Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7872
Peer-review started: February 27, 2022
First decision: April 13, 2022
Revised: April 14, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: August 6, 2022
Processing time: 144 Days and 17 Hours
Anti-inflammation drugs were uncovered to be a potential therapy for depression. Celecoxib as a selective COX2 inhibitor is also one anti-inflammation drugs. Celecoxib is widely used in the clinic, which is well known by medical workers. It is uncertain whether celecoxib has efficacy in improving depression.
To estimate the effect of celecoxib on improving depression.
All literature was searched until 2022. The databases included PubMed, OVID database, Cochrane library, Web of Science, CNKI, Clinicaltrials.gov database and Wanfang database. The random effects model was used to estimate the standar
Twenty-nine randomized controlled studies were included in the meta-analysis (including 847 subjects with depression and 810 control subjects). The meta-analysis showed that celecoxib had an effect of anti-depression. At the same time, heterogeneity was observed (I2 = 82.1%, P = 0.00), and meta-regression was implemented to estimate the source of heterogeneity, which showed that the type of depression scale and depression type may lead to the heterogeneity. Subgroup analysis with respect to depression scale and depression type suggested that depression type was the possible main source of heterogeneity. Moreover, Egger’s test, Begg’s test, funnel plot and Doi plot was implemented, and publication bias was found to be significant. Next, the trim and fill method was used to estimate the influence of publication bias on the outcome of the meta-analysis, which showed that the outcome of the meta-analysis was reliable. Sensitivity analysis was estimated by deleting a study one by one, and the outcome of the meta-analysis was significantly stable. The quality of all randomized controlled trial studies was assessed by risk of bias, which indicated the rank of evidence in the meta-analysis was high.
Celecoxib could be effective for improving depression.
Core Tip: There is inconsistency about the efficacy of celecoxib in improving depression. This is an updated systematic review and meta-analysis that includes more than 10 additional clinical trials compared to the previous meta-analysis. We compared the depression scale scores between the celecoxib group and the control group, and celecoxib had a significant reduction in depression scale scores and could be effective in improving depression.
- Citation: Wang Z, Wu Q, Wang Q. Effect of celecoxib on improving depression: A systematic review and meta-analysis. World J Clin Cases 2022; 10(22): 7872-7882
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7872.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7872
Depression as a psychiatric disorder severely threatens human health and life quality. The World Health Organization reported that over 300 million people are currently living with depression in 2018[1]. Depression has a wide array of symptoms affecting somatic, cognitive, affective and social processes[2]. Depression is closely associated with suicide[3]. In addition, depression is associated with morbidity and mortality of cardiovascular disease[4]. According to the number, type and severity of symptoms, depressive disorder is classified as mild, moderate and major depression. Depression disorder also includes bipolar depression. The pathology of depression is still uncovered. Recently, the relationship between inflammation and depression is gaining more attention. Inflammation is likely a critical disease modifier, promoting susceptibility to depression[5]. Inflammation as a potential target in the treatment of depression has led to the exploration of clarifying the efficacy of anti-inflammation drugs on improving depression.
Celecoxib is a COX2 inhibitor and an anti-inflammation drug. Celecoxib has an Food and Drug Administration indication for the management of acute pain in adult women and primary dysmenorrhea[6]. Celecoxib is widely used in inflammation diseases such as rheumatoid arthritis, and celecoxib is widely used in the clinic. Due to its clinical popularity, celecoxib is well known by many doctors and patients. Interestingly, if celecoxib has an effect of anti-depression, it would be meaningful to uncover a new function in the clinic. In fact, depression is often linked with other diseases especially inflammation diseases such as inflammatory bowel diseases[7]. From the view of anti-inflammation, it is necessary to explore the efficacy of anti-depression.
The data on the efficacy of celecoxib on improving depression are inconsistent. Some studies showed celecoxib could improve depression[8,9]. On the contrary, a study showed that celecoxib was not superior to placebo for the treatment of bipolar depression[10]. A meta-analysis[11] about celecoxib on depression was published in 2014, and the number of randomized controlled trials (RCT) was only five. Another meta-analysis[12] in 2019 estimated the efficacy of celecoxib on bipolar depression, and the number of RCT was only three. Obviously, the number of RCT included in previous meta-analyses was not enough. Therefore, it is necessary to estimate the effect of celecoxib on depression by including more clinical trials. This meta-analysis aimed to estimate whether celecoxib could improve depression including bipolar depression, major depression and so on.
The meta-analysis was made up of four parts including search strategy, study selection, quality assessment and data extraction and data synthesis.
Conducting and reporting meta-analysis data were strictly in accordance with PRISMA statement guidelines. The PICOS scheme was followed in the selected studies. A systematic literature search was implemented by two researchers (Wang Z and Wu Q). Retrieval fields included “celecoxib,” “celebrex,” “depression” and so on. Retrieval mode included basic retrieval and advanced retrieval. The process of retrieval was presented in Supplementary Table 1. We searched databases including PubMed, OVID database, Cochrane library, Web of Science, CNKI, Clinicaltrials.gov database and Wanfang database. There was no language restriction in the retrieval process. No restrictions about humans, clinical trials or RCT were used, which was aimed at the comprehensiveness of retrieval. In addition, we retrieved the references using the Reference Citation Analysis database. For searching all databases, the latest time was until 2022.
Studies that reported celecoxib and depression were screened.
Inclusive criteria: (1) RCT included celecoxib group and control group; (2) With determined criteria, patients were diagnosed with depression including bipolar depression or unipolar depression or major depression and so on; and (3) Patients diagnosed with depression were comorbid with other non-mental diseases such as cancer.
Exclusive criteria: (1) With the diagnostic depression, patients were also diagnosed with other mental diseases such as Alzheimer’s disease; (2) Clinical trials that lacked a control group; (3) Case reports, letters, editorials and conference abstracts; and (4) Data about depression scores could be not obtained.
To retrieve more relevant studies, the references were also searched. According to the PRISMA literature-searching method, the primary inclusions were obtained through scanning titles and abstracts. Then, the full texts were screened carefully. Two researchers (Wang Z and Wu Q) searched the literature and determined the selected studies independently. The final inclusions were decided through consultations.
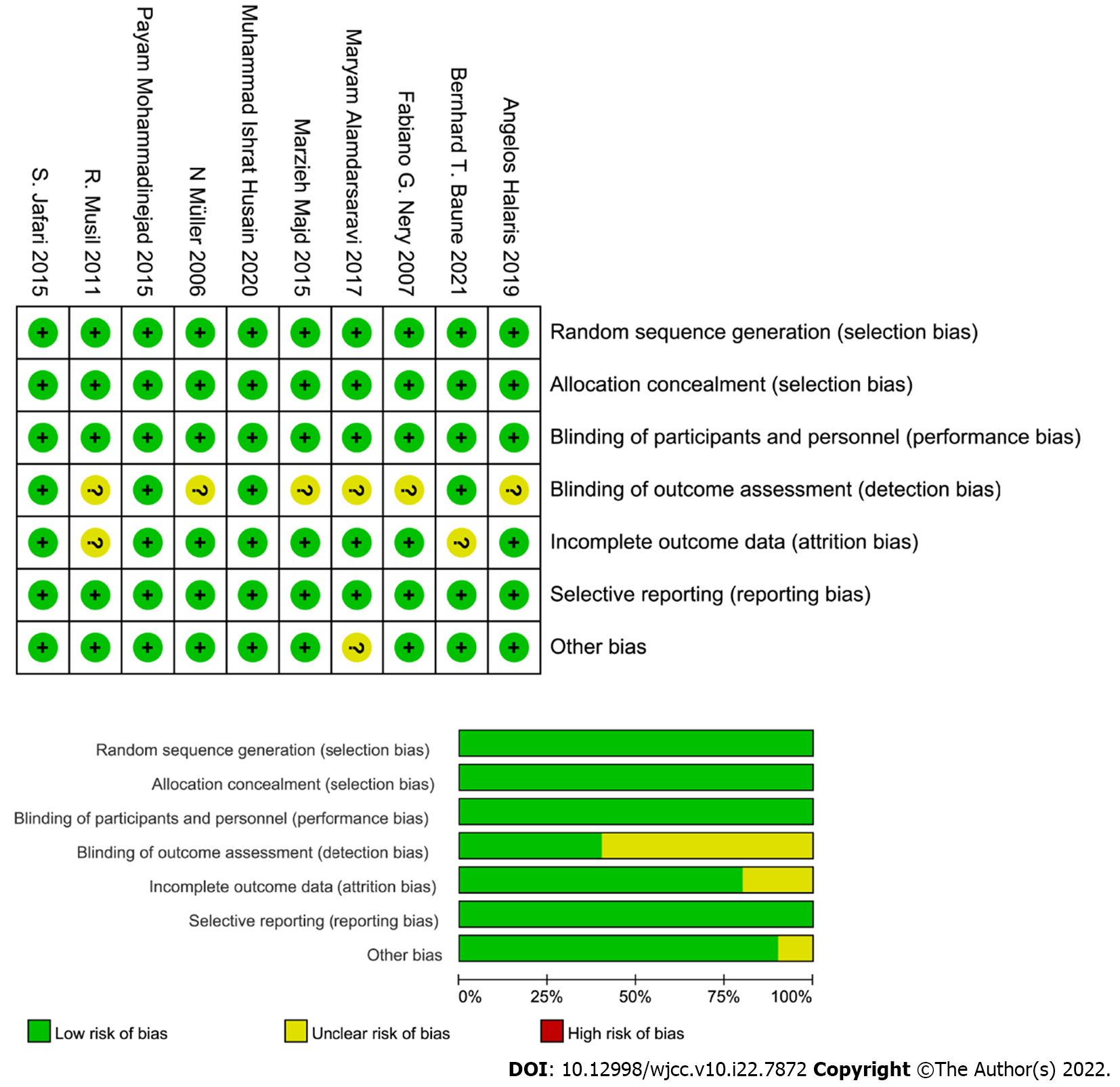
Based on the Cochrane Handbook for Systematic Reviews, risk of bias was used to evaluate the quality of all selected studies. Bias evaluation was conducted by estimating seven items including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment, incomplete outcome data (attrition bias), selection reporting (reporting bias) and other bias. All selected studies were evaluated according to above seven items. Finally, risk of bias graph and risk of bias summary plot were plotted by RevMan 5.3 software.
All data were extracted from all selected studies. A standardized data extraction form was used: name of the first author, year of publication, diagnostic criteria, study design, number of the celecoxib group and control group, type of depression scale and depression scale scores in the celecoxib group and control group. If the clinical trial included multiple treatment groups (different intervention), we only extracted data about the celecoxib and control groups. Based on the Cochrane Handbook for Systematic Reviews, if the clinical trial contained different doses and intervention periods, the trial will be divided into different trials with the same control group. The process of abstraction was administered by two researchers (Wang Z and Wu Q). They were in agreement with the outcome of the extraction.
We collected data including mean ± SD and n from selected studies. If the study provided mean ± SEM, data transformation would be implemented by the formula: SD = SEM × square root n.
All processes included forest plots, meta-regression analysis, funnel plot and Egger’s tests and were finished by STATA 16. Heterogeneity was assessed by the Cochran’s Q statistic and the I2 score. Heterogeneity was divided into homogeneity, moderate heterogeneity and high heterogeneity by I2 values of 0%-25%, 25%-50% and > 50%, respectively. If heterogeneity was significant, the random effects model was applied to estimate the standardized mean differences with 95%CI. Meta-regression and Galbraith plot were used to find the source of heterogeneity. With I2 values less than 50%, heterogeneity was considered to be small, and the fixed effects model was used.
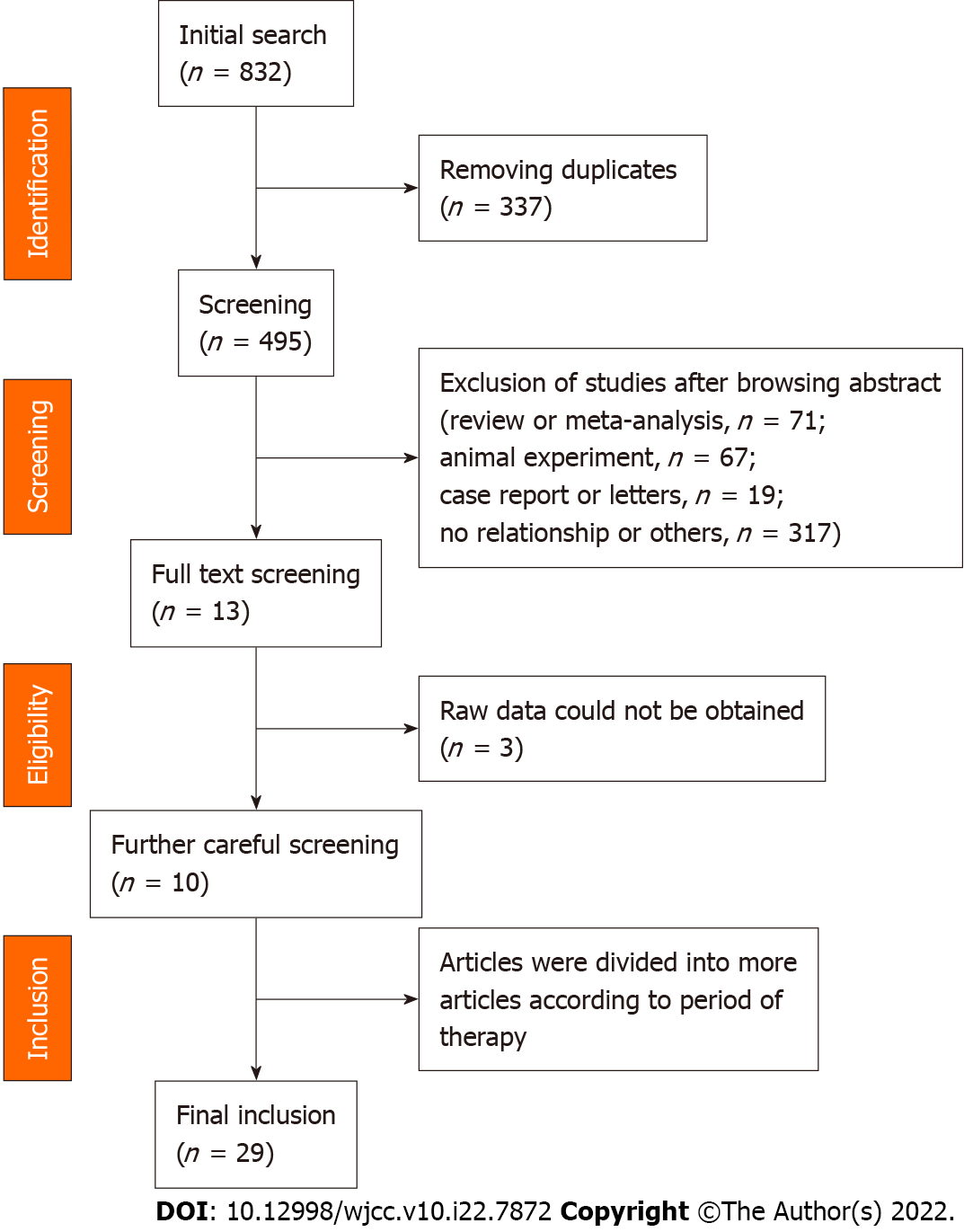
In total, 825 potentially relative records were identified, which was the sum of each database mentioned in the search strategy. After screening the titles, 338 duplicates were removed. Then, 474 records (review or meta-analysis, 71; animal experiment, 67; case report or letters, 19; no relationship or others, 317) were removed, and 13 records were obtained after screening the abstract. Because we could not obtain the raw data, three articles[13-15] were removed. Then, 10 records[8-10,16-22] were included in the meta-analysis. Except one study[19], the other studies were divided into separate studies according to a different period of therapy. Finally, 29 studies were included in the meta-analysis. All procedures were shown in Figure 1. The baseline characteristics in all included studies were presented in Supple
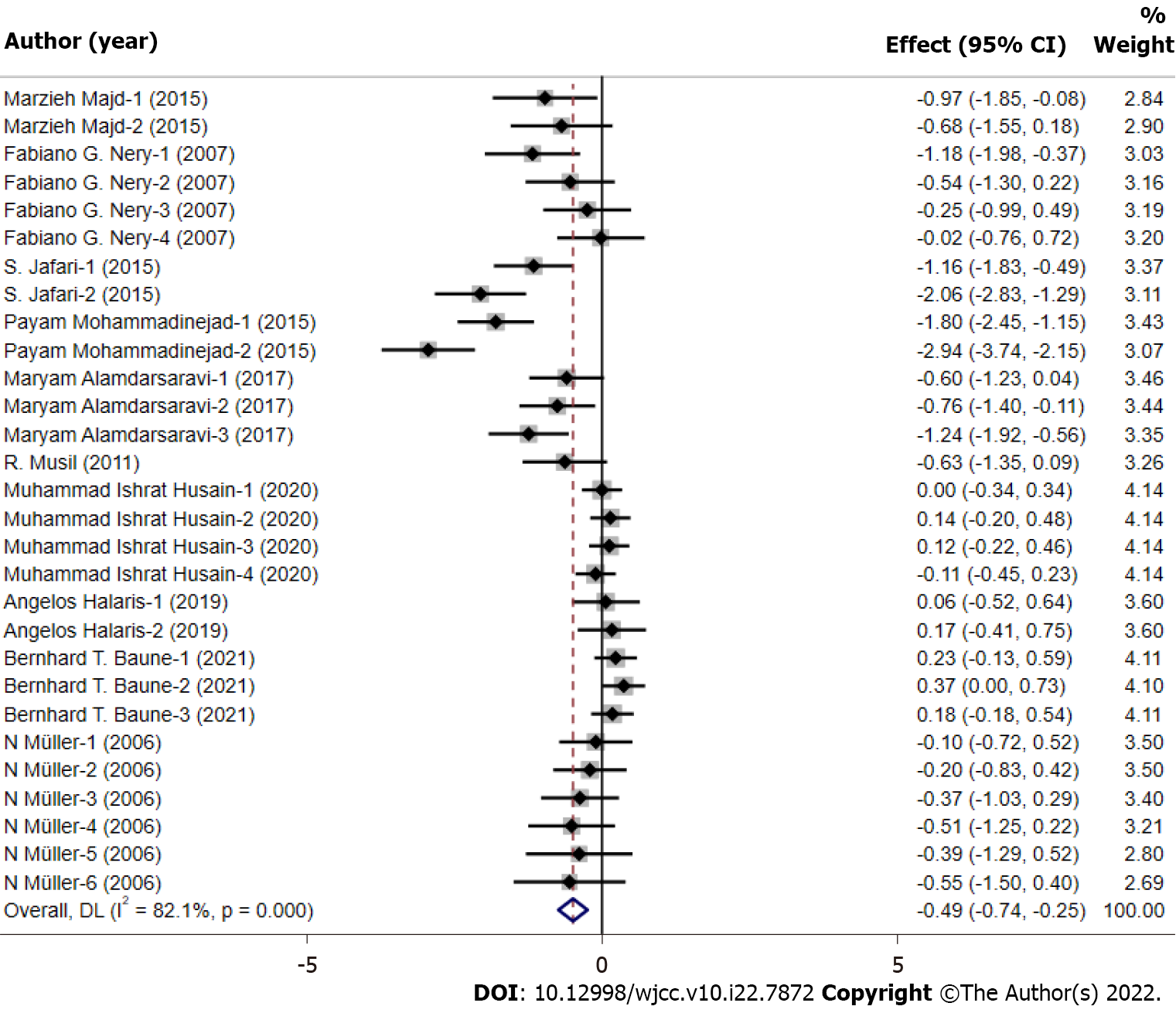
All data of the 29 studies were pooled in the meta-analysis. The outcome was shown in the forest plot (Figure 3). The depression scores in the celecoxib group were significantly lower than the control group (standardized mean difference = -0.49, 95%CI: -0.74 to -0.25, P < 0.05). Heterogeneity was observed to be severe (I2 = 82.1% and P < 0.001), and the random effect model was applied.
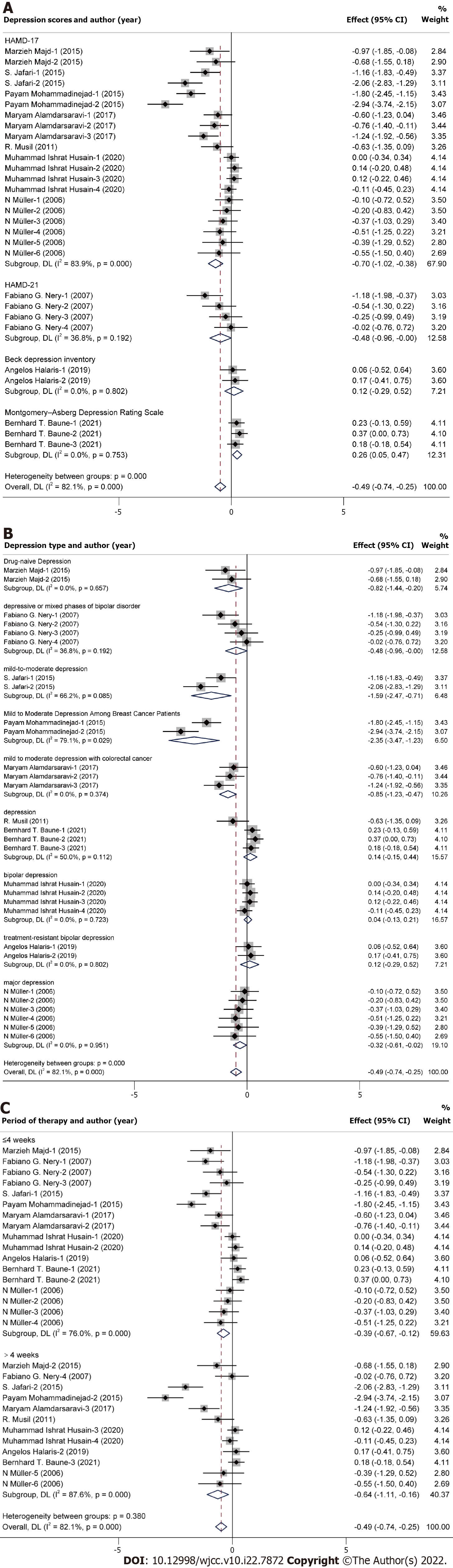
A multivariate meta-regression analysis was used to estimate the source of heterogeneity. We conducted meta-regression including three aspects (study design, depression scale and depression type). The results showed that the depression scale (regression coefficient: 0.268; P = 0.016; 95%CI: 0.054-0.483) and depression type (regression coefficient: 0.157; P = 0.020; 95%CI: 0.027-0.287) were the possible main source of heterogeneity.
After meta-regression, subgroup analysis about the depression scale and depression type was implemented to identify the possible source of heterogeneity (Figure 4A and B). Heterogeneity in the subgroup analysis about depression type was decreased, which showed that depression type may be the main source of heterogeneity. Moreover, subgroup analysis about the period of therapy was plotted (Figure 4C), which indicated that celecoxib could improve depression whether the period was ≤ 4 wk or > 4 wk.
Sensitivity analysis was conducted by deleting the studies one by one, and the outcome of meta-analysis was significantly stable.
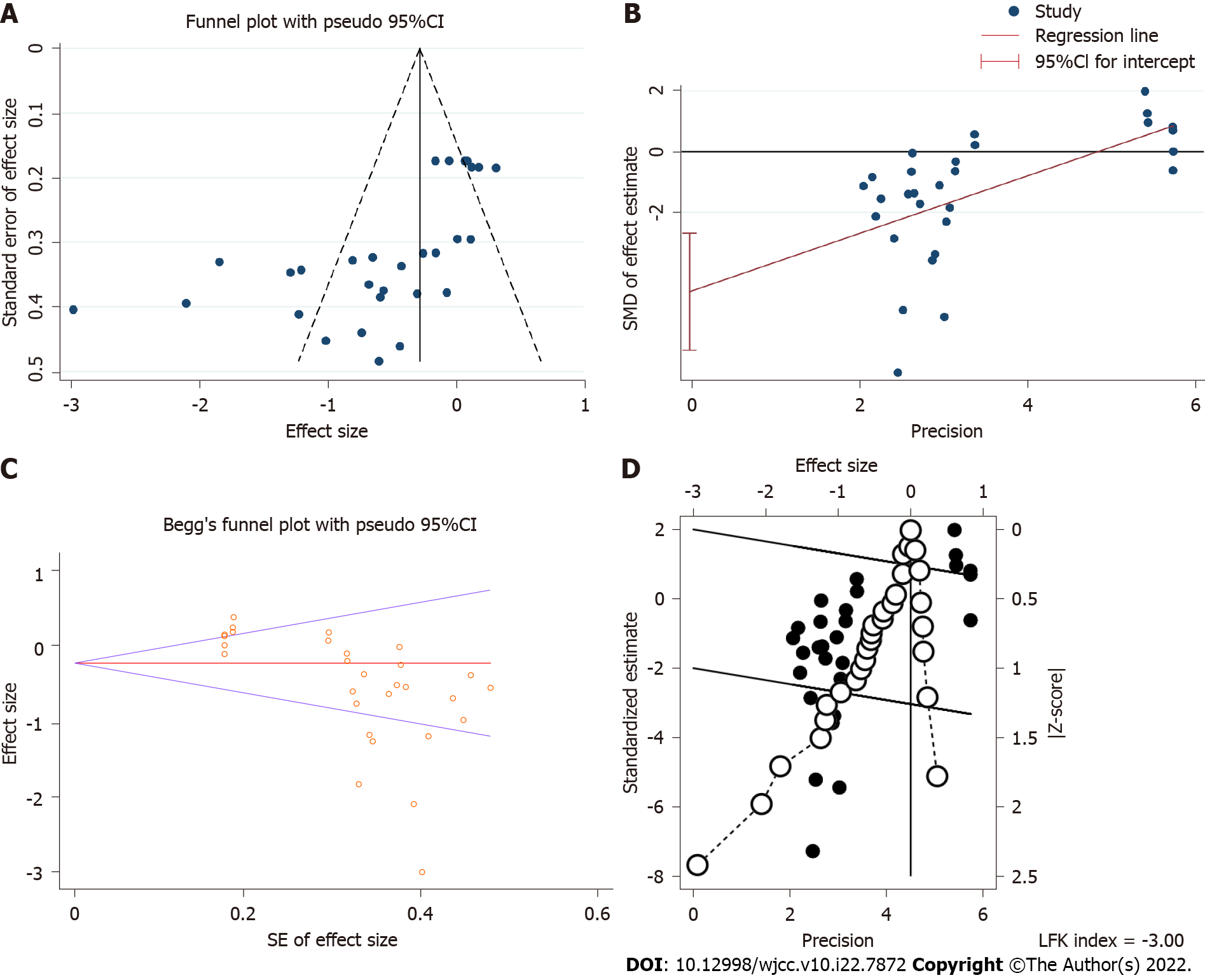
Funnel plot (Figure 5A), Egger’s test (Figure 5B), Begg’s test (Figure 5C) and Doi plot (Figure 5D) were implemented to estimate publication bias. Funnel plot, Egger’s test, Begg’s test and Doi plot showed publication bias was significant. Further, the trim and fill method was used to estimate the influence of publication bias on the outcome of the meta-analysis. The result of the trim and fill method (standardized mean difference = -0.679, 95%CI: -0.961 to -0.398, P < 0.01) indicated the outcome of the meta-analysis was reliable.
The result of the meta-analysis showed that celecoxib could improve depression. Depression type in all studies was different. This meta-analysis aimed to estimate the efficacy of celecoxib on depression. Future meta-analyses of celecoxib based on the specific type of depression should be implemented when the number of RCT studies increases. In this meta-analysis, the publication bias was significant. The result of the trim and fill method showed that this meta-analysis was still reliable. Obviously, heterogeneity was significant, and the depression scale and depression type were the main sources of heterogeneity by meta-regression and subgroup analysis. The result of the meta-analysis was likely interpreted by obvious heterogeneity. More studies would decrease the heterogeneity.
The results indicated that the anti-inflammation may be the potential target of anti-depression. Celecoxib, a COX2 inhibitor and a nonsteroidal anti-inflammatory drug, was used in the clinic. Other nonsteroidal anti-inflammatory drugs were shown to be effective for improving depression in some studies[23,24]. Extensive studies have confirmed the proinflammatory status in depression and causal relationships with neurotransmitter dysregulation[25]. On the contrary, a trial failure of anti-inflammation drugs in depression was published in 2020[26]. According to the trial failure, the authors replied and indicated that drug selection and certain inflammation status in depression status were the necessary consideration. This meta-analysis did not estimate the inflammation status for celecoxib in depression due to lack of inflammation data in most studies. Therefore, the relationship between inflammation and depression for celecoxib needs to be analyzed in the future.
On the other hand, not all depression patients coexist with abnormal inflammation levels. In these patients, it is possible that celecoxib would not improve depression. Of course, the above issues are weaknesses in the meta-analysis. Currently, there are not enough studies to support the meta-analysis regarding celecoxib on improving depression with inflammation status or without inflammation status, which is also the possible source that caused the heterogeneity. Comparing with other anti-inflammation drugs such as aspirin, data on the efficacy of improving depression are lacking. Before comparing the efficacy between celecoxib and other anti-inflammation drugs on improving depression, the issue whether inflammation status or non-inflammation status are associated with the efficacy of anti-inflammation should be resolved. If the issue is not resolved, then the result of the comparison between celecoxib and other anti-inflammation drug is not credible.
The relationship between inflammation and depression was explored by more studies. Inflammation is usually a reflection of cell damage caused by infections, physical injury or the response of tissues to an antibody challenge[27]. However, it has become apparent that psychological stress can also initiate the inflammatory response, thereby linking inflammation to both physical and mental ill health recently[27]. The inflammosome complex is expressed in microglia located in the hippocampus and other mood regulating regions that are particularly vulnerable to the effects of chronic stress, which was linked to depression[27]. Stress plays a critical role in depression, ultimately leading to pervasive mental status changes and chronic low-grade inflammatory reaction[25]. Stress-induced activation of the immune response alters neurotransmission leading to neurotransmitter imbalances such as serotonergic deficiency, which was the possible mechanism of inflammation and depression[25]. Interestingly, inflammation plays a key role in depression pathogenesis for a subset of depressed individuals[28].
Further, the bidirectional relationship between inflammation and depression was mentioned. Depression can promote intestinal permeability, i.e. greater inflammation-inducing endotoxin translocation, described as a “leaky gut” and inflammatory mediators can also induce clinical depression[28]. Therefore, the mechanism pathway between inflammation and depression is complex. Other factors such as gut microbiota, stress and so on can also participate in the complex net of inflammation and depression. The complex relationship and mechanism of inflammation and depression need more research.
Moreover, the dose of celecoxib in depression deserves exploration. Nearly all RCTs in the meta-analysis described 400 mg/d of celecoxib. No gradient of dose for celecoxib could be explored in this meta-analysis. More studies about different doses of celecoxib should be included to estimate the relationship between dose and depression. Safety of celecoxib was not mentioned in the meta-analysis due to few descriptions in the primary RCT. All in all, celecoxib is likely effective for improving depression. Weaknesses mentioned in the above context need to be resolved in the future work.
In summary, the results of this meta-analysis demonstrated that celecoxib could be effective for improving depression. Depression scale scores in the celecoxib group were less than the control group. For depression with or without inflammation, the efficacy of celecoxib on improving depression needs to be estimated separately in the future.
There is inconsistency about the efficacy of celecoxib for improving depression.
To estimate the efficacy of celecoxib for improving depression.
To provide more evidence to support the efficacy of celecoxib for improving depression.
The meta-analysis was pooled.
Depression scores in the celecoxib group were lower than the control group.
Celecoxib has an effect on improving depression.
The meta-analysis was explored from the view of a COX2 selective inhibitor, an anti-inflammation drug.
The authors would like to thank all the participants for their valuable contribution.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Badri M, Iran; Karcioglu Ö, Turkey S-Editor: Zhang H L-Editor: Filipodia CL P-Editor: Zhang H
| 1. | Hamel C, Lang E, Morissette K, Beck A, Stevens A, Skidmore B, Colquhoun H, LeBlanc J, Moore A, Riva JJ, Thombs BD, Colman I, Grigoriadis S, Nicholls SG, Potter BK, Ritchie K, Robert J, Vasa P, Lauria-Horner B, Patten S, Vigod SN, Hutton B, Shea BJ, Shanmugasegaram S, Little J, Moher D. Screening for depression in women during pregnancy or the first year postpartum and in the general adult population: a protocol for two systematic reviews to update a guideline of the Canadian Task Force on Preventive Health Care. Syst Rev. 2019;8:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 2. | Hauenstein EJ. Depression in adolescence. J Obstet Gynecol Neonatal Nurs. 2003;32:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Rihmer Z, Rihmer A. Depression and suicide - the role of underlying bipolarity. Psychiatr Hung. 2019;34:359-368. [PubMed] |
| 4. | Zhang Y, Chen Y, Ma L. Depression and cardiovascular disease in elderly: Current understanding. J Clin Neurosci. 2018;47:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 296] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 5. | Beurel E, Toups M, Nemeroff CB. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron. 2020;107:234-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 1259] [Article Influence: 251.8] [Reference Citation Analysis (0)] |
| 6. | Cohen B, Preuss CV. Celecoxib. 2022 May 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. [PubMed] |
| 7. | Salazar G. Depression and IBD. J Pediatr Gastroenterol Nutr. 2014;58:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Majd M, Hashemian F, Hosseini SM, Vahdat Shariatpanahi M, Sharifi A. A Randomized, Double-blind, Placebo-controlled Trial of Celecoxib Augmentation of Sertraline in Treatment of Drug-naive Depressed Women: A Pilot Study. Iran J Pharm Res. 2015;14:891-899. [PubMed] |
| 9. | Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, Bowden CL, Soares JC. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psychopharmacol. 2008;23:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Husain MI, Chaudhry IB, Khoso AB, Husain MO, Hodsoll J, Ansari MA, Naqvi HA, Minhas FA, Carvalho AF, Meyer JH, Deakin B, Mulsant BH, Husain N, Young AH. Minocycline and celecoxib as adjunctive treatments for bipolar depression: a multicentre, factorial design randomised controlled trial. Lancet Psychiatry. 2020;7:515-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 11. | Faridhosseini F, Sadeghi R, Farid L, Pourgholami M. Celecoxib: a new augmentation strategy for depressive mood episodes. A systematic review and meta-analysis of randomized placebo-controlled trials. Hum Psychopharmacol. 2014;29:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Bavaresco DV, Colonetti T, Grande AJ, Colom F, Valvassori SS, Quevedo J, da Rosa MI. Efficacy of Celecoxib Adjunct Treatment on Bipolar Disorder: Systematic Review and Meta-Analysis. CNS Neurol Disord Drug Targets. 2019;18:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, Mohebbi-Rasa S, Raznahan M, Kamalipour A. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. 2009;26:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 14. | Abbasi SH, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J Affect Disord. 2012;141:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 15. | Edberg D, Hoppensteadt D, Walborn A, Fareed J, Sinacore J, Halaris A. Plasma MCP-1 levels in bipolar depression during cyclooxygenase-2 inhibitor combination treatment. J Psychiatr Res. 2020;129:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Jafari S, Ashrafizadeh SG, Zeinoddini A, Rasoulinejad M, Entezari P, Seddighi S, Akhondzadeh S. Celecoxib for the treatment of mild-to-moderate depression due to acute brucellosis: a double-blind, placebo-controlled, randomized trial. J Clin Pharm Ther. 2015;40:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Mohammadinejad P, Arya P, Esfandbod M, Kaviani A, Najafi M, Kashani L, Zeinoddini A, Emami SA, Akhondzadeh S. Celecoxib Versus Diclofenac in Mild to Moderate Depression Management Among Breast Cancer Patients: A Double-Blind, Placebo-Controlled, Randomized Trial. Ann Pharmacother. 2015;49:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Alamdarsaravi M, Ghajar A, Noorbala AA, Arbabi M, Emami A, Shahei F, Mirzania M, Jafarinia M, Afarideh M, Akhondzadeh S. Efficacy and safety of celecoxib monotherapy for mild to moderate depression in patients with colorectal cancer: A randomized double-blind, placebo controlled trial. Psychiatry Res. 2017;255:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Musil R, Schwarz MJ, Riedel M, Dehning S, Cerovecki A, Spellmann I, Arolt V, Müller N. Elevated macrophage migration inhibitory factor and decreased transforming growth factor-beta levels in major depression--no influence of celecoxib treatment. J Affect Disord. 2011;134:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Halaris A, Cantos A, Johnson K, Hakimi M, Sinacore J. Modulation of the inflammatory response benefits treatment-resistant bipolar depression: A randomized clinical trial. J Affect Disord. 2020;261:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Baune BT, Sampson E, Louise J, Hori H, Schubert KO, Clark SR, Mills NT, Fourrier C. No evidence for clinical efficacy of adjunctive celecoxib with vortioxetine in the treatment of depression: A 6-week double-blind placebo controlled randomized trial. Eur Neuropsychopharmacol. 2021;53:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Möller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 558] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 23. | Rosenblat JD, Kakar R, Berk M, Kessing LV, Vinberg M, Baune BT, Mansur RB, Brietzke E, Goldstein BI, McIntyre RS. Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2016;18:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Köhler-Forsberg O, N Lydholm C, Hjorthøj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139:404-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 25. | Halaris A. Inflammation and depression but where does the inflammation come from? Curr Opin Psychiatry. 2019;32:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Miller AH, Pariante CM. Trial failures of anti-inflammatory drugs in depression. Lancet Psychiatry. 2020;7:837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 271] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 28. | Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172:1075-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 566] [Article Influence: 56.6] [Reference Citation Analysis (0)] |