Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7665
Peer-review started: February 26, 2022
First decision: April 16, 2022
Revised: May 1, 2022
Accepted: June 30, 2022
Article in press: June 30, 2022
Published online: August 6, 2022
Processing time: 145 Days and 15.2 Hours
More than 200000 hospital admissions happen per year for acute pancreatitis and more than 50000 for chronic pancreatitis in the United States of America. Necrotizing pancreatitis accounts for 20%-30% of the cases. One-quarter of the patients with pancreatitis develop vascular complications, which carries a high mortality. This mini-review will address these complications that can help primary care physicians and hospitalists in managing their patients effectively.
Core Tip: Vascular complications of acute pancreatitis carry high morbidity and mortality. Physicians must be aware of the possible complications, proper diagnosis, and treatment options. Herein, we review the vascular complications and up-to-date management.
- Citation: Kalas MA, Leon M, Chavez LO, Canalizo E, Surani S. Vascular complications of pancreatitis. World J Clin Cases 2022; 10(22): 7665-7673
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7665.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7665
Acute pancreatitis is a disorder characterized by inflammation of the pancreas secondary to variable etiologies, with the most common being gallstones and alcohol abuse. This disorder is the leading cause of gastrointestinal disease hospital admissions in the United States, with an incidence of 13-45 per 100000. Since 2000, the incidence of pancreatitis has risen by approximately 30%[1]. Smoking was found to be associated with an increased risk of developing pancreatitis, specifically among alcohol users. Moreover, the most reported complication of endoscopic retrograde cholangiopancreatography (ERCP) was found to be pancreatitis, with an incidence of approximately 3.5%[1].
Acute pancreatitis can be classified into mild, moderate, and severe based on organ failure and its duration (greater than 48 h or less than 48 h), presence or absence of local complications such as pseudocysts, acute peripancreatic fluid collections, acute necrotic collections, or walled-off necrosis[1]. The diagnosis is made on the basis of clinical, laboratory, and radiological evidence of pancreatitis. Management of acute pancreatitis is primarily supportive with volume resuscitation, analgesia, and bowel rest with or without nutritional support. Management should be started step-wise, and surgical options should be considered at the later stages if needed. Vascular complications of pancreatitis carry high morbidity and mortality. It is estimated that one-quarter of patients with pancreatitis may develop vascular complications[2-4]. Hence this review aims to address the common vascular complications associated with pancreatitis.
Pseudoaneurysm (PSA) associated with pancreatitis is rare but potentially a serious complication with the risk of rupture and lethal hemorrhage. It is caused by reactive local arteritis induced by pancreatic proteolytic enzymes. The exposure of visceral arteries to the enzymes may cause autodigestion of the wall leading to necrotizing arteritis, resulting in the weakening of the vessel lumen, causing a PSA formation. They are usually associated with a preexisting pseudocyst but can also occur without a pseudocyst[5,6].
This complication can present in both acute and chronic pancreatitis. Pseudoaneurysm usually develops 3.5 wk after the onset of acute pancreatitis[7]. The true incidence is unknown, but it ranges from 4% to 17% in single-center reports, with a higher incidence in males[7-9]. PSA is reported mostly in alcohol-induced pancreatitis, but the etiologic factor is not exclusive, and pediatric/hereditary pancreatitis cases have also been reported[5,8]. Patients present with abdominal pain or gastrointestinal bleeding secondary to rupture. Intra-abdominal bleeding, retroperitoneal bleeding, bleeding into the pancreatic duct, or common bile duct is another less common presentation[9]. The size at diagnosis may vary from a few mm to 5.6 cm[7].
The affected arteries are in proximity to the pancreas. The splenic artery (30%-50%) is most affected, followed by the left gastric, gastroduodenal artery, and pancreaticoduodenal artery. The superior mesenteric, proper hepatic artery, and small intrapancreatic arteries may also be affected[7,9,10]. Early diagnosis of PSA is important to avoid life-threatening hemorrhage. Clinicians should be aware of PSA as a pancreatitis complication. Diagnosis based on clinical presentation encompasses a challenge; abdominal pain can be from pancreatitis per se. Since PSA is more common in chronic alcohol abuse, gastrointestinal bleeding may mislead the diagnosis as a complication of cirrhosis[6].
PSA diagnosis is achieved by ultrasound (US), computed tomography (CT), and angiography. The US is a quick and easy tool for diagnosis that uses the color doppler, which shows a mass with the pulsatile, swirling flow which fills during systole unless filled with thrombus. Clot-filled and small PSA are difficult to diagnose by the US alone. The US operator must recognize sonographic appearance and thrombus age[7]. CT of the abdomen can be suggestive, but CT angiography has a higher sensitivity (95%). Angiography is considered the gold standard for diagnosis and can show smaller PSA, but it is expensive and invasive. Therefore, it is reserved for identifying the bleeding site and guiding the endovascular treatment[11].
Pang et al[9] described a peripancreatic PSA classification system. They included pancreatitis-related PSA but also postoperative PSA[9]. The classification is based on the type of artery involved, communication with the gastrointestinal tract, and exposure to pancreatic juice. Type 1: Minor artery: > 5mm away from the major artery with no communication to GI tract and no exposure to pancreas; Type 2: major artery can be sacrificed. It does communicate with the GI tract and with exposure to pancreas. Type 3: major artery cannot be sacrificed. The proposed classification can assist in treatment strategy. PSA management can be endovascular or surgical. Studies published after 2000 favor embolization as the initial treatment option[9]. Surgery continues to be an important tool for selected patients in whom angiographic intervention with embolization fails or those with hemodynamic compromise. Surgical procedures may vary from direct vessel ligation to pancreas resection, gastrectomy, or small intestine resection, depending on the affected vessel and compromise of blood perfusion to adjacent organs. Patients with concomitant pancreatitis complications may need simultaneous surgical debridement, necrosectomy, or pancreatectomy[12].
Endovascular management allows for embolization if bleeding is the main presentation but also for early treatment with stent deployment. Embolization can be done with metallic coils, transcatheter thrombin injection, covered stents, gel foam, or microparticles. Usually, coils are placed distal to the PSA and then proximal, preventing collateral backfilling. Angioembolization interventions have a reported success rate of 70%-90%[7]. Although the benefits of endovascular management have been proven with an increased success rate over the years, recurrence, stent infection, displacement, migration, and splenic infarction are possible complications. Therefore, management should be tailored to individual patients, and embolization may be used only as a bridge treatment for some[9].
Thrombosis is more often associated with severe pancreatitis compared to mild cases[13]. Necrotizing pancreatitis (NP) is a severe systemic inflammatory process that may lead to splanchnic venous system thrombosis (SVT), involving splenic, portal, and superior mesenteric veins, either independently or in combinations[14]. Also, thrombosis of peripheral vasculature has been described in the literature. In most patients, a single venous territory is involved[13].
Numerous risk factors have been described, such as male gender, history of previous Venous Thromboembolism (VTE), infected necrosis, and organ failure[15]. In multivariate analysis, the development of VTE did not increase mortality in patients with NP[16]. VTE risk in NP patients is among the highest of any hospitalized patients[17]. SVT develops in approximately 50% of patients with NP, and the incidence drops significantly in the absence of necrosis (1%-17%)[13,18].
Four main mechanisms of SVT have been proposed: (1) Swelling and necrosis of the pancreas and local inflammatory infiltration leading to vascular endothelial damage; (2) Extrinsic compression of the splanchnic vein (pancreas enlargement or pseudocyst) causing stasis; (3) Increased level of inflammatory mediators triggering coagulation cascade; and (4) Activation of the coagulation system due to release of tissue factors from the damaged pancreas[19].
Clinical manifestations can be divided into secondary to acute thrombosis and complications of portal hypertension[19]. They include abdominal pain due to mesenteric extension, gastrointestinal bleeding secondary to portal hypertension, and splenomegaly.
Color Doppler ultrasound is considered the first-line diagnostic approach for screening for SVT[19]. It has a sensitivity of 83% compared with angiography for the portal vein (PV). It has a less accurate assessment of the splenic vein due to its anatomic location[13]. Contrast-enhanced CT or magnetic resonance imaging is used to confirm SVT diagnosis[19]. It has a sensitivity of 90%. CT scan findings of acute SVT include persistent, well-defined intraluminal filling defects with low central attenuation and surrounding well-defined, rim-enhancing venous walls[13]. Angiography remains the golden standard for diagnosing SVT, but it is an invasive approach.
Approximately one-third of patients present with spontaneously splanchnic vein recanalization. The other two-thirds are at risk of developing severe complications such as bowel ischemia and liver failure. At this time, there is no consensus on whether patients with SVT should receive therapeutic anticoagulation since the theoretical risk of bleeding exists. According to a meta-analysis by Chandan et al[20], therapeutic anticoagulation can recanalize the involved vessels without increasing the risk of bleeding in the setting of SVT due to acute pancreatitis. Also, a retrospective study of 273 patients with AP-induced SVT reported a reduction in SVT incidence and improvement in the clinical outcomes without increasing the risk of bleeding[21]. Consistent with these findings, in a meta-analysis, the use of therapeutic anticoagulation resulted in a statistically significant increase in recanalization rate with no increase in hemorrhagic complications or mortality. However, there was no difference in overall mortality between the treated and control group to justify anticoagulation therapy[22]. High-quality randomized trials with an adequate sample size should be encouraged to elucidate this topic.
The incidence and prevalence of arterial thrombosis have not been reported due to the rarity of the condition. Several cases of arterial thrombosis associated with pancreatitis have been reported in the literature (Table 1). Arterial thrombosis is the result of atheromatous plaque rupture. In pancreatitis, several theories have been proposed, such as the release of proteolytic and lipolytic enzymes, which results in endothelial injury and the activation of the coagulation cascade with resultant thrombosis. In addition, adjacent inflammation can result in localized vascular inflammation, third spacing, necrosis, and mass effect, which increases the risk of thrombus development[23].
| Ref. | Vessel involved | Necrotizing pancreatitis | Management |
| Mishreki et al[22], 2011 | Juxtarenal abdominal aorta, left renal artery, aortic arch, right innominate and left common carotid artery | + | Intravenous heparin drip followed by subcutaneous enoxaparin |
| Thajudeen et al[26], 2013 | Bilateral renal arteries | + | Intravenous heparin drip and switch to oral anticoagulation |
| Chong et al[24], 2016 | Ascending aorta with renal embolus | + | Surgical removal and intravenous heparin drip followed by warfarin |
| Keskin et al[23], 2017 | Left atrial appendage | Unclear | Surgical management |
| Rodriguez et al[21], 2019 | Abdominal aorta, superior mesenteric artery | - | Intravenous heparin drip followed by warfarin |
| Chait et al[25], 2019 | Superior mesenteric artery | + | Intravascular tPA, mechanical thrombectomy and intravenous heparin drip |
Cases reported thrombus development in the abdominal aorta and its branches, such as the superior mesenteric artery and renal arteries. A case report showed widespread aortic thrombi with sparing of the celiac trunk, splenic artery, common hepatic artery, and superior mesenteric artery[24]. Arterial thrombosis in pancreatitis can be unpredictable, with a case presenting with intracardiac thrombus following pancreatitis. It should be noted that the patient had valvular atrial fibrillation and was on warfarin, and the clot was adherent to the left atrial appendage. Therefore, the thrombus could be secondary to atrial fibrillation, with pancreatitis as a possible provoking factor[25]. Cases reporting evidence of arterial thrombosis were primarily noted in patients with acute necrotizing pancreatitis. Presentations are variable depending on the vessel involved with general symptoms, including; vague abdominal pain, nausea, and vomiting. Biphasic computed tomography with angiography is sufficient for the diagnosis of arterial thrombi. In cases of small pseudoaneurysms or unclear diagnoses, angiography can be performed. Management of arterial thrombosis in pancreatitis can be complex and is variable depending on the location of the thrombus[26]. In the cases reviewed, intravascular management with thrombectomy followed by intravenous heparin was used in the patient with superior mesenteric artery thrombosis[27]. Intravenous heparin drip followed by warfarin or enoxaparin was utilized in the rest of the cases. The duration of anticoagulation remains unclear. However, in most cases, treatment for 3-6 mo with correction of the underlying pathology is agreed upon. Complications of arterial thrombosis carry high morbidity and mortality, with cases reporting the development of resultant gastric necrosis in a case of splenic artery thrombus and renal dysfunction due to renal artery thrombosis resulting in end-stage renal disease[28].
Pancreatic fistula is described as the leakage of pancreatic enzymes and secretions due to an abnormal connection with adjacent structures (e.g., organs, blood vessels, or spaces). Fistulas can be classified as internal or external depending on the location of the connection. Pancreatic fistula formation following pancreatitis episodes has not been well studied, and evidence is found only through case series. Internal pancreatic fistula is more likely to occur in cases of pancreatitis, specifically in chronic pancreatitis secondary to alcohol use[29]. Pancreatic fistulas occur due to disruption of the pancreatic ducts in trauma, acute pancreatitis, chronic pancreatitis, and pancreatic resection. Initially, fluid collection can be the only finding; however, when persistent, this can lead to pseudocyst formation with potential erosion of adjacent hollow structures and fistula formation. In a review of the literature by Brown et al[30] in 2014, patients with pancreatic portal fistula had the presence of a pseudocyst, with the majority being located in the head of the pancreas.
Clinical manifestations include; abdominal pain, abdominal distention (pancreatic ascites), nausea, vomiting, shortness of breath (pleural effusion), fever, and life-threatening hemorrhage in a small subset of patients. Disseminated fat necrosis has been reported in the literature with presentations of peripheral subcutaneous fat necrosis, which is thought to be secondary to the introduction of pancreatic enzymes into the systemic circulation. An elevated amylase level (> 6000) was found in all patients with disseminated fat necrosis[30]. Several diagnostic modalities have been performed in the literature ranging from the US to ERCP. Although there have been no studies to accurately assess the sensitivity and specificity of these tests for pancreatic fistula detection, most case series have an ERCP performed for definitive diagnosis and possible therapeutic intervention, and hence is considered the most accurate testing modality by clinicians[31]. The US can show the complex fluid collection in the portal vein with no flow on the doppler. CT with contrast generally demonstrates a fluid-attenuated portal vein with possible collateral periportal vessels and pseudocyst. Magnetic resonance cholangiopancreatography findings on T2 weighted sequences include; hyperintense portal vein and fluid signal; occasionally, visualization of the hyperintense fistulous tract could be seen[32]. In contrast, in cases of portal vein thrombosis only, ultrasonography would show decreased or absent flow on the doppler without complex fluid collection. Narrowing of the portal vein can be seen in cases of extrinsic compression; on CT with contrast, the portal vein would be hypodense with no contrast enhancement, and on MRCP, T1 weighted sequences would show an isointense portal vein with no fluid signal. Secretin enhanced MRCP can also help further characterize pancreatic ducts in cases of diagnostic uncertainty.
Percutaneous transhepatic portography (PTP) is another diagnostic modality, especially in uncertain diagnosis, and can provide an accurate assessment of the portal circulation anatomy and the extent of portal venous invasion, which has been correlated with surgical procedures findings. In addition, PTP allows for fluid extraction and examination[30].
The management of pancreatic-portal vein fistulas is variable and ranges from conservative management to surgical management involving partial or complete pancreatectomy with fistula repair. Conservative management has been successful, as reported in several cases in the literature. However, it was primarily done among stable patients with minimal clinical manifestations[33-37]. Endoscopic management with endoscopic ultrasound cyst drainage and ERCP with pancreatic stent placement has been successful in hemodynamically stable patients. Surgical management with partial or total pancreatectomy is another mode of management, specifically in patients with disseminated fat necrosis or those who are not candidates for endoscopic management or failed endoscopic management. However, there is no current treatment algorithm for pancreatic-portal fistulas due to the acute nature of the disease[38].
Hemosuccus pancreaticus (HP) is a rare cause of gastrointestinal bleeding secondary to pancreatic pathology. It is characterized by bleeding through the ampulla of Vater due to the presence of blood in the main pancreatic duct. In some cases, bleeding could be through the accessory pancreatic duct and drain through the minor duodenal papilla[39].
The bleeding source could be from the pancreas, pancreatic duct, or surrounding vessels such as the splenic artery or gastroduodenal artery. The incidence of HP as the cause of upper gastrointestinal bleed (GI) is estimated at 1 in 1500 cases of upper GI bleed with a strong male predilection (approximately 7:1). Several etiologies have been proposed in the literature, such as pancreatic inflammation, arterial aneurysm or PSA, pancreatic masses, iatrogenic, congenital, or trauma[40].
Pancreatic inflammation (acute, chronic, or hereditary) was found in approximately 80% of cases of HP. It is likely due to ductal inflammation-promoting vascular wall rupture. Local irritation due to gallstones and pseudocyst also contributes to local vascular inflammation and potential hemorrhage. Pseudocysts contain activated lytic enzymes such as elastase which can result in erosion of adjacent vessel walls and hemorrhage. Intrapancreatic or extrapancreatic arterial aneurysm or PSA is another major cause of HP, with the commonly involved vessels being splenic, gastroduodenal, pancreaticoduodenal, or hepatic arteries[41].
A review of the literature and cases between 1977 and 2020 by Cui et al[42] in 2021 reviewed the variable clinical presentations of HP. GI bleed (melena) was the most commonly reported symptom at 52%, followed by abdominal pain at 46%. Other symptoms include; nausea, vomiting, loss of appetite, weight loss, and lower GI bleeding (hematochezia).
GI bleed is generally intermittent, likely secondary to clot formation in the pancreatic duct leading to the cessation of GI bleed but persistent abdominal pain. As time passes, clot resolution occurs, leading to rebleeding and abdominal pain improvement due to decreased intraductal pressure[42].
Diagnosis and evaluation of HP remain a challenge due to the vague and variable presentation of HP. A retrospective single-center study by Yashavanth et al[43] in 2021 evaluated patients with suspected HP over a 10-year period. The study showed that the median duration of bleeding prior to diagnosis was ten days, with 40.2% of patients exhibiting symptoms for > 1 mo. The study showed that 62% of patients with HP had evidence of visceral artery aneurysms. Upper GI endoscopy showed evidence of bleeding in 64.4% of patients, and angiography was successful in localizing the source of bleeding in 94.2% of cases[43].
Serum testing (amylase, lipase, or bilirubin levels) in cases of HP is of limited use with possible hyperbilirubinemia due to pancreaticobiliary reflux and elevations in amylase and lipase in cases of pancreatitis. Therefore, the primary modes of diagnosis are through imaging modalities. Upper GI endoscopy is crucial in patients with HP and can show evidence of bleeding through the ampulla of Vater via the use of side-facing endoscopy in addition to ruling out other causes of GI bleed. Bleeding in the second part of the duodenum should prompt the suspicion of HP. Rates of upper GI endoscopy for detection of HP have been variable and range between 30%-65%. This can be explained due to the intermittent nature of the bleeding[44].
ERCP could be used as it can view filling defects in pancreatic ducts, which can aid in the diagnosis; however, this modality is not commonly utilized by clinicians. Ultrasonography with Doppler can reveal the presence of aneurysms or pseudocyst; however, it is only positive in approximately 38% of cases. Abdominal CT with contrast is commonly used in pancreatic pathology to diagnose and can show intraductal blood clots, aneurysmal opacification, pseudocyst presence, and/or contrast persistence following the arterial phase. Abdominal CT with contrast often aids in the diagnosis in 90% of cases of HP cases. However, angiography remains the gold standard for diagnosis of HP due to accurate localization of the bleeding or abnormal vessel, visualization of the arterial anatomy, and potential for therapeutic intervention.
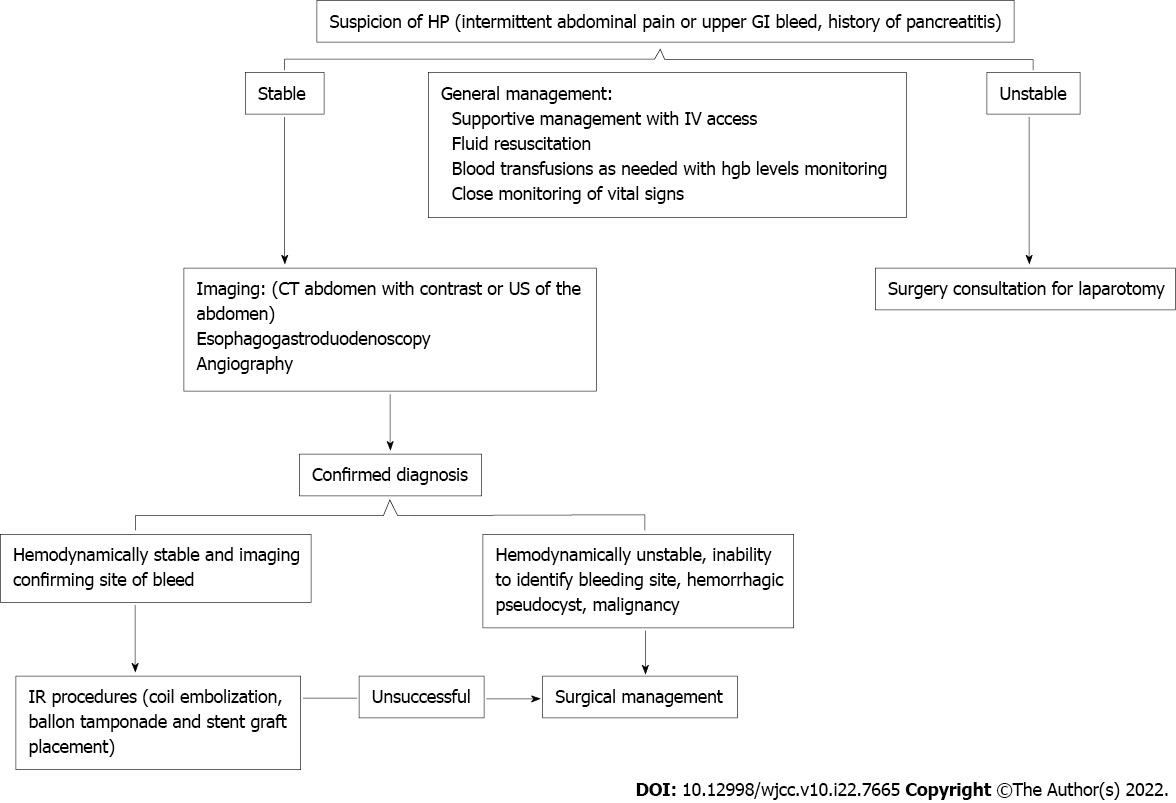
The management of HP should focus on maintaining stable hemodynamics, intravenous hydration, blood transfusion as needed, and controlling the source of bleeding (Figure 1). Therefore, supportive management is not recommended and is associated with high mortality (up to 90%). The approach to HP management is dependent on patients’ hemodynamics. Interventional radiology (IR) procedures such as coil embolization, balloon tamponade, and stent-graft placement can be performed in stable patients with immediate positive results in 79%-100% of patients and an overall success rate of 67%. Recurrence rates following interventional radiology procedures are estimated to be 30%. Recurrence etiology remains unclear. However, one theory proposed bleeding from collateral vessels in the adjacent diseased pancreas[45].
In cases with hemodynamic instability, unclear angiography findings, hemorrhagic pseudocyst, or IR procedure failure, a surgical approach should be pursued. Surgical procedures include; pancreatectomy (complete or partial) with or without splenectomy, pseudocyst excision, culprit blood vessel ligation, or bypass graft placement. The surgical approach carries high success rates of 70%-80%; however, this comes with a high mortality rate of 10%-50%. Nonetheless, surgical management has lower bleeding recurrence rates of 0-5%[39,46,47].
Intraabdominal hemorrhage can also occur in cases of pancreatitis. Several case reports have been published in the literature, with few studies done to address this issue. A retrospective study in a single center in Germany by Phillip et al[48] in 2013 evaluated spontaneous bleeding in pancreatitis patients and the treatment by transcatheter arterial embolization. In their study, intraabdominal hemorrhage was reported in < 1% of patients with pancreatitis, and 73% of those with hemorrhage had evidence of necrotizing pancreatitis. The most common source of bleeding was arterial, with one case of splenic vein hemorrhage. The diagnosis was made with contrast-enhanced CT in all patients, and angiography showed evidence of active bleeding in 57% of the cases. Patients were treated with transcatheter coiled embolization, and the in-hospital mortality was 36%; however, only 7% of the mortality was attributed directly to intraabdominal hemorrhage. Rebleeding following coil embolization was noted to be 14%[48].
A retrospective study by Chen et al[49] in 2017 evaluated the prevalence and characteristics of pancreatitis patients with intraabdominal hemorrhage. The prevalence of intraabdominal bleeding in pancreatitis patients in the study was 3.4%, with the risk factors being a high CT severity index and creatinine elevation with statistical significance. The median time between pancreatitis onset and bleeding was 17.5 d. Management of intraabdominal bleeding depends on the patient’s hemodynamic stability and can be through IR or surgical procedures.
Vascular complications of pancreatitis carry high morbidity and mortality if untreated. The prevalence of the vascular complications of pancreatitis has been studied primarily through radiological research and is estimated to be present in up to 25% of the patients. Knowledge of the possible complications can lead to timely diagnosis and management. A multidisciplinary approach tailored for individual patient care is the best approach. Over the years, minimally invasive procedures have shown good results in select patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society of Critical Care Medicine.
Specialty type: Medicine, general and internal
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Covino M, Italy; Chi T, China S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 820] [Article Influence: 82.0] [Reference Citation Analysis (1)] |
| 2. | Ahmed M, Aziz MU, Mansoor MA, Anwar S. Vascular complications in cases of acute pancreatitis - CT scan based study. J Pak Med Assoc. 2016;66:977-989. [PubMed] |
| 3. | Jiang ZQ, Xiao B, Zhang XM, Xu HB. Early-phase vascular involvement is associated with acute pancreatitis severity: a magnetic resonance imaging study. Quant Imaging Med Surg. 2021;11:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 4. | Mortelé KJ, Mergo PJ, Taylor HM, Wiesner W, Cantisani V, Ernst MD, Kalantari BN, Ros PR. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol. 2004;52:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Maatman TK, Heimberger MA, Lewellen KA, Roch AM, Colgate CL, House MG, Nakeeb A, Ceppa EP, Schmidt CM, Zyromski NJ. Visceral artery pseudoaneurysm in necrotizing pancreatitis: incidence and outcomes. Can J Surg. 2020;63:E272-E277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Hoilat GJ, Mathew G, Ahmad H. Pancreatic pseudoaneurysm. In: StatPearls. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Dörffel T, Wruck T, Rückert RI, Romaniuk P, Dörffel Q, Wermke W. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas. 2000;21:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Udd M, Leppäniemi AK, Bidel S, Keto P, Roth WD, Haapiainen RK. Treatment of bleeding pseudoaneurysms in patients with chronic pancreatitis. World J Surg. 2007;31:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Pang TC, Maher R, Gananadha S, Hugh TJ, Samra JS. Peripancreatic pseudoaneurysms: a management-based classification system. Surg Endosc. 2014;28:2027-2038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Gurala D, Polavarapu AD, Idiculla PS, Daoud M, Gumaste V. Pancreatic Pseudoaneurysm from a Gastroduodenal Artery. Case Rep Gastroenterol. 2019;13:450-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr. 2015;39:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Bergert H, Hinterseher I, Kersting S, Leonhardt J, Bloomenthal A, Saeger HD. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery. 2005;137:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 13. | Nadkarni NA, Khanna S, Vege SS. Splanchnic venous thrombosis and pancreatitis. Pancreas. 2013;42:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Junare PR, Udgirkar S, Nair S, Debnath P, Jain S, Modi A, Rathi P, Rane S, Contractor Q. Splanchnic Venous Thrombosis in Acute Pancreatitis: Does Anticoagulation Affect Outcome? Gastroenterology Res. 2020;13:25-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Butler JR, Eckert GJ, Zyromski NJ, Leonardi MJ, Lillemoe KD, Howard TJ. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB (Oxford). 2011;13:839-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 16. | Roch AM, Maatman TK, Carr RA, Colgate CL, Ceppa EP, House MG, Lopes J, Nakeeb A, Schmidt CM, Zyromski NJ. Venous Thromboembolism in Necrotizing Pancreatitis: an Underappreciated Risk. J Gastrointest Surg. 2019;23:2430-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Maatman TK, McGuire SP, Lewellen KA, McGreevy KA, Ceppa EP, House MG, Nakeeb A, Nguyen TK, Schmidt CM, Zyromski NJ. Prospective Analysis of the Mechanisms Underlying Ineffective Deep Vein Thrombosis Prophylaxis in Necrotizing Pancreatitis. J Am Coll Surg. 2021;232:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Easler J, Muddana V, Furlan A, Dasyam A, Vipperla K, Slivka A, Whitcomb DC, Papachristou GI, Yadav D. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Pancreas Study Group; Chinese Society of Gastroenterology; Chinese Medical Association. . Practice guidance for diagnosis and treatment of pancreatitis-related splanchnic vein thrombosis (Shenyang, 2020). J Dig Dis. 2021;22:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Chandan S, Buddam A, Khan SR, Mohan BP, Ramai D, Bilal M, Dhindsa B, Bhogal N, Kassab LL, Goyal H, Perisetti A, Facciorusso A, Adler DG. Use of therapeutic anticoagulation in splanchnic vein thrombosis associated with acute pancreatitis: a systematic review and meta-analysis. Ann Gastroenterol. 2021;34:862-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Zhou J, Zhang H, Mao W, Ke L, Li G, Ye B, Zhang J, Lin J, Gao L, Tong Z, Li W. Efficacy and Safety of Early Systemic Anticoagulation for Preventing Splanchnic Thrombosis in Acute Necrotizing Pancreatitis. Pancreas. 2020;49:1220-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Anis FS, Adiamah A, Lobo DN, Sanyal S. Incidence and treatment of splanchnic vein thrombosis in patients with acute pancreatitis: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 23. | Garcia-Rodriguez V, Jacob R, daSilva-deAbreu A. A Rare Case of Pancreatitis-Induced Thrombosis of the Aorta and Superior Mesenteric Artery. Methodist Debakey Cardiovasc J. 2019;15:220-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Mishreki AP, Bowles MJ. A case of widespread aortic thrombosis secondary to acute severe pancreatitis. Ann R Coll Surg Engl. 2011;93:e17-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Keskin M, Gümüşdağ A, Börklü EB, Dayı ŞÜ, Avcı İİ, Güvenç TS, Güngör B, Karabay CY, Kozan Ö. An unexpected complication of acute pancreatitis: Intra-cardiac thrombus. Am J Emerg Med. 2017;35:801.e1-801.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Chong BK, Yun JK, Kim JB, Park DH. Multiple Ascending Aortic Mural Thrombi and Acute Necrotizing Mediastinitis Secondary to Acute Pancreatitis. Korean J Thorac Cardiovasc Surg. 2016;49:401-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Chait J, Duffy E, Marks N, Rajaee S, Hingorani A, Ascher E. Superior Mesenteric Artery Thrombosis after Necrotizing Pancreatitis. Ann Vasc Surg. 2019;59:307.e17-307.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Thajudeen B, Budhiraja P, Bracamonte ER. Bilateral renal artery thrombosis secondary to acute necrotizing pancreatitis. Clin Kidney J. 2013;6:503-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Kaman L, Behera A, Singh R, Katariya RN. Internal pancreatic fistulas with pancreatic ascites and pancreatic pleural effusions: recognition and management. ANZ J Surg. 2001;71:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Brown A, Malden E, Kugelmas M, Kortz E. Diagnosis of pancreatic duct-portal vein fistula; a case report and review of the literature. J Radiol Case Rep. 2014;8:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Rasmussen IC, Karlson BM, Löfberg AM. Biliary pancreatic portal fistula as a complication of chronic pancreatitis: a case report with review of the literature. Ups J Med Sci. 2006;111:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Makary MS, Gayou EL, Kyrouac D, Shah ZK. Pancreaticoportal Fistula Formation as a Consequence of Recurrent Acute Pancreatitis: Clinical and Imaging Considerations. J Clin Imaging Sci. 2018;8:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Van Steenbergen W, Ponette E. Pancreaticoportal fistula: a rare complication of chronic pancreatitis. Gastrointest Radiol. 1990;15:299-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Yamamoto T, Hayakawa K, Kawakami S, Nishimura K, Katsuma Y, Hayashi N, Maeda M, Ishii Y. Rupture of a pancreatic pseudocyst into the portal venous system. Abdom Imaging. 1999;24:494-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Chang LH, Francoeur L, Schweiger F. Pancreaticoportal fistula in association with antiphospholipid syndrome presenting as ascites and portal system thrombosis. Can J Gastroenterol. 2002;16:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Dawson BC, Kasa D, Mazer MA. Pancreatic pseudocyst rupture into the portal vein. South Med J. 2009;102:728-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Riddell A, Jhaveri K, Haider M. Pseudocyst rupture into the portal vein diagnosed with MRI. Br J Radiol. 2005;78:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Liu H, Phillips A, Sholosh B, Novelli P, Romutis S, D'Alesio M, Lebowitz S, Singh H, Yadav D, Zureikat A, Lee K, Paniccia A, Dasyam AK. Pancreatic-Portal Vein Fistula: a Rare Diagnosis with Wide-Ranging Complications-13-Year Experience of a Pancreas Center of Excellence. J Gastrointest Surg. 2021;25:3137-3148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Yu P, Gong J. Hemosuccus pancreaticus: A mini-review. Ann Med Surg (Lond). 2018;28:45-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Toyoki Y, Hakamada K, Narumi S, Nara M, Ishido K, Sasaki M. Hemosuccus pancreaticus: problems and pitfalls in diagnosis and treatment. World J Gastroenterol. 2008;14:2776-2779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Cui HY, Jiang CH, Dong J, Wen Y, Chen YW. Hemosuccus pancreaticus caused by gastroduodenal artery pseudoaneurysm associated with chronic pancreatitis: A case report and review of literature. World J Clin Cases. 2021;9:236-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Yashavanth HS, Jagtap N, Singh JR, Ramchandani M, Lakhtakia S, Tandan M, Gupta R, Vamsi M, Bhaware B, Rao GV, Reddy DN. Hemosuccus Pancreaticus: A systematic approach. J Gastroenterol Hepatol. 2021;36:2101-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Vimalraj V, Kannan DG, Sukumar R, Rajendran S, Jeswanth S, Jyotibasu D, Ravichandran P, Balachandar TG, Surendran R. Haemosuccus pancreaticus: diagnostic and therapeutic challenges. HPB (Oxford). 2009;11:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Rammohan A, Palaniappan R, Ramaswami S, Perumal SK, Lakshmanan A, Srinivasan UP, Ramasamy R, Sathyanesan J. Hemosuccus Pancreaticus: 15-Year Experience from a Tertiary Care GI Bleed Centre. ISRN Radiol. 2013;2013:191794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Evans RP, Mourad MM, Pall G, Fisher SG, Bramhall SR. Pancreatitis: Preventing catastrophic haemorrhage. World J Gastroenterol. 2017;23:5460-5468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Kempeneers MA, Issa Y, Ali UA, Baron RD, Besselink MG, Büchler M, Erkan M, Fernandez-Del Castillo C, Isaji S, Izbicki J, Kleeff J, Laukkarinen J, Sheel ARG, Shimosegawa T, Whitcomb DC, Windsor J, Miao Y, Neoptolemos J, Boermeester MA; Working group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. International consensus guidelines for surgery and the timing of intervention in chronic pancreatitis. Pancreatology. 2020;20:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 48. | Phillip V, Rasch S, Gaa J, Schmid RM, Algül H. Spontaneous bleeding in pancreatitis treated by transcatheter arterial coil embolization: a retrospective study. PLoS One. 2013;8:e72903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Chen Y, Zhou J, Li G, Tong Z, Dong J, Pan Y, Ke L, Li W, Li J. Early Spontaneous Abdominal Bleeding is associated with Poor Outcome in Moderate to Severe Acute Pancreatitis Patients: A Propensity Matched Study. Sci Rep. 2017;7:42607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |