Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5470
Peer-review started: October 14, 2021
First decision: December 17, 2021
Revised: January 18, 2022
Accepted: April 4, 2022
Article in press: April 4, 2022
Published online: June 6, 2022
Processing time: 230 Days and 23.4 Hours
Hepatocellular carcinoma (HCC) is a primary liver cancer with high prevalence and mortality. There are many cases of advanced HCC at the time of diagnosis. Treatment methods and prognosis are different depends on whether metastasis is present. Thus, it is necessary to make an accurate evaluation at the time of diagnosis. Extrahepatic metastases of HCC usually occur through hematogenous spread or through adjacent organs such as the peritoneum. Metastasis to the urinary bladder alone is rare. Here, we report a rare case of biopsy-proven solitary metastasis of HCC to the bladder in a 60-year-old woman.
A 60-year-old female patient was found to be positive for hepatitis B surface antigen by chance after abdominal ultrasonography showed abnormal findings. Thus, liver dynamic computed tomography (CT) was performed. The patient visited the hospital for further examination. Ultrasound and CT showed 3.6 cm sized arterial enhancing mass in segment 5 and an infiltrative mass in segment 8. The patient was diagnosed with HCC through liver dynamic magnetic resonance imaging and liver biopsy. Afterwards, she underwent two transcatheter arterial chemoembolizations within five months for HCC. During follow-up, a newly appeared bladder tumor was found on liver dynamic CT. She underwent transurethral resection of the bladder tumor for diagnosis and treatment. The tissue was confirmed as metastatic HCC.
Although rare, metastasis to urinary bladder from HCC can occur without evidence of other distant metastases. Thus, regular follow-up imaging examination and clinical attention are required.
Core Tip: Without any distant metastasis, metastasis to urinary bladder from hepatocellular carcinoma can occur, which requires clinical attention.
- Citation: Kim Y, Kim YS, Yoo JJ, Kim SG, Chin S, Moon A. Rare case of hepatocellular carcinoma metastasis to urinary bladder: A case report . World J Clin Cases 2022; 10(16): 5470-5478
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5470.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5470
HCC is the sixth most common cancer worldwide and the third leading cause of cancer-related mortality[1]. In addition, because its symptoms are not severe until the disease has progressed, most HCCs at the time of diagnosis are in an advanced stage.
In HCC, not only intrahepatic metastasis, but also extrahepatic metastases to other sites such as lung, abdominal lymph nodes, bone, and adrenal glands are common [2-4]. The main routes of metastasis are vascular and lymphatic spread. Other routes such as spread through the bile duct and direct invasion are also possible[2,4-6]. However, metastasis of HCC into the urinary bladder is very rare. Here, we report a case of a solitary metastasis in the bladder found during transcatheter arterial chemoembolization (TACE) in a 60-year-old Korean woman previously diagnosed with chronic hepatitis B and HCC.
A 60-year-old female who underwent TACE twice for HCC visited our clinic after a bladder mass was found in a follow-up computed tomography (CT) scan.
A 60-year-old female was found to be positive for hepatitis B surface antigen in a laboratory test. However, the patient denied both a history of hepatitis B and a family history. Therefore, HBV DNA test and abdominal ultrasonography were performed to work up the first diagnosis of hepatitis B. The HBV DNA test was confirmed to be positive. Liver cirrhosis and 3.6 cm sized HCC in segment 5 with infiltrative HCC in segment 8 were founded on abdominal ultrasound and liver dynamic CT (Figure 1).
For accurate diagnosis of the hepatic mass lesion suspected of HCC, we carried out enhanced liver dynamic magnetic resonance imaging (MRI) and liver biopsy on the S5 mass. As a result, HCC was diagnosed without evidence of portal vein invasion, lymph node metastasis, or distant metastasis (Figure 2). For multiple intrahepatic masses, we performed TACE immediately after diagnosis and at 5 mo after the 1st TACE on the main mass. TACE was performed by infusion of Adriamycin (50 mg) and lipiodol (10 mL) mixture, followed by embolization with gelfoam. After the second TACE, a urinary bladder tumor was newly discovered on liver dynamic CT performed to confirm treatment response (Figure 3).
The patient had a history of hysterectomy for uterine myoma 20 years ago. She was taking amlodipine 10 mg and olmesartan 40 mg as antihypertensive drugs.
No special notes.
At the time of admission, the patient was 152.0 cm tall with a weight of 61.8 kg. Regarding her blood pressure, her systolic blood pressure was 124 mmHg and her diastolic blood pressure was 83 mmHg. Her heart rate (65 bpm) and body temperature (36.4°C) were normal. Her respiratory rate was also normal at 20 breaths per minute. Her mental status was alert. At the time of admission, the patient did not complain of any symptoms including abdominal pain. Abnormal findings such as ascites were not observed on abdominal examination.
In the liver function test performed during hospitalization, her aspartate aminotransferase and alanine aminotransferase levels were 24 U/L and 15 U/L, respectively, and her total bilirubin level was 0.78 mg/dL, all of which were normal. Her prothrombin time international normalized ratio was 1.05, which was within the normal range. Her albumin level was at 4.0 g/dL, which was also within the normal range.
Her HBV DNA test was negative (< 10 IU/mL) from 46.0 IU/mL after entecavir administration. No other coinfections including HCV were identified. Levels of alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) as tumor markers were found to be 3.9 ng/mL and 24.0 mAU/mL, respectively.
She underwent TACE for HCC at S5 and S8 previously confirmed on CT. After the second TACE, a follow-up CT was taken one month later. On CT, a polypoid mass of 1 cm in the bladder which had not been seen in the previous study was observed without any viable HCC (Figure 3). Follow-up CT was taken three months later. After 3 mo, the bladder mass previously seen showed an increase in size from 1 cm to 1.8 cm. We additionally performed liver MRI, chest CT, and Positron emission tomography (PET)-CT. In these studies, probable viable HCCs at S8 were found. There were no other prominent distant metastases.
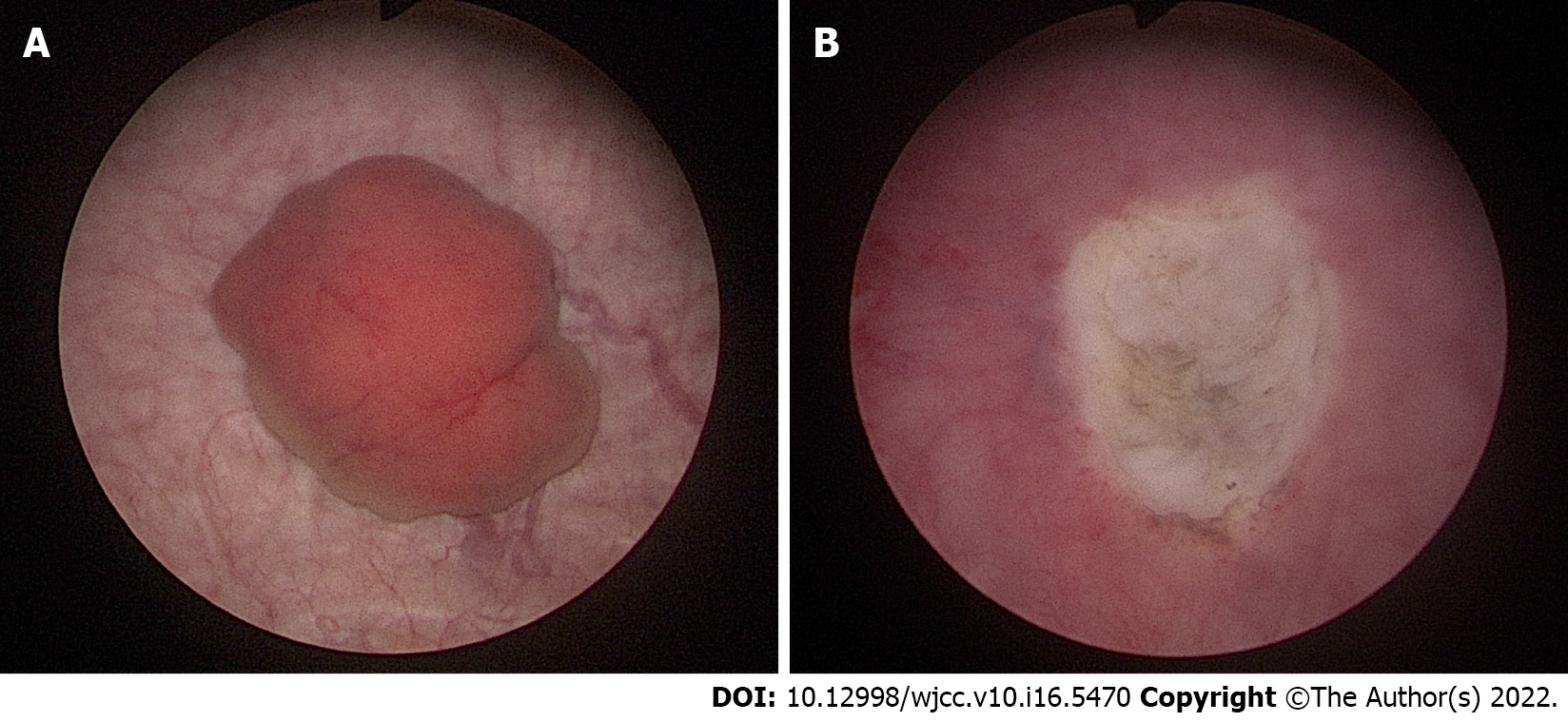
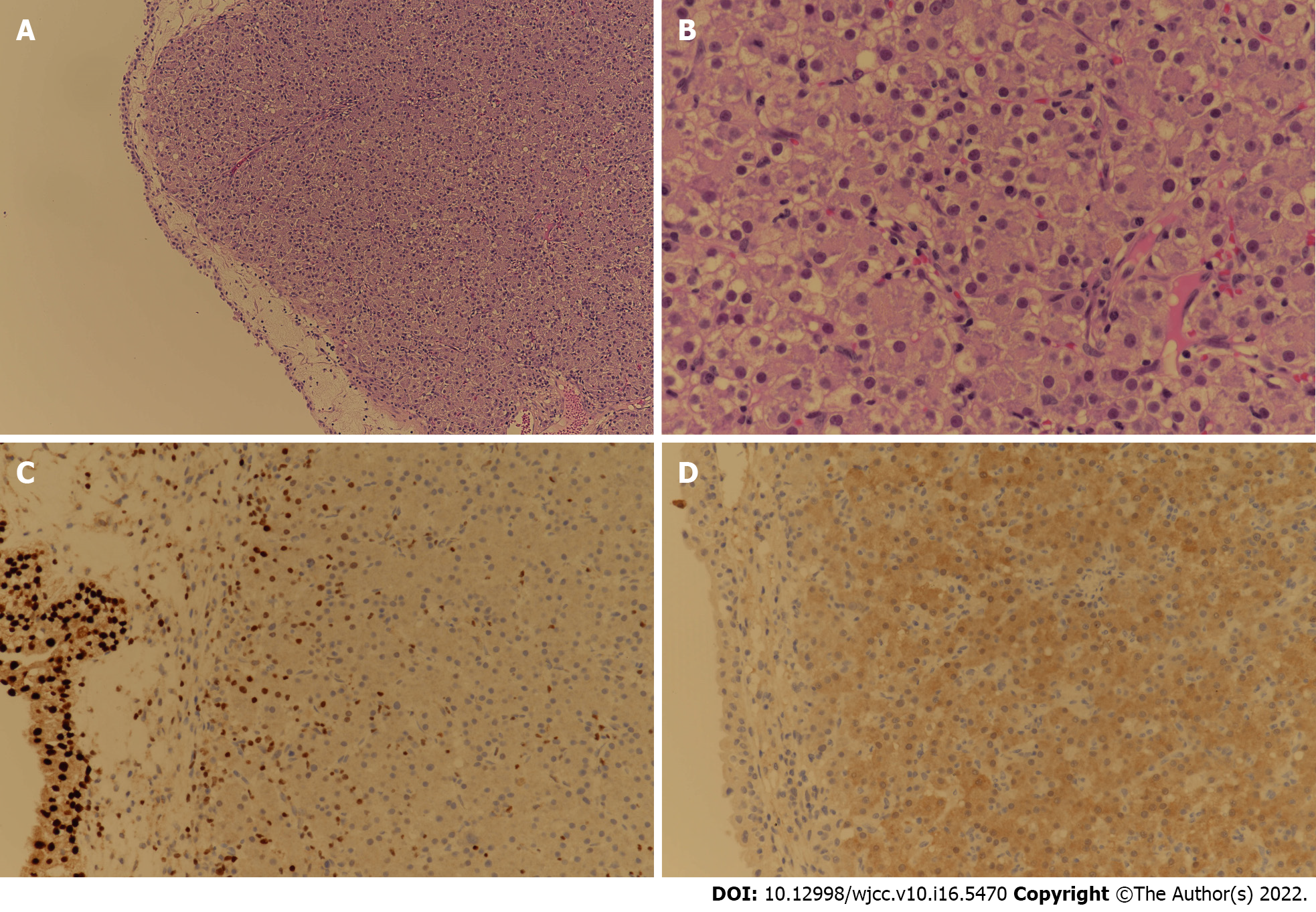
Transurethral resection of the bladder tumor was performed for the newly discovered bladder mass in the Urology Department. Cystoscopic findings revealed a 2 cm-sized well-defined reddish polypoid tumor on the posterior area of the bladder (Figure 4). The mass was removed using a hot loop. The pathology department confirmed that this tissue was metastatic HCC. In the case of HCC, it can be confirmed only by imaging study. She additionally underwent liver biopsy. Result was comparable to tissue of metastatic HCC. For the bladder tumor tissue, GATA binding protein 3 (GATA3) immunohistochemical staining that shows positive expression in the urothelium was negative, while arginase-1, which is significant in HCC, was positive. Thus, it was diagnosed as metastatic HCC (Figure 5).
There were multinodular intrahepatic masses of S5 and S8. Because her Eastern Cooperative Oncology Group performance status was 0-1 and her Child-Pugh score was A, local treatment through TACE was first attempted on the main mass. Systemic chemotherapy was then planned. TACE was performed twice. Her AFP level was 3.5 ng/mL and her PIVKA-II level was 23.4 mAU/mL, which did not rise during the treatment period. A new urinary bladder tumor was found during regular follow-up with a contrast-enhanced liver CT. Transurethral resection of bladder tumor (TUR-BT) was performed for the diagnosis and treatment.
After TUR-BT, TACE, to which the mass had previously responded well, was performed again. Lenvatinib was then added as a systemic chemotherapy. Since then, she has been followed up with regular close monitoring in an outpatient clinic without any special problems.
Treatment for HCC differs depending on its stage. The prognosis of HCC can vary greatly. Therefore, it is important to check for metastases at the first diagnosis. In addition to intrahepatic metastases for HCC, extrahepatic metastases are also known to be common[2]. Recently, PET-CT has been used so that small-sized early metastatic HCC will not be overlooked[7]. Extrahepatic metastasis occurs mainly in the lungs, lymph nodes, bones, adrenal glands, peritoneum, and omentum through direct invasion, vascular spread, or lymphatic spread[2-6]. The lung as the most metastatic site of HCC can be metastasized from the liver due to hematogenous spread through the pulmonary capillary network. If lung metastasis is present, hematogenous metastasis in other organs can also occur. Metastasis of the peritoneum and/or omentum is also important since tumor cells can propagate through ascites. Hematogenous spread through variceal collaterals and direct invasion of cancer are all possible[2]. However, metastasis to the urinary bladder alone without metastasis to other organs is known to be rare[8].
Bladder cancer is the most common malignancy of the urinary tract. Its subtypes include urothelial carcinoma, squamous cell carcinoma, and adenocarcinoma. In the United States and Europe, urothelial (formerly transitional cell) carcinoma accounts for more than 90% of bladder cancer[9]. Most patients show symptoms of gross or microscopic hematuria. They are diagnosed with biopsy through urine cytology and cystoscopy. Treatment and prognosis of urothelial carcinoma depend on the stage and muscle invasion. Transurethral resection is the basic treatment for a non-muscle invasive disease. Intravesical therapy may be additionally considered for intermediate to high-risk patients[10]. Neoadjuvant or adjuvant chemotherapy can be considered based on radical cystectomy for a muscle invasive disease. Platinum-based chemotherapy and immunotherapy can be implemented for a metastatic bladder cancer[11].
Based on previous reports[12-16], primary bladder cancer and metastatic HCC do not show significant difference in morphology under imaging study or cystoscopy. Thus, it is necessary to distinguish them through histological evaluation. For the diagnosis of metastatic HCC, it is necessary to histologically confirm HCC tumor cells in a bladder tumor. HCC tumor cells are similar to hepatocytes in morphology and function. They are polygonal and sinusoidal with a trabecular pattern. They can also secrete bile[17]. Metastatic HCC shows hepatocellular differentiation similar to HCC tumor cells.
In the present case, HCC was suspected as a liver mass with an early enhancing and delayed washout pattern on contrast-enhanced liver CT and MRI. HCC was confirmed by liver biopsy. Considering that the multinodular HCC showed no extrahepatic metastases and that the patient’s condition was stable, TACE was first tried to treat the main tumor mass. Systemic chemotherapy was then performed. After performing TACE twice, a newly appeared bladder tumor was found on follow-up liver dynamic CT. PET-CT performed at this time confirmed no prominent extrahepatic metastases. However, it was difficult to identify the bladder lesion due to its physiological uptake. Therefore, two possibilities, HCC metastasis to the bladder and primary bladder cancer, were considered. Considering their incidence, primary bladder cancer was considered first. After consulting with a urologist, it was considered that bladder tumor invasion was not deep in the imaging study. Thus, TUR-BT was performed for diagnosis and treatment.
Since liver biopsy is often not performed when HCC is first diagnosed, histological comparison with metastatic lesion is difficult. However, since this patient underwent liver biopsy, it was possible to perform histological comparison of the HCC tissue and the metastatic lesion sample obtained by TUR-BT. Hepatocyte antigen and GATA3 were negative in the immunohistochemical staining performed for the lesion of the bladder tumor. Unlike the primary HCC lesion, hepatocytes were not stained. Other immunohistochemical staining performed on bladder tumor tissue, Arginase-1 was positive and glypican-3 was weakly positive. Arginase-1 is the most sensitive and specific hepatocellular marker. It can be used to differentiate HCC from other tumors and HCC. If glypican-3 is positive, which is not expressed in normal or benign hepatocellular lesions, most HCC can be diagnosed[18,19]. With the help of immunohistochemical staining, bladder tumor could be diagnosed as metastatic HCC.
In the present case, metastatic HCC to the bladder was diagnosed. However, hepatocytes of HCC might be well stained or not well stained in metastatic HCC as properties of HCCs might not be the same. Such molecular heterogeneity with primary tumor and its corresponding metastases showing different molecular marker expression is frequently found in HCC. This heterogeneity can affect treatment options and clinical outcome. It can also cause treatment failure[20]. Thus, accurate histological diagnosis and immunohistochemical staining through biopsy are needed. Recently, in addition to immunohistochemistry, comparison between HCC and metastatic lesions has been attempted by identifying properties of HCC and metastatic sites through genetic analysis using next generation sequencing[21].
This case is an independent bladder metastasis case of hepatocellular carcinoma, which is very rare worldwide. Thus, this case report itself is meaningful. However, based on previous cases listed in Table 1, some variables such as bladder cancer after liver transplantation and hemorrhagic cystitis after radiotherapy could affect metastasis[12,14]. In the present case, there were no additional factors to consider in the primary site or the metastatic site. In addition, unlike previous studies, it is the only case report that compares biopsies from both primary and bladder metastasis sites. Because there is no established prognosis or optimal treatment modality due to its rarity, this case provides important data for future studies on HCC metastasis to the bladder.
| Case report | Location | Sex | Age (yr) | HCC Etiology | Previous treatment | Other metastasis site | Comorbidity | Liver biopsy | Bladder biopsy | Treatment | Prognosis | |
| Current case | Rep. of Korea | F | 60 | HBV | TACE #2 | None | None | O | O | TUR-BT, systemic CTx | Under follow up | |
| Kurimoto et al[13], 1993 | Japan | M | 74 | Unknown | None | Chest wall | History of stomach bleeding | X | O | TUR-BT | Died 6 months later | |
| Franks et al[14], 1999 | USA | M | 51 | HCV, Alcoholic LC | LT, Adjuvant CTx | Lung | None | X | O | TUR-BT, systemic CTx | Unknown | |
| Al-Brahim et al[15], 2004 | Canada | M | 83 | Unknown | None | Left adrenal gland? | Synchronous transitional cell carcinoma | X | O | Unknown | Unknown | |
| Chung et al[12], 2007 | Taiwan | F | 58 | C-viral LC | None | Unknown | Cervical cancer, Hemorrhagic cystitis after RTx | X | O | TUR-BT | Died 5 months later | |
| Yasutomi et al[16], 2020 | Japan | M | 89 | Alcoholic LC | TACE | None | None | X | O | TUR-BT | Unknown | |
HCC is often an advanced disease with metastases. It is clinically, molecularly, and biologically heterogenous. Even if extrahepatic metastases are not detected at the time of initial diagnosis of HCC, metastasis might progress during treatment. It is necessary to differentiate between primary cancer of other lesions and metastatic HCC. To distinguish between these two, histologic confirmation is recommended. Molecular expression and genetic analysis can also be considered for precise diagnosis. As in this case, distant metastasis to an uncommon organ may occur during HCC treatment. Thus, regular follow-up examinations and clinical attention are required even after treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hou L, China; Lashen SA, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris). 2010;58:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 2. | Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 514] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 3. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 396] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 4. | Kanda M, Tateishi R, Yoshida H, Sato T, Masuzaki R, Ohki T, Imamura J, Goto T, Hamamura K, Obi S, Kanai F, Shiina S, Omata M. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008;28:1256-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Becker AK, Tso DK, Harris AC, Malfair D, Chang SD. Extrahepatic metastases of hepatocellular carcinoma: A spectrum of imaging findings. Can Assoc Radiol J. 2014;65:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Arora S, Harmath C, Catania R, Mandler A, Fowler KJ, Borhani AA. Hepatocellular carcinoma: metastatic pathways and extra-hepatic findings. Abdom Radiol (NY). 2021;46:3698-3707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Lee JE, Jang JY, Jeong SW, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Jin SY, Choi DL. Diagnostic value for extrahepatic metastases of hepatocellular carcinoma in positron emission tomography/computed tomography scan. World J Gastroenterol. 2012;18:2979-2987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Boldo E, Santafe A, Mayol A, Lozoya R, Coret A, Escribano D, Fortea-Sanchis C, Muñoz A, Pastor JC, Perez de Lucia G, Bosch N. Rare Site Hepatocellular Carcinoma Metastasis. J Hepatocell Carcinoma. 2020;7:39-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Malats N, Real FX. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 2015;29:177-189, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Sylvester RJ, van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 224] [Reference Citation Analysis (0)] |
| 11. | Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, Lotan Y, Meeks JJ, Michalski JM, Morgan TM, Quale DZ, Rosenberg JE, Zietman AL, Holzbeierlein JM. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. 2017;198:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 663] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 12. | Chung SD, Ho CH, Hung SF, Tai HC, Yu HJ, Huang KH. Metastatic hepatocellular carcinoma in the urinary bladder with radiation-induced hemorrhagic cystitis. J Formos Med Assoc. 2007;106:861-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Kurimoto S, Komatsu H, Doi N, Wakumoto Y, Tominaga T, Nishimura Y. [Metastasis of hepatocellular carcinoma to the urinary bladder]. Urologe A. 1993;32:64-65. [PubMed] |
| 14. | Franks ME, Konety BR, Bastacky S, Gritsch HA. Hepatocellular carcinoma metastatic to the bladder after liver transplantation. J Urol. 1999;162:799-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Al-Brahim N, Alowami S, Davis I, Daya D. Synchronous transitional cell carcinoma and metastatic hepatocellular carcinoma in the urinary bladder: a case report. Can J Urol. 2004;11:2463-2466. [PubMed] |
| 16. | Yasutomi E, Yano Y, Kodama Y. Urinary bladder metastasis of hepatocellular carcinoma incidentally revealed by hematuria. Dig Liver Dis. 2020;52:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | El Jabbour T, Lagana SM, Lee H. Update on hepatocellular carcinoma: Pathologists' review. World J Gastroenterol. 2019;25:1653-1665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (4)] |
| 18. | Nguyen T, Phillips D, Jain D, Torbenson M, Wu TT, Yeh MM, Kakar S. Comparison of 5 Immunohistochemical Markers of Hepatocellular Differentiation for the Diagnosis of Hepatocellular Carcinoma. Arch Pathol Lab Med. 2015;139:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Choi WT, Kakar S. Immunohistochemistry in the Diagnosis of Hepatocellular Carcinoma. Gastroenterol Clin North Am. 2017;46:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Zheng H, Pomyen Y, Hernandez MO, Li C, Livak F, Tang W, Dang H, Greten TF, Davis JL, Zhao Y, Mehta M, Levin Y, Shetty J, Tran B, Budhu A, Wang XW. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology. 2018;68:127-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 252] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 21. | Jin K, Lan H, Wang X, Lv J. Genetic heterogeneity in hepatocellular carcinoma and paired bone metastasis revealed by next-generation sequencing. Int J Clin Exp Pathol. 2017;10:10495-10504. [PubMed] |