Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5365
Peer-review started: August 4, 2021
First decision: December 10, 2021
Revised: December 23, 2021
Accepted: April 2, 2021
Article in press: April 2, 2022
Published online: June 6, 2022
Processing time: 301 Days and 20.3 Hours
Umbilical cord milking (UCM) is an alternative placental transfusion method for delayed umbilical cord clamping in routine obstetric practice, allowing prompt resuscitation of an infant. Thus, UCM has been adopted at some tertiary neonatal centers for preterm infants to enhance placental-to-fetal transfusion. It is not suggested for babies less than 28 wk of gestational age because it is associated with severe brain hemorrhage. For late preterm or term infants who do not require resuscitation, cord management is recommended to increase iron levels and prevent the development of iron deficiency anemia, which is associated with impaired motor development, behavioral problems, and cognitive delays. Concerns remain about whether UCM increases the incidence of intraventricular hemorrhage. However, there are very few reports of late preterm infants presenting with neonatal hemorrhage stroke (NHS) and severe coagulopathy after receiving UCM. Here, we report a case of a late preterm infant born at 34 wk of gestation. She abruptly deteriorated, exhibiting signs and symptoms of NHS and severe coagulopathy after receiving UCM on the first day of life.
A female preterm infant born at 34 wk of gestation received UCM after birth. She was small for her gestational age and described as vigorous with Apgar scores of 9 and 10 at one minute and five minutes of life, respectively. After hospitalization in the neonatal intensive care unit, she showed hypoglycemia and metabolic acidosis. The baby was administered glucose and sodium bicarbonate infusions. Intramuscular vitamin K1 was also used to prevent vitamin K deficiency. The baby developed umbilical cord bleeding and gastric bleeding on day 1 of life; a physical examination showed bilateral conjunctival hemorrhage, and a blood test showed thrombocytopenia, prolonged prothrombin time, prolonged activated partial thromboplastin time, low fibrinogen, raised D-dimer levels and anemia. A subsequent cranial ultrasound and computed tomography scan showed a left parenchymal brain hemorrhage with extension into the ventricular and subarachnoid spaces. The patient was diagnosed with NHS in addition to disseminated intravascular coagulation (DIC). Fresh frozen plasma (FFP) and prothrombin complex concentrate were given for coagulopathy. Red blood cell and platelet transfusions were provided for thrombocytopenia and anemia. A bolus of midazolam, intravenous calcium and phenobarbital sodium were administered to control seizures. The baby’s clinical condition improved on day 5 of life, and the baby was hospitalized for 46 d and recovered well without seizure recurrence. Our case report suggests that preterm infants who receive UCM should undergo careful clinical assessment for intracranial hemorrhage, NHS and severe coagulopathy that may develop under certain circumstances. Supportive management, such as intensive care, FFP and blood transfusion, is recommended when the development of massive NHS and associated DIC is suspected.
Our case report suggests that for late preterm infants who are small for gestational age and who receive UCM for alternative placental transfusion, neonatal health care professionals should be cautious in assessing the development of NHS and severe coagulopathy. Neonatal health care professionals should also be more cautious in assessing the complications of late preterm infants after they receive UCM.
Core Tip: We report a case of a premature infant born at 34 wk of gestation who developed neonatal hemorrhage stroke and severe coagulopathy after receiving umbilical cord milking (UCM). The baby was small for her gestational age. She developed hypoglycemia and metabolic acidosis after hospitalization. Umbilical cord bleeding, gastric bleeding, seizure and pulmonary hemorrhage were noticed in the following three days, and physical examination showed bilateral conjunctival hemorrhage. A blood test confirmed the diagnosis of disseminated intravascular coagulation. A cranial ultrasound and a computed tomography scan showed left parenchymal brain hemorrhage with extension into the ventricular and subarachnoid spaces. Our case suggests that late preterm infants who are small for gestational age who receive UCM should undergo careful clinical assessment for intracranial hemorrhage. Supportive management such as intensive care and blood transfusion may be indicated in light of seizures and coagulopathy.
- Citation: Lu Y, Zhang ZQ. Neonatal hemorrhage stroke and severe coagulopathy in a late preterm infant after receiving umbilical cord milking: A case report. World J Clin Cases 2022; 10(16): 5365-5372
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5365.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5365
Umbilical cord milking (UCM) refers to the transfer of blood to the infant from the time of birth to the time of umbilical cord clamping by gently grasping the umbilical cord and squeezing the cord from the placenta several times toward the infant[1]. This cord management is considered to enhance placental-to-fetal transfusion and could be a suitable alternative placental transfusion method for delayed cord clamping if a newborn infant requires resuscitation in the delivery room to avoid delayed resuscitation[2]. Some clinical trials and meta-analyses have suggested benefits of UCM vs immediate cord clamping without apparent complications[3]. It has been adopted at some tertiary neonatal centers, especially for late preterm or term infants, to increase iron levels and prevent the development of iron deficiency anemia, which is associated with impaired motor development, behavioral problems, and cognitive delays[4]. However, concerns remain about whether UCM increases the incidence of intracranial hemorrhage in preterm infants[5]. Its use has been recommended to be avoided in babies less than 28 wk gestational age because it is associated with severe brain hemorrhage[6]. However, few reports have shown late preterm infants presenting with neonatal hemorrhage stroke (NHS) and severe coagulopathy after receiving UCM.
We report a case of a preterm infant born at 34 wk of gestation. After receiving UCM after birth, she developed NHS 11 h after birth, and a subsequent blood test showed severe coagulopathy. The preterm infant developed umbilical cord bleeding, gastric bleeding, and disseminated intravascular coagulation (DIC) on day 1 of life, and pulmonary hemorrhage and seizures on day 3 of life. A bolus of midazolam and phenobarbital sodium were used to control seizures, and fresh frozen plasma (FFP), red blood cell and platelet transfusions were provided to correct coagulopathy, thrombocytopenia and anemia.
A female premature infant born at 34 wk of gestation with a very low birth weight was sent to the neonatal intensive care unit (NICU) after receiving UCM.
A preterm newborn at 34 wk gestation was delivered via cesarean section to a 38-year-old mother due to a premature rupture of the membrane and absence of blood flow in the umbilical artery diastolic period. After birth, the obstetrics doctor milked the cord by squeezing it toward the infant for 2 s and repeated the same action 3 times before clamping and cutting the cord. After hospitalization, the baby was found to have hypoglycemia and mild metabolic acidosis. The baby was administered a glucose infusion for hypoglycemia and sodium bicarbonate for metabolic acidosis. Intramuscular vitamin K1 was administered for 3 d to prevent vitamin K deficiency coagulopathy. Eleven hours after birth, the baby developed apnea with decreased peripheral oxygen saturation levels. The baby was diagnosed with respiratory distress syndrome. Thus, the baby started nasal continuous positive airway pressure (nCPAP). Fourteen hours after birth, the baby was noticed to be bleeding at the umbilical cord site and the location where the blood was drawn. As blood was found in the gastric tube, FFP was given for coagulopathy, and an omeprazole and prothrombin complex concentrate was administered for gastric bleeding. On day 3 of life, an increased inspired oxygen concentration was needed, and blood was found in the endotracheal tube. The baby presented with repetitive, rhythmic movements of the limbs; thus, pulmonary hemorrhage and seizure were considered. The baby was started on mechanical ventilation with synchronized intermittent mandatory ventilation to achieve adequate oxygenation. An epinephrine and prothrombin complex concentrate was flushed into the trachea, and FFP (20 mL/kg × 3), red blood cell (20 mL/kg × 2) and platelet (30 mL/kg) transfusions were administered for coagulopathy and anemia.
The patient had no past illness history.
The baby weighed 1270 g, was described as vigorous, and cried at birth, with Apgar scores of 9 and 10 at one minute and five minutes of life, respectively. The mother did not take precautions or receive checks regularly during pregnancy. She was diagnosed with preeclampsia with high blood pressure without a history of hypertension on admission. An intrauterine fetal ultrasound revealed fetal distress and fetal growth restriction.
On the baby’s admission to the NICU, a physical examination revealed a temperature of 36.1 °C, a heart rate of 164 bpm, a blood pressure of 51/35 (41) mmHg, and a respiratory rate of 48 breaths/min. Her baseline oxygen saturation was maintained at approximately 85%. She was found to have bilateral conjunctival hemorrhage.
Table 1 shows the laboratory findings for the first three days of life. After hospitalization, a quick bedside blood glucose reading revealed a level of 2.4 mmol/L; a blood gas test revealed a pH level of 7.13, a PCO2 Level of 22.5 mmHg, an ABE of 20.6 mmol/L, and a lactate level of 13.4 mmol/L; and a blood test showed a high hemoglobin level of 207 g/L, a platelet level of 119 × 109/L and a C-reactive protein level of 11 mg/L (normal 0-8 mg/L). The coagulation study results were as follows: A prothrombin time (PT) of 36.2 s (normal 10-14), an international normalized ratio (INR) of 3.18 s (normal 0.8-1.4), an activated partial thromboplastin time (APTT) of 70.8 s (normal 24-40), a thrombin time of 29.6 s (normal 14-21), a fibrinogen level of 0.52 g/L (normal 2-4), a D-dimer (DD) level of 4040 µg/L (0-500), and a calcium level of 1.36 mmol/L (2-2.6). A blood test showed a decreased platelet level from 47 × 109/L on day 2 of life to 26 × 109/L on day 3 of life (Table 1). A blood test also showed rapidly decreased hemoglobin levels to 113 g/L and platelet levels from 62 × 109/L on day 4 of life to 15 × 109/L on day 5 of life; the blood calcium level was 1.37 mmol/L (2-2.6). On Day 9 of life, there was an improvement in pulmonary hemorrhage and an increase in the platelet level to 41 × 109/L.
| Variables | Day 1 | Day 2 | Day 3 |
| WBC (109/L) | 13.7 | 5.2 | 4.3 |
| Hemoglobin (g/dL) | 207 | 193 | 113 |
| Platelets (109/L) | 119 | 47 | 26 |
| CRP (mg/L) | 11 | < 1 | < 1 |
| PT (s) | 36.2 | 14.1 | |
| APTT (s) | 70.8 | 37.9 | |
| INR | 3.18 | 1.23 | |
| Fibrinogen (g/L) | 0.52 | 1.91 | |
| DD (μg/L) | 4040 | 7340 |
A chest X-ray revealed a diffuse ground glass appearance. A cranial ultrasound showed high density in the left frontal and parietal lobes.
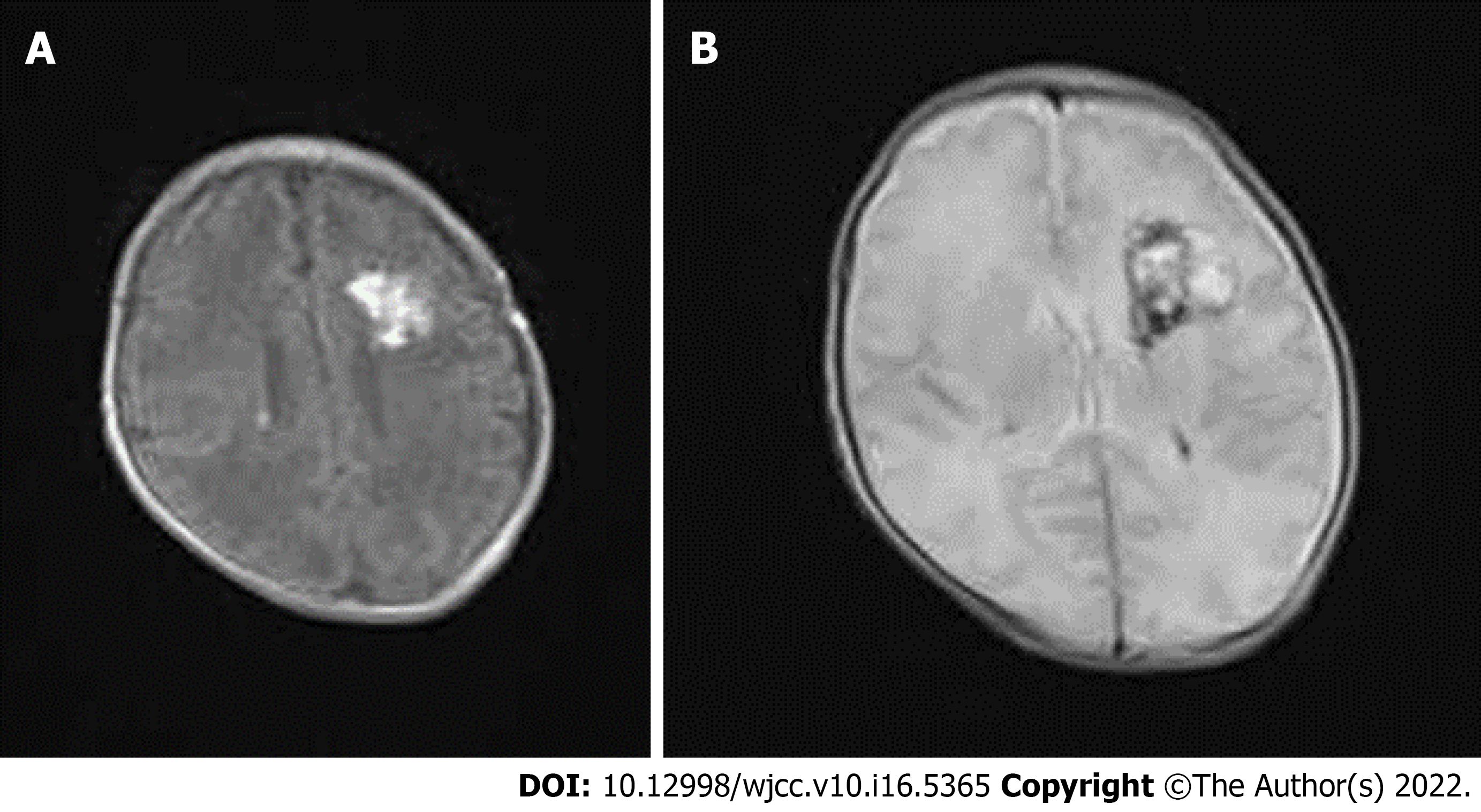
A subsequent cranial computed tomography (CT) scan showed a left parenchymal brain hemorrhage with extension into the ventricular and subarachnoid spaces. Brain edema was massive (Figure 1). The blood test showed thrombocytopenia, prolonged PT, prolonged APTT, low fibrinogen, raised DD levels and anemia, confirming the diagnosis of DIC.
On admission to the NICU, the baby received a glucose infusion for hypoglycemia and sodium bicarbonate for metabolic acidosis after hospitalization. Intramuscular vitamin K1 was administered for 3 d to prevent vitamin K deficiency coagulopathy. Eleven hours after birth, the baby developed apnea with a decreased peripheral oxygen saturation level, while bleeding was noticed at the baby’s umbilical cord site and the location where the blood was drawn. Blood was also found in the gastric tube. The baby received nCPAP, and FFP (20 mL/kg) was given for coagulopathy. On day 3 of life, blood was found in the endotracheal tube, and the baby presented with repetitive, rhythmic movements of the limbs. A chest radiograph demonstrated bilateral alveolar infiltrates; thus, pulmonary hemorrhage and seizures were considered. A change was made to high-frequency ventilation with an epinephrine and prothrombin complex concentrate flushed in the trachea and FFP (20 mL/kg × 2), red blood cell (20 mL/kg × 2) and platelet (30 mL/kg) transfusions for coagulopathy and anemia. A bolus of midazolam for 3 d, intravenous calcium for 11 d and phenobarbital sodium for 5 d was administered to control the seizures.
On day 5 of life, the seizures finally disappeared with resolution of the gastric bleeding. On day 9 of life, with improvement of the clinical condition, the baby was changed to nasal oxygen and started enteral feeding. The antinuclear antibody test was all negative. Later hospitalization was unremarkable. No paroxysmal discharge or asymmetrical attenuation was found on the electroencephalogram on day 40 of life. Magnetic resonance imaging (MRI) was performed on day 25 of life and showed improvement of the left parenchymal brain hemorrhage and subarachnoid hemorrhage (Figure 2). We did not perform angiography by CT or MRI to exclude underlying vascular abnormalities because angiography was not available in our hospital. The baby was hospitalized for 46 d and recovered well without seizure recurrence. The patient was followed up at 8 months old with correction of gestational age, and her neurodevelopment progressed according to her age.
Placental transfusions have become standard procedures in neonatal care. UCM provides a placental transfusion by pushing blood with a duration similar to immediate umbilical cord clamping, allowing prompt resuscitation of an infant[1,2]. It is an alternative placental transfusion method for delayed umbilical cord clamping in obstetric practice[7]. Three or four repetitions of milking the intact cord deliver approximately 14 mL/kg of blood[8]. A previous meta-analysis of UCM showed benefits and no adverse effects in the immediate postnatal period in infants with a gestational age of less than 33 wk. Higher hemoglobin levels and hematocrit and a reduced risk for intraventricular hemorrhage of all grades were identified in the UCM group[9]. However, a multicenter noninferiority randomized clinical trial of preterm infants (born at 23-31 wk gestation) showed that severe intracerebral hemorrhage was significantly higher in the UCM group than in the delayed umbilical cord clamping group (8% vs 3%). For infants within 23 to 27 wk gestation, compared with 6% of infants who received delayed cord clamping, the incidence rate of severe brain bleeding was 22% in the UCM group[10]. Thus, the benefit and safety of UCM remain uncertain in preterm infants, especially in infants less than 28 wk gestational age. It is recommended that cord milking be avoided in babies less than 28 wk gestational age[6]. For late preterm or term infants who do not require resuscitation, cord management is recommended to increase iron levels and prevent the development of iron deficiency anemia. Concerns remain about whether UCM increases the incidence of intraventricular hemorrhage in late preterm or term infants. However, few reports have shown that late preterm infants can develop NHS and severe coagulopathy after receiving UCM.
NHS is diagnosed in a neonate with a focal accumulation of blood within the brain parenchyma confirmed by autopsy or imaging. It usually occurs in term infants presenting with encephalopathy, seizures, and/or neurologic deficits in the first 28 d of life[11]. Evidence suggests that neonatal hemorrhagic stroke affects approximately 1 in 6300 Live births. Maternal etiologies have been implicated in NHS, including infection, anticoagulant use or coagulopathy, medication side effects, substance misuse, seizure disorders, trauma, cholestasis and febrile illness[12]. Definitive causes of NHS include structural lesions and bleeding diatheses. However, such definitive causes account for few cases, and the mechanisms of idiopathic NHS are not well understood. A population-based case–control study found associations with intrapartum variables such as difficult fetal transition and being small for gestational age[13]. There are biologically plausible explanations for the adverse effects of UCM in preterm infants related to the fragile germinal matrix[14]. Premature infants lack adequate cerebral autoregulation compared with mature infants[15]. Fetal growth restriction preterm infants more frequently display impaired autoregulation compared with appropriate for gestational age on days 2 and 3 of life, which may predispose them to brain injuries[16]. Furthermore, changes in systemic blood flow with repeat UCM may be transferred to cerebral blood flow, causing rupture[17]. A study in anesthetized 128-d-old preterm lambs (equivalent to a human at 26 wk gestation) demonstrated that UCM caused large fluctuations in mean carotid artery pressures and blood flows[18]. In our case, the infant was small for her gestational age, and our previous ultrasound examination did not find intrauterine intracranial hemorrhage. After receiving UCM, she developed left parenchymal brain hemorrhage with extension into the ventricular and subarachnoid spaces. We assumed that the fluctuation in blood flow with repeat UCM may cause rupture, especially for late preterm infants who are small for gestational age.
The most common causes of coagulation activation are severe sepsis or shock due to circulatory collapse or extensive tissue damage from trauma[19,20]. However, bleeding disorders such as inherited coagulopathy and vitamin K deficiency can also lead to coagulopathy[21]. Preterm infants are of particular concern for bleeding because of immature hepatic and hemostatic function, and they are thought to have lower serum levels of vitamin K-dependent clotting factors, including factors II, VII, IX, and X[22]. The current practice is to routinely administer intramuscular vitamin K1 shortly after birth to all infants to prevent hemorrhagic diseases in newborns. Therefore, infants are less likely to develop early vitamin K deficiency bleeding. This research hypothesized that the infant had consumptive coagulopathy owing to extensive tissue damage from trauma. The UCM procedure may change systemic blood flow and cause rupture of fragile brain vessels. Blood loss then produces profound hypovolemic instability, DIC, and unresponsive metabolic acidosis. Thus, it is important to enhance the implementation of the procedures associated with clinical UCM guideline availability, knowledge of UCM benefits and strict cooperation within the delivery team. When UCM is performed for late preterm infants, especially those who are small for their gestational age or have a difficult fetal transition, neonatal health care professionals need to assess the occurrence of intracranial hemorrhage within the first three days of life because it can affect infants with higher hemodynamic and respiratory instabilities[7]. It is important to recognize the complications. Supportive management, such as intensive care and blood transfusion, may be indicated in light of seizures and coagulopathy.
Our case suggested that for late preterm infants who are small for gestational age and who receive UCM for alternative placental transfusion, neonatal health care professionals should be cautious in assessing the development of NHS and severe coagulopathy. Immediate recognition of NHS development by ultrasound and cranial CT scans plays a vital role in the recommendations of neonatal neurointensive care and seizure monitoring. A complete blood count with platelet count and coagulation evaluations in addition to imaging are important in intensive care management. Immediate FFP and blood transfusion are recommended when the development of coagulopathy and associated DIC are suspected. This case study strongly recommends that neonatal health care professionals be more cautious in assessing late preterm infants who are small for gestational age and receive UCM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciarambino T, Italy; Mallis P, Greece S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Shirk SK, Manolis SA, Lambers DS, Smith KL. Delayed clamping vs milking of umbilical cord in preterm infants: a randomized controlled trial. Am J Obstet Gynecol. 2019;220:482.e1-482.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Katheria AC. Umbilical Cord Milking: A Review. Front Pediatr. 2018;6:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Ortiz-Esquinas I, Gómez-Salgado J, Rodriguez-Almagro J, Arias-Arias Á, Ballesta-Castillejos A, Hernández-Martínez A. Umbilical Cord Milking in Infants Born at <37 Weeks of Gestation: A Systematic Review and Meta-Analysis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Katheria A, Garey D, Truong G, Akshoomoff N, Steen J, Maldonado M, Poeltler D, Harbert MJ, Vaucher YE, Finer N. A Randomized Clinical Trial of Umbilical Cord Milking vs Delayed Cord Clamping in Preterm Infants: Neurodevelopmental Outcomes at 22-26 Months of Corrected Age. J Pediatr. 2018;194:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Kumbhat N, Eggleston B, Davis AS, DeMauro SB, Van Meurs KP, Foglia EE, Lakshminrusimha S, Walsh MC, Watterberg KL, Wyckoff MH, Das A, Handley SC; Generic Database Subcommittee of the National Institute of Child Health and Human Development Neonatal Research Network. Umbilical Cord Milking vs Delayed Cord Clamping and Associations with In-Hospital Outcomes among Extremely Premature Infants. J Pediatr. 2021;232:87-94.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Aziz K, Lee CHC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, Magid DJ, Niermeyer S, Schmölzer GM, Szyld E, Weiner GM, Wyckoff MH, Yamada NK, Zaichkin J. Part 5: Neonatal Resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2021;147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 7. | Basile S, Pinelli S, Micelli E, Caretto M, Benedetti Panici P. Milking of the Umbilical Cord in Term and Late Preterm Infants. Biomed Res Int. 2019;2019:9185059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | McAdams RM, Fay E, Delaney S. Whole blood volumes associated with milking intact and cut umbilical cords in term newborns. J Perinatol. 2018;38:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Al-Wassia H, Shah PS. Efficacy and safety of umbilical cord milking at birth: a systematic review and meta-analysis. JAMA Pediatr. 2015;169:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Katheria A, Reister F, Essers J, Mendler M, Hummler H, Subramaniam A, Carlo W, Tita A, Truong G, Davis-Nelson S, Schmölzer G, Chari R, Kaempf J, Tomlinson M, Yanowitz T, Beck S, Simhan H, Dempsey E, O'Donoghue K, Bhat S, Hoffman M, Faksh A, Arnell K, Rich W, Finer N, Vaucher Y, Khanna P, Meyers M, Varner M, Allman P, Szychowski J, Cutter G. Association of Umbilical Cord Milking vs Delayed Umbilical Cord Clamping With Death or Severe Intraventricular Hemorrhage Among Preterm Infants. JAMA. 2019;322:1877-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 11. | Dunbar M, Kirton A. Perinatal Stroke. Semin Pediatr Neurol. 2019;32:100767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Putbrese B, Kennedy A. Findings and differential diagnosis of fetal intracranial haemorrhage and fetal ischaemic brain injury: what is the role of fetal MRI? Br J Radiol. 2017;90:20160253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Cole L, Dewey D, Letourneau N, Kaplan BJ, Chaput K, Gallagher C, Hodge J, Floer A, Kirton A. Clinical Characteristics, Risk Factors, and Outcomes Associated With Neonatal Hemorrhagic Stroke: A Population-Based Case-Control Study. JAMA Pediatr. 2017;171:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Atienza-Navarro I, Alves-Martinez P, Lubian-Lopez S, Garcia-Alloza M. Germinal Matrix-Intraventricular Hemorrhage of the Preterm Newborn and Preclinical Models: Inflammatory Considerations. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Rhee CJ, da Costa CS, Austin T, Brady KM, Czosnyka M, Lee JK. Neonatal cerebrovascular autoregulation. Pediatr Res. 2018;84:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Cohen E, Baerts W, Caicedo Dorado A, Naulaers G, van Bel F, Lemmers PMA. Cerebrovascular autoregulation in preterm fetal growth restricted neonates. Arch Dis Child Fetal Neonatal Ed. 2019;104:F467-F472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Katheria AC, Szychowski JM, Essers J, Mendler MR, Dempsey EM, Schmölzer GM, Arnell K, Rich WD, Hassen K, Allman P, Varner M, Cutter GR, Finer N. Early Cardiac and Cerebral Hemodynamics with Umbilical Cord Milking Compared with Delayed Cord Clamping in Infants Born Preterm. J Pediatr. 2020;223:51-56.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Blank DA, Polglase GR, Kluckow M, Gill AW, Crossley KJ, Moxham A, Rodgers K, Zahra V, Inocencio I, Stenning F, LaRosa DA, Davis PG, Hooper SB. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Arch Dis Child Fetal Neonatal Ed. 2018;103:F539-F546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol. 2021;192:803-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 20. | Modanlou H, Hutson S, Merritt AT. Early Blood Transfusion and Resolution of Disseminated Intravascular Coagulation Associated with Massive Subgaleal Hemorrhage. Neonatal Netw. 2016;35:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Araki S, Shirahata A. Vitamin K Deficiency Bleeding in Infancy. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 22. | Ardell S, Offringa M, Ovelman C, Soll R. Prophylactic vitamin K for the prevention of vitamin K deficiency bleeding in preterm neonates. Cochrane Database Syst Rev. 2018;2:CD008342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |