a metastatic NEC, especially in view of CK7 and TTF1 positivity. She therefore underwent an 18F-FDG PET/CT scan 1.5 mo after the surgery, which showed no metabolic activity in a previously described 4 mm peripheral lesion in the laterobasal segment of the left lower pulmonary lobe. Furthermore, no metabolically active lesions were found elsewhere in the body suggestive of distant NEC metastases.
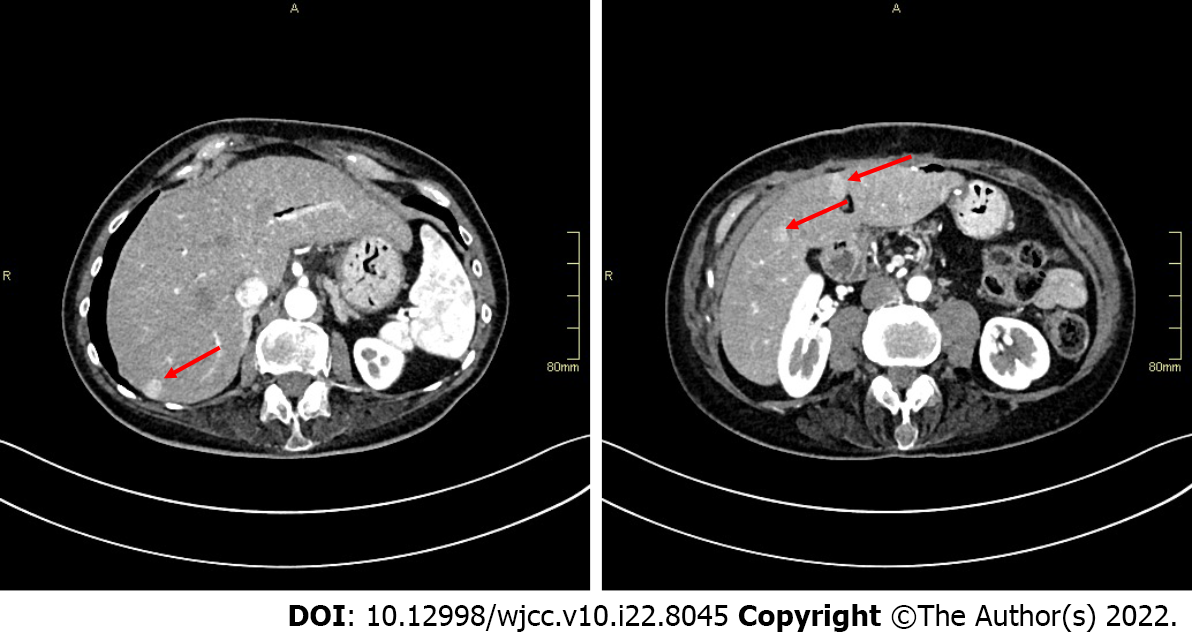
Postoperatively, the patient attended regular follow-up visits and CT evaluation every 3 mo. Follow-up chest CT scans showed no evidence of disease spread to the lungs and no changes to a previously described pulmonary lesion. However, several small (the largest one measuring 15 mm) hypervascular lesions in the right liver lobe, suggestive of liver metastases, were observed on abdominal CT scan 9 mo after the surgery (Figure 4). Systemic therapy with cisplatin and etoposide was therefore initiated.
Simultaneous coexistence of two distinct tumors can result from either proliferation of a single precursor cell with divergent differentiation (composite tumors), or combined growth of two different neoplastic clones arising from distinct precursor cells (collision tumors)[10]. Mixed neuroendo
Whether the tumor described in our patient represents a true mixed neoplasm with both exocrine and endocrine differentiation, or whether it is a coincidental collision tumor seems unclear. In our case, NEC is associated with IAPN, which is a preinvasive neoplasm. Similarly, individual reports can be found in the literature concerning NEC associated with intracholecystic papillary-tubular neoplasm (ICPN) in the gallbladder[13-16] or IPMN in the pancreas[17-19]. However, the literature data regarding the histogenesis of such tumors is not clear. Alternatively to the concurrent existence of two distinct independent lesions, some authors suggest that the two components of the tumor potentially arise from either a common progenitor capable of differentiation in several directions or by transdifferentiation of one tumor cell to another[20,21]. Meguro et al[14] described a case of mixed adenoneuroendocrine carcinoma arising from ICPN associated with pancreaticobiliary maljunction. Based on the histopathologic appearance, they proposed a transdifferentiation from poorly differentiated adenocarcinoma to NEC as the most possible histogenesis of the tumor[14]. Furthermore, Sciarra et al[15] performed immunohistochemical and molecular analysis of a gallbladder MiNEN composed of ICPN, adenocarcinoma, and NEC and revealed the same mutation profile, namely, TP53 mutation c.700T>C in all three components, supporting the hypothesis of their monoclonal origin. On the other hand, Stukavec et al[17] studied chromogranin A and CD57 as markers of neuroendocrine differentiation in pancreatic NEC combined with IPMN and, based on the pattern of immunoreactions, refuted the hypothesis that the two components share a common origin from one progenitor neoplastic cell. However, in most previously described cases of IPMN or ICPN presumably related with a neuroendocrine component, the papillary component showed variable areas of high-grade dysplasia together with invasive carcinoma[14,16,18,19]. In our case, a full differentiation spectrum is lacking since IAPN shows mainly low-grade dysplasia with only small foci of high-grade dysplastic changes, comprising less than 25% of the tumor and no invasive component. We could postulate that the IAPN component gave rise to invasive adenocarcinoma, which very early transdifferentiated to NEC, as has been shown in colorectal NEC with adjacent glandular adenoma or adenocarcinoma components. In these tumors, extensive molecular analysis has provided evidence that the two components share a common clonal origin and that their separation occurs early during malignant transformation, with subsequent independent mutational evolution[10,22]. Moreover, typical genetic founder mutations of the classical colorectal adenoma-carcinoma sequence found in colorectal NECs strongly suggest their evolution from colonic mucosa through a similar malignant transformation process, with additional subsequent transdifferentiation into a neuroendocrine cell phenotype[22]. Genetic data allowing definite conclusions regarding the molecular origin of ampullary NEC are non-existent. In our case, therefore, NEC arising from IAPN could be suggested but remains hypothetical, allowing a strong possibility that the two tumor components derived from two distinct pathologic events and their co-occurrence is only coincidental.
Due to the rarity of such tumors, their clinical and pathological behavior remains largely unknown, as do appropriate therapeutic measures. In the case of MiNENs, their outcome is highly dependent on the type of neuroendocrine and non-neuroendocrine components, giving rise to different prognostic categories according to the grade of malignancy of each component[11]. In the present case, whether it is a true mixed neoplasm or not, the tumor’s pure counterparts are associated with contrasting clinical outcomes. Non-invasive IAPNs show a favorable prognosis with 3- and 5-year survival of 100% after successful removal[1]. The prognosis of invasive IAPNs is still significantly better than that of conventional invasive carcinomas of the ampulla, although the difference in survival rate at 5 years did not reach statistical significance (3-year survival rate 69% vs 44%, P < 0.01 and 5-year survival rate 45% vs 28%, P = 0.06 for invasive IAPNs vs other invasive ampullary carcinomas, respectively)[1]. On the other hand, NECs are highly aggressive neoplasms, usually even more so than the common types of carcinoma arising at the same site[2]. Reported overall survival for patients with localized disease was 38 mo in a Surveillance, Epidemiology and End Results data analysis of 2546 patients with high-grade gastrointestinal NECs[23]. In comparison, the overall survival of 28.6 mo was reported for localized gastro-entero-pancreatic MiNENs in a large multi-center series[24]. Specific data on ampullary NEC or MiNEN survival are lacking, but their prognosis seems dismal. Vanoli et al[6] collected a retrospective series of 203 duodenal and ampullary neuroendocrine neoplasms treated surgically or endoscopically, among which 22 were ampullary NECs. Most NECs caused patient death in a median of 10 mo from diagnosis, with only one patient being alive without disease 42 mo after surgery[6]. Similarly, among 18 surgically treated ampullary neuroendocrine neoplasms reported by Milanetto et al[7], disease recurrence occurred in all four cases of NECs, with a median disease-free survival of 14 mo after R0 pancreatoduodenectomy. Despite systemic treatment of recurrence, all four patients eventually died due to NEC progression, after a median follow-up of 23 mo[7]. Given the shorter survival time and high risk of recurrence after upfront surgery, the authors proposed alternative treatment approaches for ampullary NECs, provided that biopsy availability is ascertained before the final therapeutic decision[7]. Adjuvant chemotherapy after R0 resection seems to offer improved survival[25,26]; however, the clinical relevance of this finding cannot be determined solely on the basis of individual reported cases.
Since no guidelines or solid evidence exist to support the best way of adjuvant or other types of treatment, it seems reasonable to plan the treatment according to the standard of care for the most aggressive and/or predominant component of the tumor from the same site of origin, in our case ampullary NEC. Based on the European Neuroendocrine Tumor Society guidelines for gastro-entero-pancreatic NECs, surgical resection together with platinum-based postoperative chemotherapy is advised in the case of localized disease, although supported by low-level evidence[23,27]. After complete resection of localized NEC, follow-up visits with conventional imaging (CT or magnetic resonance imaging) should be scheduled every 3-6 mo during the first 2 to 3 years and then every 6-12 mo up to 5 years after surgery[27]. In the case of coexistence of two different tumors, clinical patterns might differ significantly[24]. We thus recommend that newly diagnosed cases are discussed at multidisciplinary team meetings to tailor postoperative treatment and follow-up appropriately. The present case showed significant mitotic activity and an elevated proliferation index, as confirmed by diffuse detection of Ki-67 in 100% of cells. However, no lymph node metastases were demonstrated in any of the 25 examined lymph nodes. The patient did not undergo adjuvant systemic treatment initially; however, she was kept under close surveillance. Systemic therapy with cisplatin and etoposide was initiated after liver metastases were discovered on follow-up CT 8 mo after the surgery.
We describe, to the best of our knowledge, the first case of IAPN associated with NEC. The pathogenesis of this rare entity is considerably unclear, with problems arising in differential diagnosis between mixed neuroendocrine–non-endocrine neoplasm or collision of two distinct tumors. Radical resection is the treatment of choice for resectable tumors, although the prognosis appears unpredictable. Further investigations including molecular analyses are required to advance the biological understanding of this rare disease and identify the appropriate treatment strategy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Kimura Y, Japan; Vyawahare MA, India S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | Ohike N, Kim GE, Tajiri T, Krasinskas A, Basturk O, Coban I, Bandyopadhyay S, Morohoshi T, Goodman M, Kooby DA, Sarmiento JM, Adsay NV. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol. 2010;34:1731-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 3. | Nassar H, Albores-Saavedra J, Klimstra DS. High-grade neuroendocrine carcinoma of the ampulla of vater: a clinicopathologic and immunohistochemical analysis of 14 cases. Am J Surg Pathol. 2005;29:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Albores-Saavedra J, Hart A, Chablé-Montero F, Henson DE. Carcinoids and high-grade neuroendocrine carcinomas of the ampulla of vater: a comparative analysis of 139 cases from the surveillance, epidemiology, and end results program-a population based study. Arch Pathol Lab Med. 2010;134:1692-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Dumitrascu T, Dima S, Herlea V, Tomulescu V, Ionescu M, Popescu I. Neuroendocrine tumours of the ampulla of Vater: clinico-pathological features, surgical approach and assessment of prognosis. Langenbecks Arch Surg. 2012;397:933-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Vanoli A, La Rosa S, Klersy C, Grillo F, Albarello L, Inzani F, Maragliano R, Manca R, Luinetti O, Milione M, Doglioni C, Rindi G, Capella C, Solcia E. Four Neuroendocrine Tumor Types and Neuroendocrine Carcinoma of the Duodenum: Analysis of 203 Cases. Neuroendocrinology. 2017;104:112-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Milanetto AC, Pasquali C, Da Broi M, Brambilla T, Capretti G, Zerbi A. Ampullary neuroendocrine neoplasms: surgical experience of a rare and challenging entity. Langenbecks Arch Surg. 2018;403:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Randle RW, Ahmed S, Newman NA, Clark CJ. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: a population-based study. J Gastrointest Surg. 2014;18:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Wen LJ, Chen JH, Xu HJ, Yu Q, Deng Y, Liu K. The clinical profiles, management, and prognostic factors of biliary mixed neuroendocrine nonneuroendocrine neoplasms: A systematic review of the literature. Medicine (Baltimore). 2020;99:e23271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Furlan D, Cerutti R, Genasetti A, Pelosi G, Uccella S, La Rosa S, Capella C. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. 2003;83:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | La Rosa S, Sessa F, Uccella S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol. 2016;27:284-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (2)] |
| 12. | Frizziero M, Chakrabarty B, Nagy B, Lamarca A, Hubner RA, Valle JW, McNamara MG. Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Systematic Review of a Controversial and Underestimated Diagnosis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (2)] |
| 13. | Fraga J, Caetano Oliveira R, Alexandrino H, Cipriano MA. Neuroendocrine Carcinoma and Intracystic Papillary Neoplasm: A Rare Association in the Gallbladder. GE Port J Gastroenterol. 2019;26:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Meguro Y, Fukushima N, Koizumi M, Kasahara N, Hydo M, Morishima K, Sata N, Lefor AT, Yasuda Y. A case of mixed adenoneuroendocrine carcinoma of the gallbladder arising from an intracystic papillary neoplasm associated with pancreaticobiliary maljunction. Pathol Int. 2014;64:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Sciarra A, Missiaglia E, Trimech M, Melloul E, Brouland JP, Sempoux C, La Rosa S. Gallbladder Mixed Neuroendocrine-Non-neuroendocrine Neoplasm (MiNEN) Arising in Intracholecystic Papillary Neoplasm: Clinicopathologic and Molecular Analysis of a Case and Review of the Literature. Endocr Pathol. 2020;31:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Chatterjee D, Wang H. Mixed Adenoneuroendocrine Carcinoma Arising in a Papillary Adenoma of Gallbladder. AJCC-REP. 2014;2:37-42. |
| 17. | Stukavec J, Jirasek T, Mandys V, Denemark L, Havluj L, Sosna B, Kosmahl M, Zadorova Z. Poorly differentiated endocrine carcinoma and intraductal papillary-mucinous neoplasm of the pancreas: Description of an unusual case. Pathol Res Pract. 2007;203:879-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Tewari N, Zaitoun AM, Lindsay D, Abbas A, Ilyas M, Lobo DN. Three cases of concomitant intraductal papillary mucinous neoplasm and pancreatic neuroendocrine tumour. JOP. 2013;14:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Hashimoto Y, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Sueda T, Shimamoto F, Hiyama E. Mixed ductal-endocrine carcinoma derived from intraductal papillary mucinous neoplasm (IPMN) of the pancreas identified by human telomerase reverse transcriptase (hTERT) expression. J Surg Oncol. 2008;97:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Manuel-Vazquez A, Ramia JM, Latorre-Fragua R, Valle-Rubio A, Arteaga-Peralta V, Ramiro-Pérez C, de la Plaza-Llamas R. Pancreatic Neuroendocrine Tumors and Intraductal Papillary Mucinous Neoplasm of the Pancreas: A Systematic Review. Pancreas. 2018;47:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Marrache F, Cazals-Hatem D, Kianmanesh R, Palazzo L, Couvelard A, O'Toole D, Maire F, Hammel P, Levy P, Sauvanet A, Ruszniewski P. Endocrine tumor and intraductal papillary mucinous neoplasm of the pancreas: a fortuitous association? Pancreas. 2005;31:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Woischke C, Schaaf CW, Yang HM, Vieth M, Veits L, Geddert H, Märkl B, Stömmer P, Schaeffer DF, Frölich M, Blum H, Vosberg S, Greif PA, Jung A, Kirchner T, Horst D. In-depth mutational analyses of colorectal neuroendocrine carcinomas with adenoma or adenocarcinoma components. Mod Pathol. 2017;30:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120:2814-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 253] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 24. | Frizziero M, Wang X, Chakrabarty B, Childs A, Luong TV, Walter T, Khan MS, Morgan M, Christian A, Elshafie M, Shah T, Minicozzi A, Mansoor W, Meyer T, Lamarca A, Hubner RA, Valle JW, McNamara MG. Retrospective study on mixed neuroendocrine non-neuroendocrine neoplasms from five European centres. World J Gastroenterol. 2019;25:5991-6005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Imamura N, Nanashima A, Hiyoshi M, Fujii Y. Report of two cases of large cell neuroendocrine carcinoma of duodenal ampulla with contrasting outcomes following pancreaticoduodenectomy according to the use of adjuvant chemotherapy. Int J Surg Case Rep. 2017;31:132-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Taghizadeh-Hesary F, Moradi A, Malekzadeh M. Long-term complete remission of a patient with high grade neuroendocrine carcinoma of ampulla of Vater. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, Caplin M, O'Toole D, Perren A; Vienna Consensus Conference participants. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 419] [Article Influence: 46.6] [Reference Citation Analysis (0)] |