Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7708
Peer-review started: September 15, 2021
First decision: October 18, 2021
Revised: November 30, 2021
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: August 6, 2022
Processing time: 309 Days and 20.5 Hours
The factors influencing the prognosis of patients with esophageal cancer vary among studies and are still poorly known.
To determine the factors associated with survival in patients with esophageal cancer.
This retrospective study included patients with esophageal cancer admitted between January 2017 and March 2020 at Heping Hospital Affiliated to Changzhi Medical College. All patients were treated according to the available guidelines. Follow-up was censored in October 2020. Univariable and multivariable Cox regression analyses were used to determine the independent risk factors for overall survival (OS).
In total, 307 patients were included. Their median age was 64 (range, 44-79) years, 63.5% were male, and the median disease course was 2 (0.1-36) months. The median tumor size was 3 (0-10) cm. Most patients were T3 (29.6%), N0 (70.0%). Most tumors were grade 2 (48.2%), and 87.3% were squamous cell carcinoma. The in-hospital mortality was 16.9%, the 30-day mortality was 19.9%, and the 90-day mortality was 25.4%. The cumulative OS rates at the last follow-up were 82.1% (95%CI: 67.7%-96.5%) for stage 0/I/II and 47.4% (95%CI: 16.5-78.6%) for stage III/IVA (P < 0.001). The multivariable analysis showed that creatinine levels (HR = 1.02, 95%CI: 1.00-1.03, P = 0.050), pTNM III/IVA (HR = 4.19, 95%CI: 2.19-8.01, P < 0.001), adjuvant radiotherapy and/or chemotherapy (HR = 0.23, 95%CI: 0.11-0.49), and the Comprehensive Complication Index (CCI) (HR = 1.02, 95%CI: 1.004-1.03, P = 0.011) were independently associated with OS.
The survival of patients with esophageal cancer is poor, especially those with pTNM III/IVA. pTNM stage III/IVA, CCI, and adjuvant therapy (radiotherapy and/or chemotherapy) are independently associated with OS.
Core Tip: The factors influencing prognosis in esophageal cancer vary among studies and are still poorly known. Therefore, this study aimed to determine the factors related to the survival of patients with esophageal cancer. The results showed that the in-hospital mortality was 16.9%, the 30-day mortality was 19.9%, and the 90-day mortality was 25.4%. Hence, the survival of patients with esophageal cancer is poor, especially those with pTNM III/IVA disease. pTNM stage III/IVA, Comprehensive Complication Index, and adjuvant therapy (radiotherapy and/or chemotherapy) are independently associated with overall survival. These results help delineate the factors associated with poor survival in patients with esophageal cancer.
- Citation: Shi MK, Mei YQ, Shi JL. Short- (30-90 days) and mid-term (1-3 years) outcomes and prognostic factors of patients with esophageal cancer undergoing surgical treatments. World J Clin Cases 2022; 10(22): 7708-7719
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7708.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7708
Esophageal cancer is the ninth cancer worldwide in terms of incidence but the sixth in mortality[1]. The most common histological subtypes of esophageal cancer include squamous cell carcinoma (SCC) and adenocarcinoma[2-5]. Worldwide, SCC comprises 90% of all esophageal cancer cases. In Western countries, the incidence of SCC is on the decline while adenocarcinoma incidence is rising; SCC is more common in Eastern Europe and Asia, while adenocarcinoma is more common in North America and Western Europe[3-5]. Most patients with esophageal cancer are > 50 years old[2,3], and both histologic subtypes are more common in men[4]. The most likely risk factors for esophageal cancer include tobacco use and excessive alcohol use (especially for the development of SCC), obesity (especially for the development of adenocarcinoma), and a history of gastroesophageal reflux disease (GERD) and/or Barrett esophagus (especially for the development of adenocarcinoma)[3,4].
Most tumors are diagnosed with regional or distant metastasis, and the 5-year overall survival (OS) is 39% in patients with a localized disease compared with 4% in patients with distant metastases[3]. Local recurrence after primary treatment with definitive chemoradiation may occur in 10%-30% of the patients within the first year[2]. Increased HER2-neu expression is associated with poor survival, particularly in patients with SCC[4]. The 5-year OS rate among patients treated with neoadjuvant chemotherapy for esophageal cancer in various studies ranges from 16% to 62%[6]. A Charlson score ≥ 2, history of myocardial infarction, and congestive heart failure may increase mortality risk following surgery for esophageal cancer[7]. Age > 70 years does not have prognostic significance after esophagectomy for esophageal cancer[8].
Some predictive models are available, but their value is limited. The Dutch nomogram is based on three variables and shows a concordance index of 0.76-0.77[9]. The POSSUM models can predict morbidity and mortality in patients undergoing gastroesophageal surgery, but they can overestimate the risks[10]. Other multivariable analysis studies reported various factors associated with poor prognosis[11-14]. However, beyond the traditional prognostic factors (e.g., histological grade and TNM staging[3-5,15-19]), the factors influencing prognosis in esophageal cancer are poorly known and vary among studies. Identification of the factors that could help refine prognostication is important since two patients with the same histological grade and TNM staging can have different survival.
Therefore, this study aimed to determine the factors related to the survival of esophageal cancer. The results could help delineate the factors associated with poor survival in patients with esophageal cancer.
This retrospective study included patients with esophageal cancer admitted between January 2017 and June 2020 at the Department of Gastrointestinal Surgery of Heping Hospital Affiliated to Changzhi Medical College. This study was approved by the Ethics Committee of Heping Hospital Affiliated to Changzhi Medical College [approval number: 2020 (037), approval date: July 22, 2020]. The requirement for informed consent was waived by the committee due to the retrospective study design.
The inclusion criteria were: (1) > 18 years of age; (2) underwent surgical treatments; and (3) confirmed with esophageal cancer by postoperative pathological examination. The exclusion criteria were: (1) incomplete clinical data; and (2) follow-up < 90 days.
Each patient was treated according to the available guidelines for the treatment of esophageal cancer[4,5,15,16], the physicians’ clinical experience, and the discussion with the patient. The treatment regimens included radiotherapy alone, chemotherapy alone (paclitaxel + cisplatinum, paclitaxel + nedaplatin, oxaliplatin, tegafur/gimeracil/oteracil, oxaliplatin + docetaxel/tegafur/gimeracil/oteracil, and nedaplatin/docetaxel), and radiotherapy combined with chemotherapy.
The type of surgery was selected according to the tumor’s location and size (the most important factor), infiltration depth, invasive degree, and general condition of the patients (whether they could tolerate open surgery). The surgery methods included endoscopic submucosal dissection (ESD), mediastinoscopy/laparoscopy/thoracoscopy, laparothoracoscopy combined palliative resection of esophageal cancer, laparothoracoscopy combined esophageal cancer radical operation, and open surgery. All the procedures were performed by experienced surgeons and followed standard protocols.
The patients were followed at 1, 3, 6, 9, 12, 18, 24, 30, 36, 48, and 72 months after the operation. For this study, follow-up was censored in October 2020. The follow-up was completed by the investigators and the medical team routinely. Routine follow-up included telephone, SMS, email, and outpatient visits. All follow-up data were extracted from the patient charts. The patients were not contacted for the purpose of this study.
The following data were collected from the medical records: demographic data, past medical history, and concomitant diseases; site, size, stage, and type of esophageal cancer; hematological examination results within 1 week before the operation, treatment strategies, operation-related parameters, postoperative complications, Comprehensive Complication Index (CCI)[20]; survival, recurrence, and metastasis.
The continuous variables were tested for normality using the Kolmogorov-Smirnov test. The continuous variables were not normally distributed in this study and are presented as medians (ranges). Categorical and ordinal variables are presented as frequencies and percentages. Univariable and multivariable Cox regression analyses (backward) were used to determine the independent risk factors for OS. The variables with P values < 0.10 in the univariable analysis were included in the multivariable analysis. The Kaplan-Meier curves of OS were plotted according to the pTNM staging results. All statistical analyses were two-sided. P values < 0.05 were considered statistically significant. SPSS 22.0 (IBM, Armonk, NY, United States) was used for statistical analyses.
Initially, 357 patients were included according to the inclusion criteria, but 26 with missing clinical information and 24 lost to follow-up were excluded, leaving 307 patients. As shown in Table 1, the median age at diagnosis was 64 (44-79) years, 63.5% were male, median BMI was 22.2 (14.9-31.6) kg/m2, median disease course was 2 (0.1-36) months, 30.9% had a history of smoking, 6.5% had a history of drinking, and 75.9% were ASA II. Table 1 also presents the biochemical characteristics of the patients.
| Characteristics | Median (range) / n (%) |
| Age (yr) | 64 (44, 79) |
| Body mass index (kg/m2) | 22.2 (14.9, 31.6) |
| Disease course (months) | 2 (0.1, 36) |
| Sex (male) | 195 (63.5%) |
| Smoking | 95 (30.9%) |
| Drinking | 20 (6.5%) |
| Family history of esophagus cancer | 22 (7.2%) |
| Hypertension | 112 (36.5%) |
| Diabetes | 21 (6.8%) |
| Coronary heart disease | 16 (5.2%) |
| ASA stage | |
| II | 233 (75.9%) |
| III | 73 (23.8%) |
| IV | 1 (0.3%) |
| Hemoglobin (g/L) | 141 (80, 180.4) |
| MCV (fl) | 93.6 (71.1, 134.1) |
| Platelets (× 109/L) | 213 (60.3, 445.9) |
| Lymphocytes (× 109/L) | 1.56 (0.07, 7.42) |
| Monocytes (× 109/L) | 0.36 (0.05, 1.01) |
| Neutrophils (× 109/L) | 3.63 (1.15, 12.94) |
| PT (s) | 13.8 (11.4, 31.9) |
| APTT (s) | 31.4 (10.6, 51.7) |
| Fibrinogen (g/L) | 3.79 (1.95, 6.3) |
| D-dimer (ng/mL) | 130 (14, 3354) |
| Total protein (g/L) | 71 (3.32, 88.1) |
| Albumin (g/L) | 42.1 (26.1, 63.5) |
| Creatinine (µmol/L) | 63 (36, 187) |
| Hematocrit (%) | 46.6 (28.2, 64.5) |
Table 2 shows the characteristics of the tumors. Most tumors were in the middle part of the esophagus (55.7%). The median tumor size was 3 (0-10) cm. Most patients were T3 (29.6%) N0 (70.0%). Most tumors were grade 2 (48.2%), and 87.3% were SCC.
| Variables | Median (range) / n (%) |
| Tumor location | |
| Upper | 15 (4.9%) |
| Middle to upper | 21 (6.8%) |
| Middle | 171 (55.7%) |
| Middle to lower | 43 (14.0%) |
| Lower | 57 (18.6%) |
| Tumor diameter (cm) | 3 (0, 10) |
| T stage | |
| Tis | 30 (9.8%) |
| 1a | 4 (1.3%) |
| 1b | 69 (22.5%) |
| 2 | 75 (24.4%) |
| 3 | 91 (29.6%) |
| 4a | 36 (11.7%) |
| 4b | 2 (0.7%) |
| N stage | |
| 0 | 215 (70.0%) |
| 1 | 61 (19.9%) |
| 2 | 28 (9.1%) |
| 3 | 3 (1.0%) |
| G stage | |
| 0 | 30 (9.8%) |
| 1 | 21 (6.8%) |
| 1-2 | 73 (23.8%) |
| 2 | 148 (48.2%) |
| 2-3 | 24 (7.8%) |
| 3 | 11 (3.6%) |
| pTNM | |
| 0 | 30 (9.8%) |
| I | 108 (35.2%) |
| II | 71 (23.1%) |
| III | 87 (28.3%) |
| IVA | 11 (3.6%) |
| Pathological type | |
| Squamous cell carcinoma | 268 (87.3%) |
| Intraepithelial neoplasia | 30 (9.8%) |
| Adenocarcinoma | 8 (2.6%) |
| Signet-ring cell carcinoma | 1 (0.3%) |
| Neoadjuvant radiotherapy and/or chemotherapy | 51 (16.6%) |
| Surgery | |
| Mediastinoscopy/ laparoscopy/thoracoscopy | 258 (84.0%) |
| Thoracotomy/laparotomy | 27 (8.8%) |
| Endoscopic submucosal dissection | 22 (7.2%) |
| Resection | |
| R0 | 304 (99.0%) |
| R1 | 3 (1.0%) |
| Operation time (min) | 270 (36, 485) |
| Intraoperative blood loss (mL) | 150 (2, 1000) |
| Lymph node dissection | 283 (92.2%) |
| Postoperative treatment | |
| None | 213 (69.4%) |
| Radiotherapy alone | 7 (2.3%) |
| Chemotherapy alone | 77 (25.1%) |
| Radiotherapy + chemotherapy | 10 (3.3%) |
| Number of metastatic lymph nodes | 0 (0, 8) |
Among the 307 patients, 16.6% received neoadjuvant treatments, 84.0% underwent mediastinoscopy/laparoscopy/thoracoscopy, 8.8% underwent open surgery, and 7.2% underwent ESD. An R0 resection was achieved in 99.0% of the patients. Operation time was 270 (36-485) min, and blood loss was 150 (2-1000) mL. Lymph node dissection was performed in 92.2% of the patients, and the median number of positive lymph nodes was 0 (0-8). Most patients (69.4%) received no adjuvant treatments, 2.3% received radiotherapy alone, 25.1% received chemotherapy alone, and 3.3% received radiotherapy and chemotherapy.
Table 3 presents the complications observed. Among the 307 patients, 35.5% had no complications, while 64.5% had complications. The in-hospital mortality was 16.9%, the 30-day mortality was 19.9%, and the 90-day mortality was 25.4%.
| Variables | Median (range) / n (%) |
| Clavien-Dindo stage | |
| None | 109 (35.5%) |
| I | 27 (8.8%) |
| II | 121 (39.4%) |
| IIIa | 28 (9.1%) |
| IIIb | 4 (1.3%) |
| IV | 4 (1.3%) |
| IVa | 8 (2.6%) |
| IVb | 6 (2.0%) |
| CCI, median (range) | 20.9 (0, 96.6) |
| Anastomotic leakage | 75 (24.4%) |
| Secondary operation | 7 (2.3%) |
| Hypoalbuminemia | 88 (28.7%) |
| Pulmonary infection | 68 (22.1%) |
| Recurrence | 11 (3.6%) |
| Metastasis | 21 (6.8%) |
| In-hospital mortality | 52 (16.9%) |
| 30-day mortality | 61 (19.9%) |
| 90-day mortality | 78 (25.4%) |
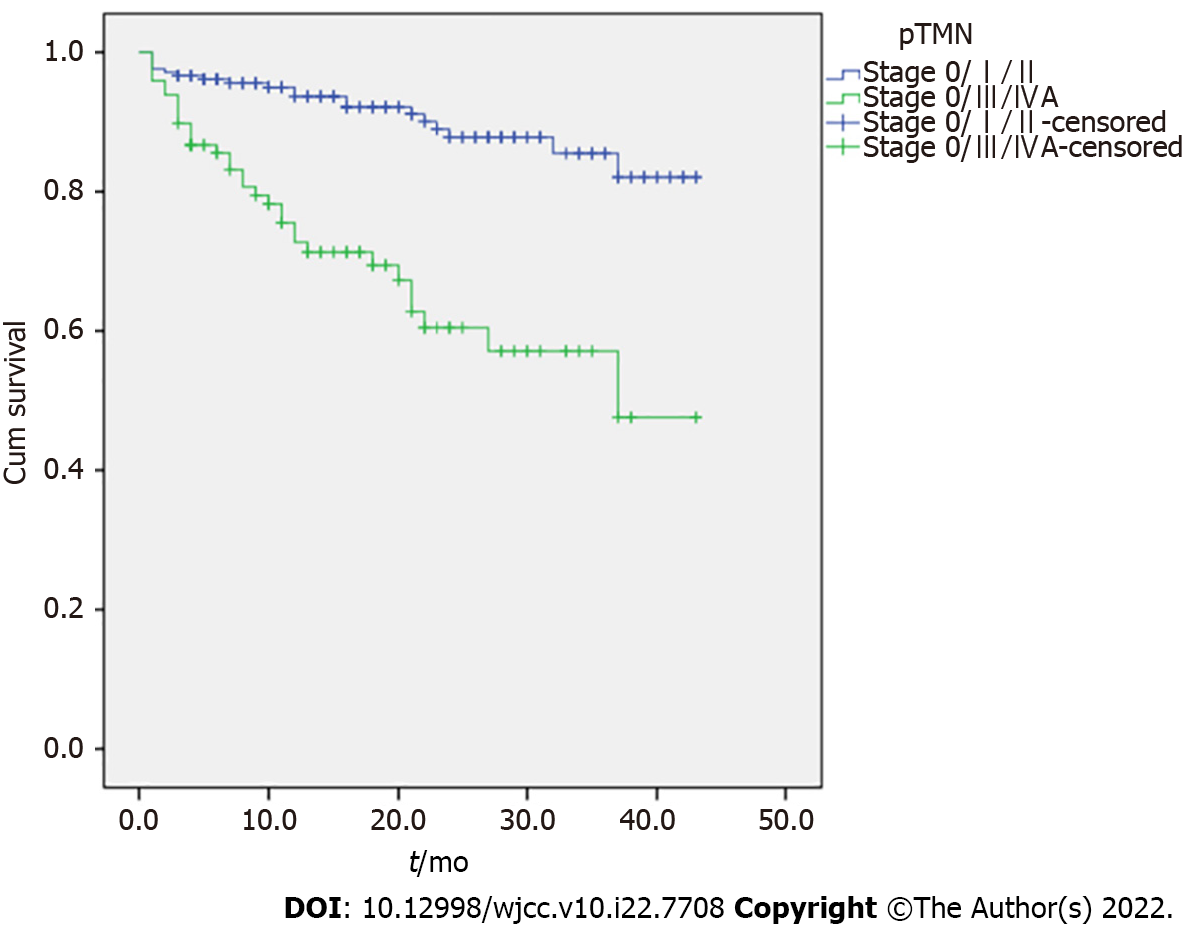
The 1-year cumulative OS rates were 93.7% (95%CI: 88.3%-99.1%) for stage 0/I/II and 72.4% (95%CI: 57.7%-87.1%) for stage III/IVA. The 2-year cumulative OS rates were 87.8% (95%CI: 79.1%-96.5%) for stage 0/I/II and 60.2% (95%CI: 41.6%-78.8%) for stage III/IVA. The 3-year cumulative OS rates were 85.5% (95%CI: 74.7%-96.3%) for stage 0/I/II and 56.9% (95%CI: 36.8%-77.0%) for stage III/IVA. The cumulative OS rates at the last follow-up were 82.1% (95%CI: 67.7%-96.5%) for stage 0/I/II and 47.4% (95%CI: 16.5%-78.6%) for stage III/IVA. The Kaplan-Meier analysis shows that the differences in survival were significant (P < 0.001) (Figure 1).
Table 4 shows that creatinine levels (P = 0.020), tumor size (P = 0.002), T3-4 (P = 0.003), N1-3 (P < 0.001), grade ≥ 2 (P = 0.032), pTNM III/IVA (P < 0.001), adjuvant therapy (P = 0.023), number of positive lymph nodes (P < 0.001), and CCI (P < 0.001) were associated with OS in the univariable analyses. The multivariable analysis showed that the creatinine levels (HR = 1.02, 95%CI: 1.00-1.03, P = 0.050), pTNM III/IVA (HR = 4.19, 95%CI: 2.19-8.01, P < 0.001), adjuvant radiotherapy and/or chemotherapy (HR = 0.23, 95%CI: 0.11-0.49), and the CCI (HR = 1.02, 95%CI: 1.004-1.03, P = 0.011) were independently associated with OS.
| Univariable | Multivariable | |||||
| HR | 95%CI | P | HR | 95%CI | P | |
| Age | 1.018 | 0.979, 1.059 | 0.377 | |||
| Sex | ||||||
| Female | ref | |||||
| Male | 1.020 | 0.58, 1.796 | 0.945 | |||
| Body mass index | ||||||
| < 28 | ref | |||||
| ≥ 28 | 1.912 | 0.816, 4.478 | 0.136 | |||
| Smoking | 1.185 | 0.663, 2.118 | 0.567 | |||
| Drinking | 0.916 | 0.285, 2.942 | 0.882 | |||
| Family history of esophagus cancer | 1.372 | 0.545, 3.452 | 0.501 | |||
| Hypertension | 0.939 | 0.530, 1.663 | 0.829 | |||
| Diabetes | 1.898 | 0.810, 4.449 | 0.140 | |||
| Hemoglobin | 0.987 | 0.971, 1.004 | 0.125 | |||
| D-dimer | 1.000 | 0.999, 1.001 | 0.782 | |||
| Albumin | 1.004 | 0.945, 1.067 | 0.897 | |||
| Creatinine | 1.020 | 1.003, 1.036 | 0.020 | 1.016 | 1, 1.032 | 0.050 |
| Tumor diameter | 1.244 | 1.083, 1.429 | 0.002 | |||
| T stage | ||||||
| Tis/0-2 | ref | ref | ||||
| 3-4 | 2.327 | 1.331, 4.068 | 0.003 | 1.869 | 0.98, 3.564 | 0.058 |
| N stage | ||||||
| 0 | ref | |||||
| 1-3 | 2.869 | 1.659, 4.962 | < 0.001 | |||
| G stage | ||||||
| < 2 | ref | |||||
| ≥ 2 | 1.990 | 1.062, 3.73 | 0.032 | |||
| pTNM stage | ||||||
| 0/I/II | ref | ref | ||||
| III/IVA | 4.117 | 2.349, 7.213 | < 0.001 | 4.189 | 2.190, 8.012 | < 0.001 |
| Pathological type | ||||||
| Squamous cell carcinoma | ref | |||||
| Others | 0.616 | 0.222, 1.711 | 0.353 | |||
| Received preoperative radiotherapy or chemotherapy | 1.157 | 0.592, 2.261 | 0.669 | |||
| Operation method | ||||||
| ESD/endoscopy/thoracoscopy/laparoscopy | ref | |||||
| Thoracotomy/laparotomy | 0.867 | 0.312, 2.405 | 0.784 | |||
| Lymph node dissection | 3.622 | 0.500, 26.255 | 0.203 | |||
| Postoperative radiotherapy and/ or chemotherapy | 0.449 | 0.225, 0.897 | 0.023 | 0.234 | 0.112, 0.488 | < 0.001 |
| Number of metastatic lymph nodes | 1.277 | 1.109, 1.471 | 0.001 | |||
| CCI | 1.029 | 1.014, 1.044 | < 0.001 | 1.018 | 1.004, 1.032 | 0.011 |
The factors influencing prognosis in esophageal cancer are still poorly known and vary among studies. Therefore, this study aimed to determine the factors related to the survival of patients with esophageal cancer. The results show that the survival of patients with esophageal cancer is poor, especially those with pTNM stage III/IVA. pTNM stage III/IVA, CCI, and adjuvant therapy (radiotherapy and/or chemotherapy) are independently associated with OS. This indicates that early clinical stage, fewer postoperative complications, and adjuvant therapy might be related to a better prognosis in patients with esophageal cancer after surgery. This study showed that the CCI is an independent risk factor affecting prognosis, indicating that postoperative nursing care to reduce postoperative complications might be helpful to improve the survival rate, while many surgeons tend to focus on surgery instead of postoperative nursing. Science-based postoperative management to reduce complications is also very important.
In this study, the 3-year cumulative OS rates were 85.5% for stage 0/I/II and 56.9% for stage III/IVA. This is consistent with the literature, as the studies indicate that a more advanced disease is associated with poorer survival[3-5,15-18]. Regarding the adjuvant treatments, this association is not surprising since the efficacy of adjuvant treatments to prevent recurrence and metastasis, and improve survival is the reason for giving adjuvant therapy in the first place[3-5,15,16,21]. Regarding the CCI, Bernardi et al[22] showed that patients with esophageal cancer who completed their treatment plan had a lower CCI than those who eventually dropped out, affecting the prognosis. Yamashita et al[23] and Aoyama et al[24] showed that the CCI was correlated with the prognosis of patients who undergo curative resection of esophageal carcinoma.
Nevertheless, a wide variety of other factors are associated with esophageal cancer prognosis in various studies. The Dutch nomogram is based on three variables independently associated with esophageal carcinoma: T stage, number of positive lymph nodes, and lymph node involvement[9]. The POSSUM score is a complex scoring system designed to determine the short-term postoperative mortality and includes 19 clinical, biochemical, and operative variables independently associated with prognosis[10]. Kawakita et al[25] showed that C-reactive protein levels and platelet distribution width could predict survival in patients with esophageal cancer. In esophageal SCC, which was the main histological subtype in the present study, Kim et al[26] showed that only the CCI was associated with survival, supporting the present study. In the study by Hauge et al[19], only the pTNM stage was independently associated with OS, supporting the present study. Hauge et al[19] also suggested that patients with R0 resection and who received adjuvant therapy had a better survival than the other subgroups of patients, but, in the present study, the number of patients with R1 resection was too small for subgroup analyses. A large meta-analysis (171 studies and 73629 patients) indicated that the factors associated with OS were the pT stage, pN stage, perineural invasion, circumferential resection margin, poor tumor grade, and a high neutrophil-to-lymphocyte ratio[27]. The differences among studies are highly dependent upon the study populations, data available for analysis (especially retrospective studies, local practice, and the treatment periods. However, specific factors identified by multiple studies might be considered more reliable, but validation studies are necessary from multiple centers.
Of note, in this study, creatinine levels were independently associated with the prognosis of esophageal cancer, but the P-value was borderline, and it is unknown whether including more patients would tip the balance one way or the other. Creatinine levels have been reported to be independently associated with prognosis in gynecological[28,29] and colorectal[30] cancers, but no previous studies have reported such an association in esophageal cancer. Further study is required to clarify this issue.
This study has limitations. First, it was a retrospective study, and some data were not collected (e.g., the patients’ postoperative nutritional status, which is known to influence prognosis[31]). In addition, the follow-up data were from the charts, and there is a possibility of unreported events. Second, the factors related to recurrence-free survival (RFS) could not be analyzed due to incomplete data. Third, it was a single-center study, and it is unknown whether the results are valid externally.
In conclusion, the pTNM stage, CCI, and postoperative radiotherapy and/or chemotherapy are independently associated with OS. The survival of patients with pTNM III/IVA disease is worse than that of patients with pTNM I/II disease. Fewer complications and adjuvant therapy are associated with better survival.
Esophageal cancer is the ninth cancer worldwide in terms of incidence but the sixth in mortality. The prognosis of esophageal cancer is poor.
The factors influencing the prognosis of patients with esophageal cancer vary among studies and are still poorly known. Some predictive models are available, but their value is limited.
This study aimed to determine the factors related to the survival of patients with esophageal cancer.
This retrospective study included patients with esophageal cancer admitted between January 2017 and March 2020 at Heping Hospital Affiliated to Changzhi Medical College. All patients were treated according to the available guidelines. Follow-up was censored in October 2020. Univariable and multivariable Cox regression analyses were used to determine the independent risk factors for overall survival (OS).
Among 307 patients, the in-hospital mortality was 16.9%, the 30-day mortality was 19.9%, and the 90-day mortality was 25.4%. The patients showed a cumulative OS rate at the last follow-up of 82.1% (95%CI: 67.7%-96.5%) for stage 0/I/II and 47.4% (95%CI: 16.5%-78.6%) for stage III/IVA (P < 0.001). Creatinine levels (HR = 1.02, 95%CI: 1.00-1.03, P = 0.050), pTNM III/IVA (HR = 4.19, 95%CI: 2.19-8.01, P < 0.001), adjuvant radiotherapy and/or chemotherapy (HR = 0.23, 95%CI: 0.11-0.49), and the Comprehensive Complication Index (CCI) (HR = 1.02, 95%CI: 1.004-1.03, P = 0.011) were independently associated with OS.
The survival of patients with esophageal cancer is poor, especially those with pTNM III/IVA. pTNM stage III/IVA, CCI, and adjuvant therapy (radiotherapy and/or chemotherapy) are independently associated with OS. These results could help manage patients by identifying those needing closer follow-up.
These results could help delineate the factors associated with poor survival in patients with esophageal cancer. Identification of the factors that could help refine prognostication is important since two patients with the same histological grade and TNM staging can have different survival. These results should be validated in large cohorts of patients from multiple centers.
Provenance and peer review: Unsolicited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim KT, Singapore; Pourhoseingholi MA, Iran S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 2. | Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 417] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 3. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 998] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 4. | Xu Y, Liu H, Chen J, Zhou Q. Comparisons between the National Comprehensive Cancer Network (NCCN) non-small-cell lung cancer (NSCLC) Clinical Practice Guidelines (Chinese version), the NCCN original edition, and the European Society for Medical Oncology NSCLC Guidelines in 2009. Thorac Cancer. 2010;1:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi51-vi56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 6. | Courrech Staal EF, Aleman BM, Boot H, van Velthuysen ML, van Tinteren H, van Sandick JW. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg. 2010;97:1482-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Backemar L, Lagergren P, Johar A, Lagergren J. Impact of co-morbidity on mortality after oesophageal cancer surgery. Br J Surg. 2015;102:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Ruol A, Portale G, Zaninotto G, Cagol M, Cavallin F, Castoro C, Sileni VC, Alfieri R, Rampado S, Ancona E. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg. 2007;133:1186-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Lagarde SM, Reitsma JB, Ten Kate FJ, Busch OR, Obertop H, Zwinderman AH, Moons J, van Lanschot JJ, Lerut T. Predicting individual survival after potentially curative esophagectomy for adenocarcinoma of the esophagus or gastroesophageal junction. Ann Surg. 2008;248:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Dutta S, Horgan PG, McMillan DC. POSSUM and its related models as predictors of postoperative mortality and morbidity in patients undergoing surgery for gastro-oesophageal cancer: a systematic review. World J Surg. 2010;34:2076-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Chao YK, Chen HS, Wang BY, Hsu PK, Liu CC, Wu SC. Factors associated with survival in patients with oesophageal cancer who achieve pathological complete response after chemoradiotherapy: a nationwide population-based study. Eur J Cardiothorac Surg. 2017;51:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Jung HK, Tae CH, Lee HA, Lee H, Don Choi K, Park JC, Kwon JG, Choi YJ, Hong SJ, Sung J, Chung WC, Kim KB, Kim SY, Song KH, Park KS, Jeon SW, Kim BW, Ryu HS, Lee OJ, Baik GH, Kim YS, Jung HY; Korean College of Helicobacter and Upper Gastrointestinal Research. Treatment pattern and overall survival in esophageal cancer during a 13-year period: A nationwide cohort study of 6,354 Korean patients. PLoS One. 2020;15:e0231456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Tustumi F, Kimura CM, Takeda FR, Uema RH, Salum RA, Ribeiro-Junior U, Cecconello I. PROGNOSTIC FACTORS AND SURVIVAL ANALYSIS IN ESOPHAGEAL CARCINOMA. Arq Bras Cir Dig. 2016;29:138-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Chen MF, Chen PT, Lu MS, Lee CP, Chen WC. Survival benefit of surgery to patients with esophageal squamous cell carcinoma. Sci Rep. 2017;7:46139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus. 2017;14:37-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 16. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 708] [Article Influence: 88.5] [Reference Citation Analysis (1)] |
| 17. | Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, Roth JA, Rashid A, Hamilton SR, Wu TT. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 387] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 18. | Gu Y, Swisher SG, Ajani JA, Correa AM, Hofstetter WL, Liao Z, Komaki RR, Rashid A, Hamilton SR, Wu TT. The number of lymph nodes with metastasis predicts survival in patients with esophageal or esophagogastric junction adenocarcinoma who receive preoperative chemoradiation. Cancer. 2006;106:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Hauge T, Amdal CD, Falk RS, Johannessen HO, Johnson E. Long-term outcome in patients operated with hybrid esophagectomy for esophageal cancer - a cohort study. Acta Oncol. 2020;59:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1299] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 21. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ; CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 776] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 22. | Bernardi D, Asti E, Aiolfi A, Bonitta G, Luporini A, Bonavina L. Outcome of Trimodal Therapy in Elderly Patients with Esophageal Cancer: Prognostic Value of the Charlson Comorbidity Index. Anticancer Res. 2018;38:1815-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Yamashita K, Watanabe M, Mine S, Fukudome I, Okamura A, Yuda M, Hayami M, Imamura Y. The impact of the Charlson comorbidity index on the prognosis of esophageal cancer patients who underwent esophagectomy with curative intent. Surg Today. 2018;48:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Aoyama T, Atsumi Y, Kawahara S, Tamagawa H, Tamagawa A, Ozawa Y, Maezawa Y, Kano K, Murakawa M, Kazama K, Segami K, Hara K, Numata M, Oshima T, Yukawa N, Masuda M, Rino Y. The Clinical Impact of the Age-adjusted Charlson Comorbidity Index on Esophageal Cancer Patients Who Receive Curative Treatment. In Vivo. 2020;34:2783-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Kawakita Y, Motoyama S, Sato Y, Wakita A, Nagaki Y, Imai K, Minamiya Y. Prognostic Significance of Combined Platelet Distribution Width and C-Reactive Protein Score in Esophageal Cancer. Anticancer Res. 2020;40:5715-5725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Kim GH, Na HK, Ahn JY, Lee JH, Jung KW, Kim DH, Kim HR, Choi KD, Song HJ, Kim YH, Lee GH, Jung HY, Park SI. Long-term Outcomes and Factors Affecting the Survival of Patients with Mucosal Esophageal Squamous Cell Carcinoma. Gut Liver. 2021;15:705-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Kamarajah SK, Marson EJ, Zhou D, Wyn-Griffiths F, Lin A, Evans RPT, Bundred JR, Singh P, Griffiths EA. Meta-analysis of prognostic factors of overall survival in patients undergoing oesophagectomy for oesophageal cancer. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Lafleur J, Hefler-Frischmuth K, Grimm C, Schwameis R, Gensthaler L, Reiser E, Hefler LA. Prognostic Value of Serum Creatinine Levels in Patients with Epithelial Ovarian Cancer. Anticancer Res. 2018;38:5127-5130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Schwameis R, Postl M, Bekos C, Hefler L, Reinthaller A, Seebacher V, Grimm C, Polterauer S, Helmy-Bader S. Prognostic value of serum creatine level in patients with vulvar cancer. Sci Rep. 2019;9:11129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Yang M, Zhang Q, Ruan GT, Tang M, Zhang X, Song MM, Zhang XW, Zhang KP, Ge YZ, Shi HP. Association Between Serum Creatinine Concentrations and Overall Survival in Patients With Colorectal Cancer: A Multi-Center Cohort Study. Front Oncol. 2021;11:710423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Takao K, Konishi H, Fujiwara H, Shiozaki A, Shoda K, Kosuga T, Kubota T, Arita T, Morimura R, Murayama Y, Kuriu Y, Ikoma H, Nakanishi M, Okamoto K, Otsuji E. Clinical Significance of Prognostic Nutritional Index in the Treatment of Esophageal Squamous Cell Carcinoma. In Vivo. 2020;34:3451-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |