Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7293
Peer-review started: March 15, 2022
First decision: April 13, 2022
Revised: April 25, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: July 26, 2022
Processing time: 117 Days and 23.1 Hours
The incidence of breast cancer in China is increasing while its mortality rate is decreasing. The annual breast cancer incidence in China is 39.2 million, accounting for two-thirds of the urban population. In China, breast cancer is the fifth most common malignant tumor overall and the most common in women, accounting for 17% of female malignant tumors.
To investigate the accuracy of strain ultrasound elastography (SUE) on the evaluation of preoperative neoadjuvant chemotherapy (NAC) in breast cancer.
Overall, 90 patients with breast cancer treated at our hospital between January 2018 and February 2019 were selected for this study. The patients received six cycles of NAC with docetaxel, epirubicin, and cyclophosphamide. Surgical treatment was also performed, and pathological reactivity was assessed. The patients were evaluated using conventional ultrasonography and SUE before biopsy. The differences between groups were analyzed to calculate the mean and standard deviation with significance measured using a t-test, while multivariate analysis was performed using logistic regression analysis.
Of the patients analyzed, 20 had a pathological complete remission (pCR) while 70 did not achieve pCR after NAC. The ratio of the elastic strain ratio (SR) and elastic score of 4–5 in patients with pCR were 5.5 ± 1.16 and 15.00%, respectively; these were significantly lower than those in patients without pCR (85%) and significantly higher than in patients without pCR (14%). SR and elastic score 4–5 were independent factors influencing NAC efficacy (OR=0.644, 1.426 and 1.366, respectively, P < 0.05). SR was positively correlated with elasticity score (rs = 0.411, P < 0.05). The area under the receiver operator characteristic curve of SR and SR combined with elastic score in predicting patients without pCR was 0.822 and 0.891, respectively (P < 0.05).
Strain ultrasonic elastography may be used to evaluate the effects of preoperative NAC in patients with breast cancer.
Core Tip: The changes in a malignant tumor should be evaluated to determine the effects of preoperative neoadjuvant chemotherapy in breast cancer. However, conventional ultrasonography is not as sensitive as the current pathological reactivity detection method and may not meet clinical needs in determining the effect of neoadjuvant chemotherapy. A relatively novel method called strain ultrasound elastography was used in 90 patients with breast cancer in this study. It was found to be more effective and sensitive in the evaluation of the effects of neoadjuvant chemotherapy in patients with breast cancer.
- Citation: Pan HY, Zhang Q, Wu WJ, Li X. Preoperative neoadjuvant chemotherapy in patients with breast cancer evaluated using strain ultrasonic elastography. World J Clin Cases 2022; 10(21): 7293-7301
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7293.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7293
There is a high incidence of breast cancer in women. The use of adjuvant chemotherapy can effectively reduce mortality from breast cancer through treatment and prevention of recurrence[1]. Neoadjuvant chemotherapy (NAC) improves the efficacy of conventional treatments for breast cancer and helps prevent metastasis. However, there are individual differences in efficacy. Among these differences, pathological reactivity is an important factor affecting the efficacy of NAC. Therefore, it is necessary to predict the pathological reactivity of patients with breast cancer before starting NAC[2].
Strain ultrasound elastography (SUE) is in the stage of observation and research, and may be applied to the breast, thyroid, prostate, and other tissues of the body. The elastic coefficient between the lesion or tumor and the surrounding normal tissue produces different strain sizes and is displayed in different colors. This ultrasound technology has helped determine the size of malignant lesions through the detection of strain sizes[3]. This study aimed to investigate the accuracy of SUE in the evaluation of preoperative NAC and its related factors in patients with breast cancer.
Ninety patients with breast cancer who were treated in our hospital between January 2018 and February 2019 were selected. They were 29–67 years old, with an average age of 49.80 ± 9.11 years. Of these, 78 patients were diagnosed with invasive ductal carcinoma and 12 with invasive lobular carcinoma. The inclusion criteria for the patients were as follows: female patients; informed consent provided by the patients and their families; SUE performed in our hospital; clinical stage II-III; completed six cycles of NAC consisting of docetaxel (75 mg/m2), epirubicin (75 mg/m2), and cyclophosphamide (500 mg/m2); underwent surgical treatment; and pathologically diagnosed with invasive carcinoma. The exclusion criteria for the patients were as follows: underwent radiotherapy, chemotherapy, and other anti-tumor treatments before the study period; had other systemic malignancies; or had other serious diseases such as immune system diseases, endocrine diseases, or liver and kidney dysfunction.
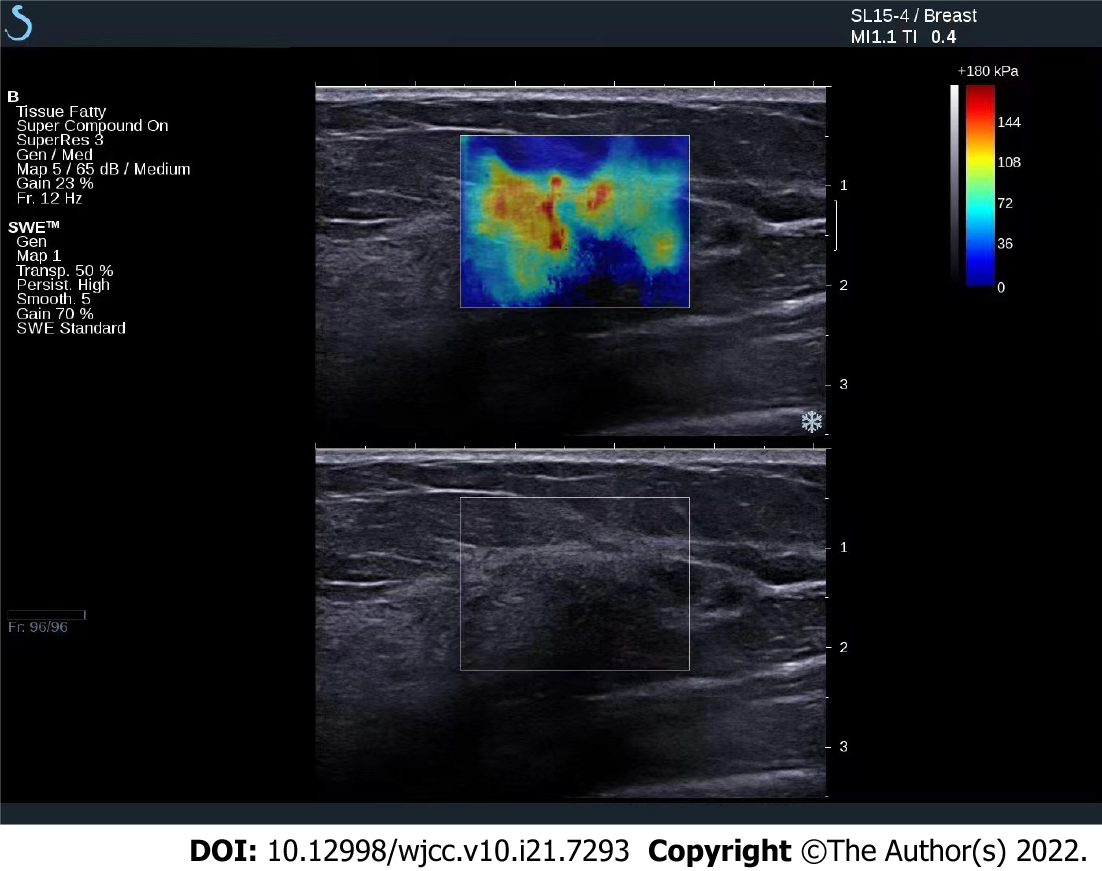
Conventional ultrasound and SUE were performed before tumor biopsy using our hospital’s HV800 ultrasound apparatus with a probe frequency of 8–12 Mhz. Conventional ultrasound examination of the breast was performed first to determine the tumor morphology, boundary, aspect ratio, surrounding tissue, echo, and blood flow changes. SUE was performed thereafter; the region of interest selected was more than twice the size of the tumor, the area with the tumor was exposed to slight vibration, compression frequency was 3–4 Hz, the depth of the probe was at the subcutaneous layer of the skin, adjacent to the muscle layer. The probe was placed perpendicular to the patient’s intact skin; the angle of the probe was adjusted in real time to maintain its perpendicular placement. Measurement of the elastic strain ratio (SR) of the axial adipose tissue to lesion was performed after stable image storage.
The images were assessed using a five-point scoring system[4], as follows: 1 point was assigned if the image was totally green, signifying a completely deformed tumor; 2 points, if the image was mainly green with visible blue, signifying that most of the tumor was deformed, but a small portion remained intact; 3 points, if the image of the area surrounding the tumor was green and deformed while the central area was blue and intact; 4 points, if the image of the tumor was blue overall and without any deformation; and 5 points, if the tumor and its surrounding tissue were blue, signifying no deformation. Hence, a score of 1 to 3 pertains to a low elasticity score, while a score of 4 to 5 pertains to a high elasticity score.
All patients received six cycles of NAC, consisting of docetaxel (75 mg/m2), epirubicin (75 mg/m2), and cyclophosphamide (500 mg/m2). They also underwent surgical treatment to determine their response to the chemotherapy through pathological examination. The patients who were assessed to have human epidermal growth factor receptor 2 positive tumors through fluorescence in situ hybridization received trastuzumab treatment. The doses of trastuzumab administered to the patients were 8 mg/kg body weight on the first cycle followed by 6 mg/kg body weight on the successive five cycles, with each cycle administered once every 3 wk.
Pathological examination of the breast tissue was performed after surgical treatment (Figure 1). The Miller–Payne classification was used to determine pathological remission of malignancy through microscopic assessment. Based on this classification, grade 1 pertains to the unchanged number of invasive cancer cells; grade 2 to the less than 30% decrease in the amount of invasive cancer cells before chemotherapy; grade 3 to the 30%–90% decrease in the amount of invasive cancer cells compared with that before chemotherapy; grade 4 to more than 90% decrease in the amount of invasive cancer cells compared with that before chemotherapy, and with only scattered small cluster cells or single cancer cells retained; and grade 5 to pathological complete remission (pCR) where there are no invasive cancer cells in the original tumor bed. Grades 1–4 are defined as without pCR, and grade 5 is defined as with pCR.
Data analysis was performed using SPSS 22.0 software. Nominal data were expressed as percentages. The differences between the groups, such as their SRs, were analyzed to calculate the mean ± SD and statistical significance analyzed using a t-test. Multivariate analysis was performed using logistic regression analysis; the prediction value of SR was analyzed using the receiver operator characteristic (ROC) curve. Statistical significance was set at P < 0.05.
Pathological results of 90 patients after NAC chemotherapy: 14 cases of grade 1, 28 cases of grade 2, 17 cases of grade 3, 11 cases of grade 4, and 20 cases of grade 5. There were 20 pCR patients and 70 non-pCR patients.
The ratio of SR and elasticity scores of 4 to 5 in pCR patients was significantly lower than that of non-pCR patients (P < 0.05, Table 1).
| Groups | Cases | SR | Elasticity score 4-5, n (%) |
| pCR | 20 | 5.50 ± 1.16 | 3 (15.00) |
| Non-pCR | 70 | 12.29 ± 6.60 | 54 (77.14) |
| t/χ2 value | -4.563 | 25.868 | |
| P value | 0.000 | 0.000 |
The clinical data were compared between patients with elasticity scores of 4 to 5 and 1 to 3 points, and the difference was not statistically significant (P > 0.05), as shown in Table 2. SR in patients with elastic score 4–5 was significantly higher than that in patients with elastic score 1–3 (P < 0.05). As shown in Table 3.
| Groups | Elasticity score 4-5 | Elasticity score 1-3 | t value | P value |
| Cases | 57 | 33 | ||
| Age (yr) | 50.06 ± 8.02 | 50.82 ± 9.11 | -0.412 | 0.681 |
| ER positive | 35 (61.40) | 18 (54.55) | 0.406 | 0.524 |
| PR positive | 30 (52.63) | 16 (48.48) | 0.144 | 0.705 |
| Ki-67 > 14% | 32 (56.14) | 19 (57.58) | 0.018 | 0.895 |
| HER2 positive | 14 (24.56) | 5 (15.15) | 1.111 | 0.292 |
| Lesion diameter (cm) | 4.35 ± 1.13 | 4.30 ± 1.10 | 0.204 | 0.839 |
| Group | Cases | SR | t value | P value |
| Elasticity score 4-5 | 57 | 11.28 ± 1.43 | 4.271 | 0.000 |
| Elasticity score 1-3 | 33 | 9.92 ± 1.50 |
The proportion of Ki-67 > 14% in pCR patients was significantly higher than that in non-pCR patients (P < 0.05). The age, lesion diameter, and estrogen receptor positivity were compared between pCR patients and non-pCR patients, and the difference was not statistically significant (P > 0.05, Table 4).
| General clinical data | pCR | Non-pCR | t/χ2 value | P value |
| Cases | 20 | 70 | ||
| Age (yr) | 49.02 ± 8.19 | 51.02 ± 9.92 | -0.824 | 0.412 |
| Lesion diameter (cm) | 4.42 ± 1.23 | 4.12 ± 1.20 | 0.981 | 0.329 |
| ER positive | 12 (60.00) | 41 (58.57) | 0.013 | 0.909 |
| PR positive | 11 (55.00) | 35 (50.00) | 0.156 | 0.693 |
| Ki-67 > 14% | 17 (85.00) | 34 (48.57) | 8.407 | 0.004 |
| HER2 positive | 7 (35.00) | 12 (17.14) | 2.003 | 0.157 |
| Pathological type | 0.386 | 0.534 | ||
| Invasive ductal carcinoma | 16 (80.00) | 62 (88.57) | ||
| Invasive lobular carcinoma | 4 (20.00) | 8 (11.43) | ||
| Molecular subtypes | 2.548 | 0.467 | ||
| Triple negative | 3 (15.00) | 10 (14.29) | ||
| HER2 | 6 (30.00) | 23 (32.86) | ||
| Luminal B | 11 (55.00) | 30 (42.86) | ||
| Luminal A | 0 (0.00) | 7 (10.00) |
Spearman rank correlation analysis of the above indicators showed that SR was positively correlated with the elasticity score (rs = 0.411, P < 0.05), and there was no correlation between SR and elasticity score (Table 5).
| Index | SR | |
| rs value | P value | |
| Age | 0.193 | 0.304 |
| Lesion diameter | 0.182 | 0.353 |
| ER positive | 0.082 | 0.405 |
| PR positive | 0.100 | 0.722 |
| Ki-67 > 14% | 0.092 | 0.565 |
| HER2 positive | 0.182 | 0.672 |
| Elasticity score | 0.411 | 0.000 |
The clinical data of patients were used as independent variables, and the NAC efficacy was used as the dependent variable (pCR assignment 0, non-pCR assignment 1) for logistic regression analysis. The results showed that Ki-67 > 14%, SR, and elasticity scores 4–5 were independent influencing factors of NAC efficacy (OR = 0.644, 1.426, and 1.366, respectively, P < 0.05, Table 6).
| Factor | β | SE | Walds | P value | OR (95%CI) |
| Ki-67 > 14% | -0.440 | 0.132 | 11.111 | 0.000 | 0.644 (0.497-0.834) |
| SR | 0.355 | 0.122 | 8.467 | 0.000 | 1.426 (1.123-1.811) |
| Elasticity score 4-5 | 0.312 | 0.2112 | 7.760 | 0.000 | 1.366 (1.097-1.702) |
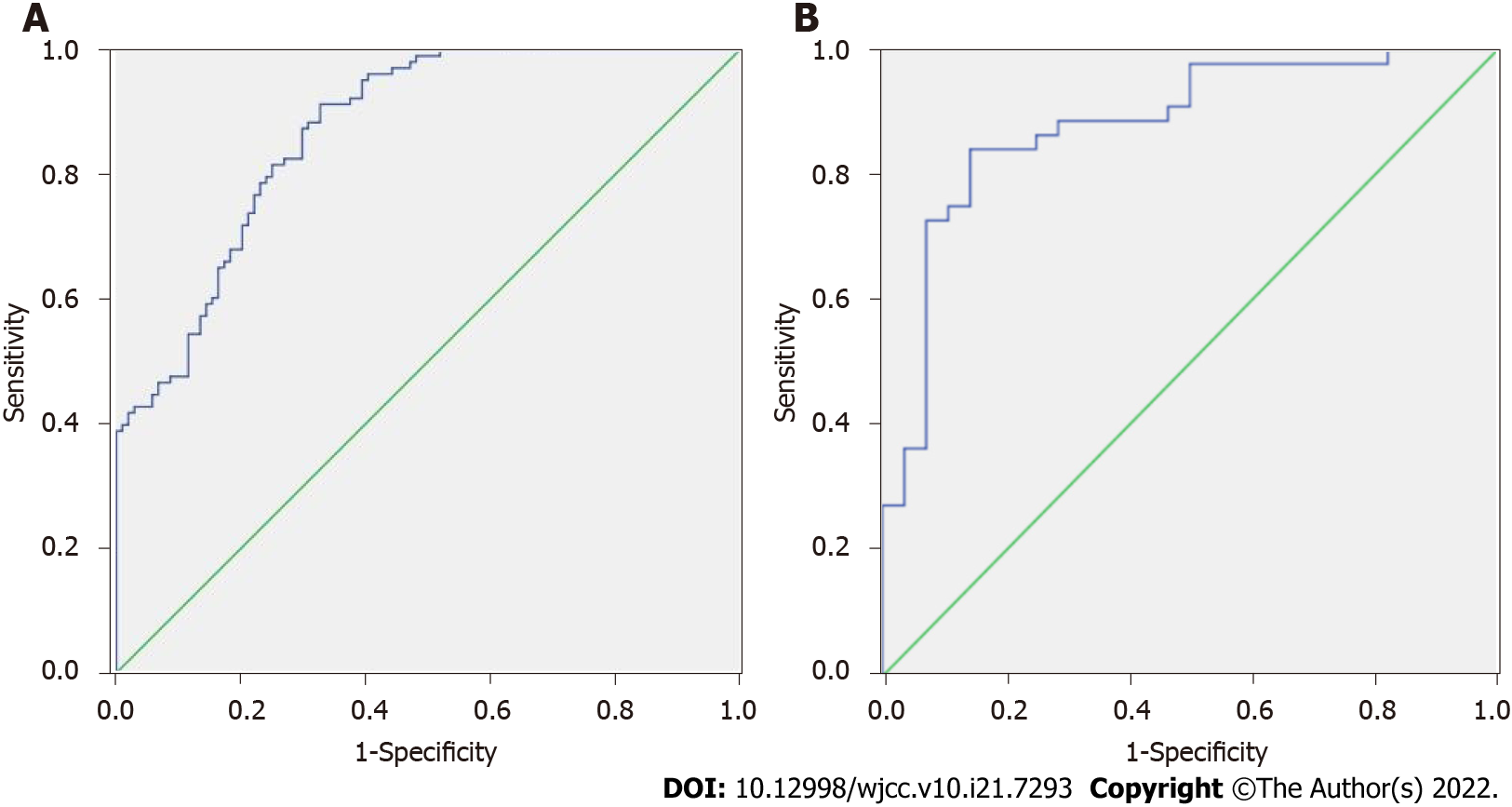
The area under ROC curve of SR and SR combined with elasticity score in predicting non-pCR was 0.822 and 0.891, respectively (P < 0.05, Figure 2A and B, Table 7).
| Index | Area under curve | P value | Sensitivity | Specificity |
| SR | 0.802 | 0.000 | 80.00% | 70.00% |
| SR combined flexibility score | 0.891 | 0.000 | 82.00% | 83.00% |
NAC is an important supplement to the treatment of breast cancer and can significantly reduce the recurrence of malignant tumors in patients. It helps improve the prognosis of patients and the attainment of pCR. However, some previous studies[5-7] have found significant differences in the pathological response of individuals after NAC. The diagnosis of pathological remission is of great significance for individualized treatment and prognosis. However, there are differences in the sensitivity of the current NAC pathological reactivity detection methods, and simple conventional ultrasound cannot meet the clinical needs.
Previous literature[8-10] suggests that ultrasound elastography may have potential value in determining the pathological reactivity of malignant tumors after NAC, as patients with low elasticity scores were more likely to achieve complete tumor remission. Other studies[11,12] also suggested that the hardness of the tissue in breast cancer evaluated by ultrasound elastography was closely related to NAC reactivity. This study found that the SR and elasticity scores of 4–5 in patients with pCR were significantly lower than those in patients without pCR. The SR of patients with elasticity score of 4–5 was significantly higher than that of patients with elasticity score of 4–5. These results suggest that there is a low elasticity score in patients with breast cancer with complete remission; the proportion of SR lesions also decreased significantly. The SUE examination used the characteristics of uneven elastic coefficient distribution in the tissue; consequently, the strain distribution will also vary due to the unevenness of the elasticity in the tissue. The strain coefficient is small in an area with a large elastic coefficient; on the contrary, the strain coefficient is large in an area with a small elastic coefficient.
The Ki-67 antigen is a popular biological indicator in the study of various malignant tumors, especially breast cancer. Currently, Ki-67 antigen detection is generally used to describe cell proliferation in malignant tumors and to achieve targeted personalized treatment[13-15]. In this study, the comparison of clinical general data between patients with and without pCR showed that the proportion of Ki-67 was > 14% in patients with pCR and was significantly higher than that in patients without pCR. This suggests that the amount of Ki-67 expression in the tumor may be an important factor that affects the prognosis of NAC treatment in patients with breast cancer. The expression of Ki-67 in tumor proliferation covers all phases of the cycle except the G0 phase, hence the proportion of Ki-67 >14% in patients with pCR is higher. Related studies[16-18] also suggested that proliferating-cell nuclear antigen and Ki-67 could be used to detect the proliferation of breast cancer cells, and that they could be used as substitutes for each other. However, Ki-67 is more useful since it is expressed in all phases of the proliferative cycle of the malignant cells.
Multivariate analysis showed that Ki-67 > 14%, SR, and elasticity scores 4–5 independently influenced the efficacy of NAC. The clinical data of patients were the independent variables, and the NAC efficacy was the dependent variable. The SR values measured by SUE showed a high sensitivity and specificity in predicting pCR in patients with breast cancer. The expression of Ki-67 in low-differentiated adenocarcinoma tissues was significantly higher than that in medium and highly differentiated adenocarcinoma tissues; this indicates that the positive degree of Ki-67 staining was correlated with histological grade and was related to the occurrence and development of breast cancer. High Ki-67 expression signifies poor prognosis and is an important reference value for the diagnosis, treatment, and evaluation of breast cancer. SUE is an important supplement to the two-dimensional gray-scale ultrasound and can better evaluate the hardness of breast masses with good repeatability[19,20]. Unlike conventional two-dimensional gray-scale ultrasound, SUE can evaluate the hardness of lesions and indirectly reflect the composition of the tissue matrix. Therefore, the main potential benefit of SUE is a relatively accurate assessment of residual cancer after NAC. It is especially suitable for the assessment of cribriform, multinodular, and alternative atrophy patterns of residual cancer. Patients without pCR may have a non-uniform density of their breast mass that may be detected as a higher proportion of SR values and an elasticity score of 4–5 points. These explain the potential of the SR value and elasticity score of 4–5 points as predictors of the efficacy of NAC in patients with breast cancer.
Previous studies have mainly focused on the treatment and prognosis of patients with breast cancer after receiving NAC treatment. There are only a few studies and existing information on the prediction of pathological reactivity. This study analyzed and explored the pathological reactivity of breast cancer after NAC. It analyzed the possible mechanism of this change. This may potentially serve as a reference for the individualized treatment and prognosis of clinical NAC-assisted breast cancer treatment. In addition, this study also conducted an in-depth discussion on the diagnosis and efficacy analysis of breast cancer based on the relevant parameters of SUE technology that can provide a reference for clinical practice. However, this study also has a few shortcomings in that the sample size is relatively small and the findings will need to be confirmed by further large-sample studies.
Breast cancer has the seventh highest mortality rate of all cancers in China and has the fifth highest cancer mortality in women, accounting for 8.2% of all female malignant tumors and seriously impacting women’s health and quality of life.
Early detection, diagnosis, and treatment are key to reducing mortality and improving cure rates.
Strain ultrasound elastography (SUE) is a new type of ultrasound technology that can determine relative tissue hardness by evaluating the desmoplastic changes in a tissue to differentiate between benign and malignant tumors.
It is a way to diagnose tumors and diffuse diseases that cannot be detected by conventional ultrasound.
Of the patients analyzed, 20 had a pathological complete remission (pCR) while 70 did not achieve pCR after neoadjuvant chemotherapy (NAC).
Strain ultrasonic elastography may be used to evaluate the effects of preoperative NAC in patients with breast cancer.
SUE is a new type of ultrasound technology that can determine relative tissue hardness by evaluating the desmoplastic changes in a tissue to differentiate between benign and malignant tumors.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lynce F, United States; Teo SH, Malaysia S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Shimoyama D, Shitara H, Hamano N, Ichinose T, Sasaki T, Yamamoto A, Kobayashi T, Tajika T, Takagishi K, Chikuda H. Reliability of shoulder muscle stiffness measurement using strain ultrasound elastography and an acoustic coupler. J Med Ultrason (2001). 2021;48:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Evans A, Sim YT, Pourreyron C, Thompson A, Jordan L, Fleming D, Purdie C, Macaskill J, Vinnicombe S, Pharoah P. Pre-operative stromal stiffness measured by shear wave elastography is independently associated with breast cancer-specific survival. Breast Cancer Res Treat. 2018;171:383-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Akimoto E, Kadoya T, Kajitani K, Emi A, Shigematsu H, Ohara M, Masumoto N, Okada M. Role of 18F-PET/CT in Predicting Prognosis of Patients With Breast Cancer After Neoadjuvant Chemotherapy. Clin Breast Cancer. 2018;18:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Cabrera-Galeana P, Muñoz-Montaño W, Lara-Medina F, Alvarado-Miranda A, Pérez-Sánchez V, Villarreal-Garza C, Quintero RM, Porras-Reyes F, Bargallo-Rocha E, Del Carmen I, Mohar A, Arrieta O. Ki67 Changes Identify Worse Outcomes in Residual Breast Cancer Tumors After Neoadjuvant Chemotherapy. Oncologist. 2018;23:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Yang X, Zhai D, Zhang T, Zhang S. Use of strain ultrasound elastography versus fine-needle aspiration cytology for the differential diagnosis of thyroid nodules: a retrospective analysis. Clinics (Sao Paulo). 2020;75:e1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Ersoy YE, Kadioglu H. Review of Novel Sentinel Lymph Node Biopsy Techniques in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy. Clin Breast Cancer. 2018;18:e555-e559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 593] [Cited by in RCA: 732] [Article Influence: 104.6] [Reference Citation Analysis (0)] |
| 8. | Della Pepa GM, Menna G, Stifano V, Pezzullo AM, Auricchio AM, Rapisarda A, Caccavella VM, La Rocca G, Sabatino G, Marchese E, Olivi A. Predicting meningioma consistency and brain-meningioma interface with intraoperative strain ultrasound elastography: a novel application to guide surgical strategy. Neurosurg Focus. 2021;50:E15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Jia K, Li L, Wu XJ, Hao MJ, Xue HY. Contrast-enhanced ultrasound for evaluating the pathologic response of breast cancer to neoadjuvant chemotherapy: A meta-analysis. Medicine (Baltimore). 2019;98:e14258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Dickson DM, Smith SL, Hendry GJ. Can patient characteristics explain variance in ultrasound strain elastography measures of the quadratus femoris and patellar tendons? Knee. 2021;28:282-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Li XP, Lan JY, Liu DQ, Zhou H, Qian MM, Wang WW, Yang M. OCA2 rs4778137 polymorphism predicts survival of breast cancer patients receiving neoadjuvant chemotherapy. Gene. 2018;651:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Martín-Sánchez JC, Lunet N, González-Marrón A, Lidón-Moyano C, Matilla-Santander N, Clèries R, Malvezzi M, Negri E, Morais S, Costa AR, Ferro A, Lopes-Conceição L, La Vecchia C, Martínez-Sánchez JM. Projections in Breast and Lung Cancer Mortality among Women: A Bayesian Analysis of 52 Countries Worldwide. Cancer Res. 2018;78:4436-4442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Xue LB, Liu YH, Zhang B, Yang YF, Yang D, Zhang LW, Jin J, Li J. Prognostic role of high neutrophil-to-lymphocyte ratio in breast cancer patients receiving neoadjuvant chemotherapy: Meta-analysis. Medicine (Baltimore). 2019;98:e13842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Murayama M, Nosaka K, Inami T, Shima N, Yoneda T. Biceps brachii muscle hardness assessed by a push-in meter in comparison to ultrasound strain elastography. Sci Rep. 2020;10:20308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Shi XE, Tian JH, Yang XJ, Wang YF, Yang KH. Survival benefit of neoadjuvant chemotherapy for resectable breast cancer: A meta-analysis. Medicine (Baltimore). 2018;97:e10634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, Takashima T, Tomita S, Motomura H, Ohsawa M, Hirakawa K, Ohira M. Prediction of Treatment Response to Neoadjuvant Chemotherapy in Breast Cancer by Subtype Using Tumor-infiltrating Lymphocytes. Anticancer Res. 2018;38:2311-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Jin Z, Chenghao Y, Cheng P. Anticancer Effect of Tanshinones on Female Breast Cancer and Gynecological Cancer. Front Pharmacol. 2021;12:824531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Yun SJ, Jin W, Cho NS, Ryu KN, Yoon YC, Cha JG, Park JS, Park SY, Choi NY. Shear-Wave and Strain Ultrasound Elastography of the Supraspinatus and Infraspinatus Tendons in Patients with Idiopathic Adhesive Capsulitis of the Shoulder: A Prospective Case-Control Study. Korean J Radiol. 2019;20:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Tupper P, Leung K, Wang Y, Jongman A, Sereno JA. Characterizing the distinctive acoustic cues of Mandarin tones. J Acoust Soc Am. 2020;147:2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Hahn S, Lee YH, Lee SH, Suh JS. Value of the Strain Ratio on Ultrasonic Elastography for Differentiation of Benign and Malignant Soft Tissue Tumors. J Ultrasound Med. 2017;36:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |