Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5317
Peer-review started: December 13, 2021
First decision: January 12, 2022
Revised: January 21, 2022
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: June 6, 2022
Processing time: 171 Days and 2.7 Hours
Crouzon syndrome (CS; OMIM 123500) is an autosomal dominant inherited craniofacial disorder caused by mutations in the fibroblast growth factor receptor 2 (FGFR2) gene. CS is characterized by craniofacial dysostosis, exophthalmos, and facial anomalies with hypoplastic maxilla and relative mandibular prognathism.
Our report involves a 6-year-old fraternal twin boy with many caries in the oral cavity who presented with characteristic features of CS based on clinical and radiographic examinations along with Sanger sequencing. The fraternal girl did not show any abnormalities indicating CS. Carious teeth and poor oral hygiene were managed promptly through administering appropriate behavior guidance, orthodontic treatment was planned, and preventive procedures were described.
CS could occur in a fraternal twin caused by a de novo mutation of the FGFR2 gene. Oral hygiene instruction, preventive programs on oral hygiene, orthodontic treatment, and maxillary osteotomy were required for treatment.
Core Tip: Crouzon syndrome (CS) is an autosomal dominant inherited craniofacial disorder caused by mutations in fibroblast growth factor receptor 2, but approximately 50% of cases result from de novo mutations. This syndrome has been rarely seen and evaluated in fraternal twins, only one of whom has CS. We presented a 6-year-old fraternal twin boy diagnosed with CS who had many caries and enamel hypomineralization in the oral cavity. The boy’s parents and his fraternal twin sister did not show any abnormalities indicating CS, so we hypothesize that the fraternal twin boy’s gene mutation arose from a de novo mutation.
- Citation: Li XJ, Su JM, Ye XW. Crouzon syndrome in a fraternal twin: A case report and review of the literature. World J Clin Cases 2022; 10(16): 5317-5323
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5317.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5317
Crouzon syndrome (CS) is an autosomal dominant inherited disorder and characterized by calvarial deformities with craniofacial dysostosis, exophthalmos, and facial anomalies[1,2]. In 1912, the French physician Louis Edouard Octave Crouzon first reported this syndrome and identified a mother and her son with craniosynostosis that resulted in skull and facial deformities[3]. This syndrome is described as the mildest type of many craniosynostosis syndromes[3,4]. The birth incidence of CS is approximately 1:25000[4]. It is proven to be the most common craniosynostosis syndrome, as it accounts for nearly 4.8% of all craniosynostosis cases at birth[1,5]. It is more common in males than females (3:1)[6]. Mutations in the fibroblast growth factor receptor 2 (FGFR2) gene on chromosome locus 10q25.3-q26 are related to CS[7]. Families with CS commonly suggest an autosomal dominant inheritance pattern[8,9]. The syndrome presents incomplete penetrance and variable expressivity leading to phenotypical characteristics varying from mild to severe[7,10]. About 50% of cases result from de novo mutations of the FGFR2 gene[11].
CS is commonly observed at birth owing to characteristic facial and cranial deformities and a positive family history. The prominent manifestations of these patients can be detected upon physical examination. Brachycephaly, hypertelorism, proptosis, flattened forehead, beaked noses, and maxil
| Crouzon | Apert | Pfeiffer | Muenke | Saethre-Chotzen | |
| Gene | FGFR2 | FGFR2 | FGFR1/ FGFR2 | FGFR3 | TWIST1 |
| Craniofacial phenotype | Brachycephaly; shallow orbits; ocular proptosis; midface hypoplasia | Turribrachycephaly; large anterior fontanelle with bitemporal widening and occipital flattening; shallow orbits with ocular proptosis and horizontal grooves above supraorbital ridges; mild hypertelorism and down-slanting palpebral fissures; “parrot beak” nasal deformity | Turribrachycephaly; midface hypoplasia; proptosis; hypertelorism; strabismus; down-slanting palpebral fissures; beaked nasal deformity | Craniosynostosis of coronal sutures; uncommon to have midface hypoplasia | Brachycephaly or acrocephaly or anterior plagiocephaly; low frontal hairline; eyelid ptosis; facial asymmetry; deviated nasal septum; ear deformities; parietal foramina |
| Oral and dental phenotype | Constricted, high-arched palate; anterior open bite; Missing teeth; supernumerary; delayed tooth eruption | High arched or cleft palate; severe midface hypoplasia; anterior open bite; tooth agenesis; supernumerary teeth; dental fusion; delayed tooth eruption | Anterior open bit and bilateral posterior cross-bites; hypodontia; microdontia; dilacerations; radicular dentin dysplasia | High arched palate; the lowest incidence of cleft palate | Cleft palate |
| Limb phenotype | Normal hands and feet | Severe syndactyly (mitten hand); poor joint mobility | Broad thumbs; broad great toes; variable feature, partial soft-tissue syndactyly of the hands; ankylosed elbows; hydrocephalus | Thimble-like middle phalanges | Brachydactyly; syndactyly; radioulnar synostosis; Hallux valgus |
| Other phenotype | Low-set ears | Hearing loss; visceral anomalies | Hearing loss | Hearing loss; congenital heart malformation; short stature | |
| Cognition | Normal | Intellectual disability | Normal or nearly normal | Normal |
The most common dental problems in craniosynostosis are supernumerary teeth, hypodontia, delayed eruption, and macrodontia[23,24]. We report a 6-year-old fraternal twin boy with CS who had many caries and enamel hypomineralization in the oral cavity. The boy’s parents and his fraternal twin sister did not show any abnormalities indicating CS. Therefore, we hypothesize that the twin boy’s gene mutation arose from a de novo mutation.
A 6-year-old Chinese boy was referred to the stomatology department of the Children’s Hospital, Zhejiang University School of Medicine, with a chief complaint of dental caries.
Dental caries were observed since the patient was 4-years-old and gradually became progressively larger.
The patient underwent intracranial decompression surgery at the age of 1 due to hydrocephalus. Posterior cranial vault distraction was performed when he was 2-years-old. The patient underwent a ventriculoperitoneal shunt operation for hydrocephalus at the age of 3. One year later, the patient had cranial vault remodeling as a result of craniofacial dysostosis.
His medical and developmental history showed that he was born at full-term by cesarean delivery after an uneventful pregnancy with a 48 cm height and 2.35 kg weight, along with his fraternal twin sister with a 48 cm height and 1.8 kg weight. The family history of going back for three generations was negative for similar conditions, and his fraternal twin sister had no medical issues.
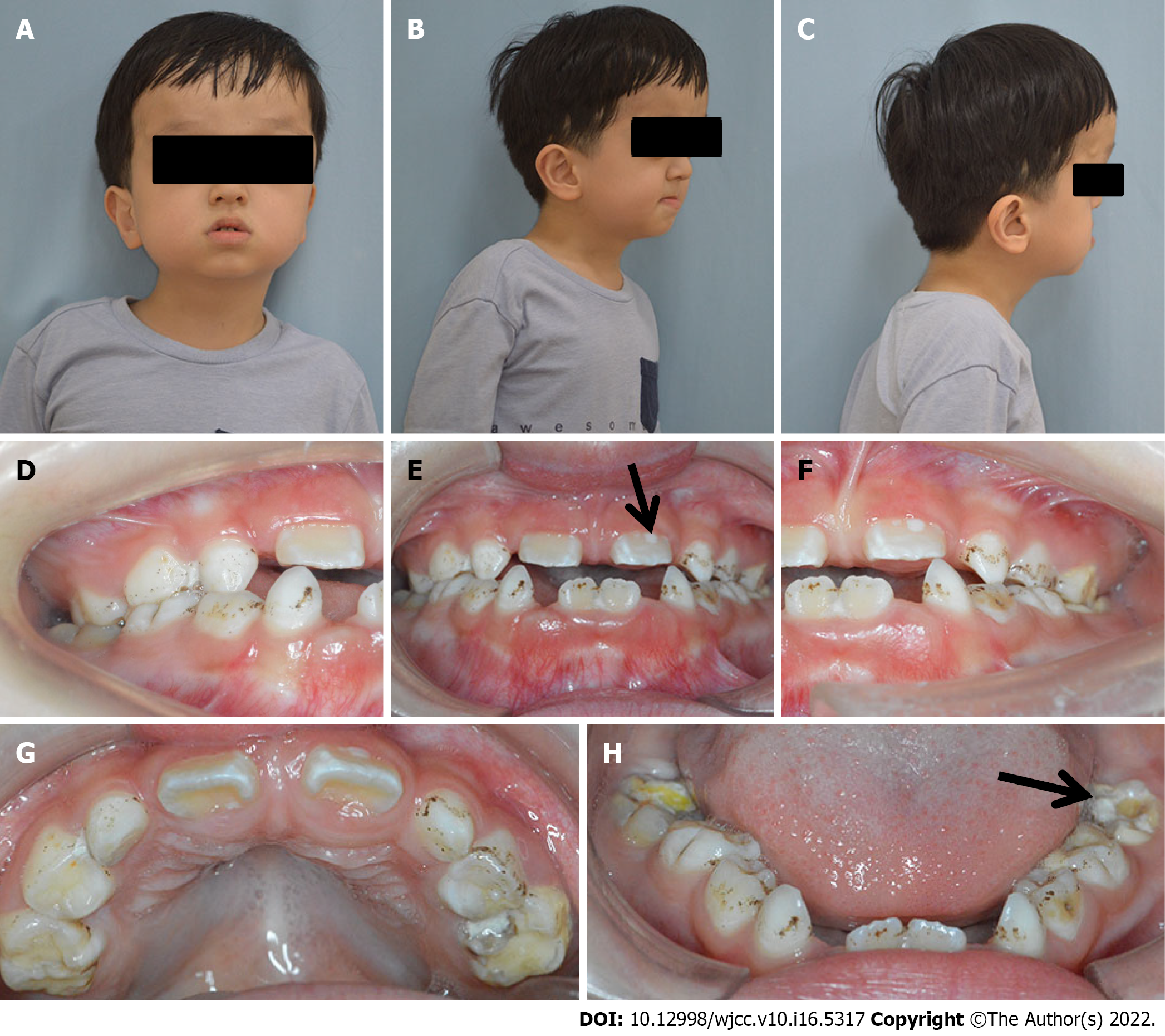
Physical examination showed normal height (108 cm) and weight (20.4 kg) compared with that of children of the same age. Clinically normal hands and feet were found. Extraoral examination revealed a prominent forehead, eyelid ptosis, ocular proptosis, shallow eye sockets, hypertelorism, midface hypoplasia, and a retrusive upper lip and protrusive lower lip (Figure 1).
Intraoral examination showed that the patient was in early mixed dentition, with all deciduous canines and molar teeth present in the maxillary and mandibular arch. The chronology of eruption and eruption status was in accordance with the child’s age (Figure 1). The oral hygiene of the child was poor with scattered pigmentation on the teeth, and permanent teeth 36 and 46 and deciduous teeth 54, 55, 65, 74, 75, and 85 were mostly decayed. The eruptive permanent teeth, including 11, 21, 36, and 46, showed enamel hypomineralization. The maxillary arch was constricted, and the anterior cross bite and open bite are shown.
Sanger sequencing showed that the pathogenic variant c.1026C>G (p.Cys342Trp) was present on the FGFR2 gene for the patient. The mutation was not present in any of the family members.
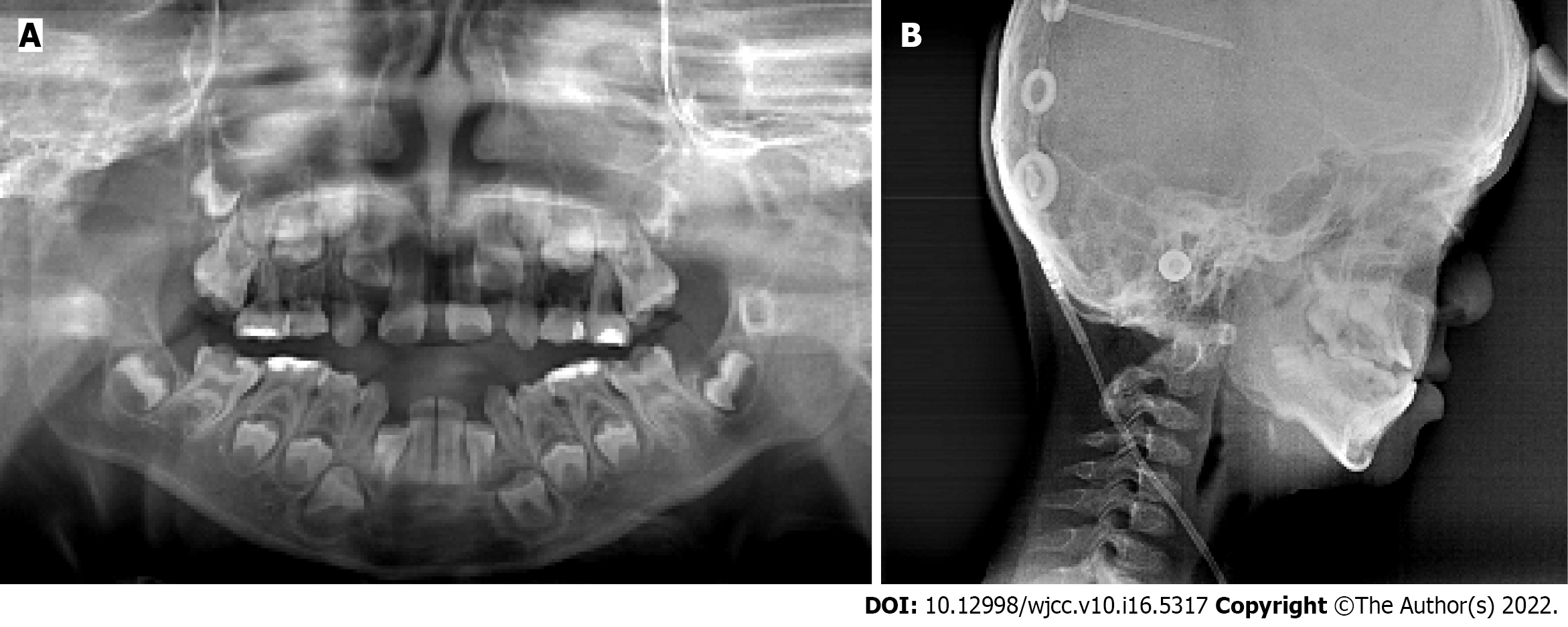
The panoramic radiograph showed that there were no supernumerary teeth or normal permanent tooth germ development. Deciduous carious teeth 55, 64, 65, 75, and 85 had once been restored and displayed further second caries at the time of the study. Permanent teeth 36 and 46 and deciduous teeth 54 and 74 were decayed (Figure 2A).
Based on the lateral cephalometric analysis, a severe skeletal Class III relationship was diagnosed with Sella-Nasion-A point angle: 63.8°, Sella-Nasion-B point angle: 71.6°, and A point-Nasion-B point angle: -7.8° (Figure 2B). A hypodivergent facial pattern was shown with Sella-Nasion/Gonion-Gnathion: 35.1°. The cephalometric analysis also revealed the maxillary skeletal size (condylion to point A) was diminished. The upper central incisors leaned toward the lip (U1/Sella-Nasion: 112.8°).
The final diagnosis of the presented case was CS, caries, and enamel hypomineralization.
A comprehensive treatment plan was made. The initial treatment involved improving the patient’s oral hygiene and restoring his carious teeth. The dental caries in teeth 54, 55, 64, 65, 74, 75, 85, 36, and 46 were treated with resin-based restorations. Topical fluoride was regularly applied to enamel hypomineralization in teeth 11, 21, 36, and 46 to promote enamel remineralization. The Skeletal Class III malocclusion was treated by orthodontic treatment and orthognathic surgery. Multidisciplinary approaches involving pediatric dentists, dental surgeons, oral and maxillofacial surgeons, and orthodontists were indispensable for this treatment.
At the first dental visit, the patient was nervous and fearful and was unwilling to open his mouth. Appropriately administered behavior management and guidance[25,26], including show-and-tell tactics, positive reinforcement, sound communication, and modeling, were used to manage the patient’s anxieties. Oral examination, radiographic assessment, oral prophylaxis, and polishing of the teeth with the rubber cup were performed during the first dental appointment. For the latter dental appointments, carious restoration was successfully completed utilizing show-and-tell tactics, positive reinforcement, and distracting the patient by showing movies during the procedure. After the procedure, the patient was praised and given a post-visit sticker, and he was agreeable for a future visit.
Three months later, the oral hygiene of the patient was good, and topical fluoride was applied in teeth 11, 21, 36, and 46 to promote enamel remineralization.
Craniosynostosis results from the premature fusion of one or multiple cranial sutures, resulting in restricted growth of the skull, brain, face, and central nervous system development[4]. CS is the most common syndromic craniosynostosis and is caused by the mutation of FGFR2[17]. FGFR2 belongs to a family of four FGFRs. The FGFR family plays a primary role in the growth and differentiation of mesenchymal and neuroectodermal cells by binding to FGF and initiating signal transduction. Additionally, the FGFR family regulates cranial suture fusion on a macroscopic level[7].
For our patient the c.1026C>G (p.Cys342Trp) mutation on the FGFR2 gene caused a cysteine-to-tryptophan substitution at amino acid 342. Mutations at amino acid 342 can cause CS and Pfeiffer syndrome. Normal hands and feet can be observed with CS, which differentiates it from Pfeiffer syndrome. The loss of this cysteine residue is one of the most common mutations in CS patients and has previously been reported in a Chinese family, a Japanese sporadic case, and 4 Caucasian cases[9,27-29]. The Chinese family and 1 Caucasian family with CS have a dominant inherited amino acid 342 mutation[9,27]. The Japanese case and the German patients with CS were sporadic[28,29]. Since the fraternal twin sister and the parents did not present gene mutations and similar presentations, our patient’s mutation was considered to be a de novo mutation. In 11 of the 21 families with CS who underwent tracing to determine the origin of the mutation by analyzing parents and the other family members, FGFR2 mutations arose de novo, representing a high mutation rate for FGFR2[29].
This case report describes a patient with CS, who presented with a high forehead, ocular proptosis, shallow eye sockets, hypertelorism, palpebral ptosis, and a skeletal Class III relationship. The carious teeth were fully restored, and oral hygiene instruction was performed in the clinic under behavior management and guidance. The child was advised to follow-up regularly with preventive care for teeth. The boy was satisfied with his good oral hygiene. Interceptive orthodontics was contemplated. Midfacial advancement with a Le Fort III, Le Fort II plus zygomatic repositioning, monobloc, or facial bipartition is required for midface retrusion[30]. The appropriate time of surgery is deemed at 8 years of age[31]. He has been referred to the Department of Oral and Maxillofacial Surgery for orthognathic surgical procedures in the next few years. He has also been referred to the Departments of Neurosurgery, Ophthalmology, Otolaryngology, and Psychology to prevent complications.
In summary, CS could occur in a fraternal twin caused by a de novo mutation of the FGFR2 gene and is characterized by craniosynostosis, a prominent forehead, midface hypoplasia, and proptosis. Oral hygiene instruction and preventive programs on oral hygiene should be performed regularly. A multidisciplinary approach involving oral and maxillofacial surgeons and orthodontists was necessary for the treatment of midface hypoplasia.
We sincerely thank the patient and his parents for their participation and permission to publish this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Malekzadegan A, Iran; Tsou HK, Taiwan S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Bowling EL, Burstein FD. Crouzon syndrome. Optometry. 2006;77:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Khandelwal R, Agrawal P, Majumdar MR. Crouzon syndrome. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Padmanabhan V, Hegde AM, Rai K. Crouzon's syndrome: A review of literature and case report. Contemp Clin Dent. 2011;2:211-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Aviv RI, Rodger E, Hall CM. Craniosynostosis. Clin Radiol. 2002;57:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Wang JC, Nagy L, Demke JC. Syndromic Craniosynostosis. Facial Plast Surg Clin North Am. 2016;24:531-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Gothwal S, Nayan S, Kumar J. Crouzon syndrome with bony upper airway obstruction: case report and review literature. Fetal Pediatr Pathol. 2014;33:199-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Al-Namnam NM, Hariri F, Thong MK, Rahman ZA. Crouzon syndrome: Genetic and intervention review. J Oral Biol Craniofac Res. 2019;9:37-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Lin Y, Gao H, Ai S, Eswarakumar JVP, Zhu Y, Chen C, Li T, Liu B, Jiang H, Liu Y, Li Y, Wu Q, Li H, Liang X, Jin C, Huang X, Lu L. FGFR2 mutations and associated clinical observations in two Chinese patients with Crouzon syndrome. Mol Med Rep. 2017;16:5841-5846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Li ZL, Chen X, Zhuang WJ, Zhao W, Liu YN, Zhang FX, Ha RS, Wu JH, Zhao C, Sheng XL. FGFR2 mutation in a Chinese family with unusual Crouzon syndrome. Int J Ophthalmol. 2016;9:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Lu X, Forte AJ, Fan F, Zhang Z, Teng L, Yang B, Alperovich M, Steinbacher DM, Alonso N, Persing JA. Racial disparity of Crouzon syndrome in maxilla and mandible. Int J Oral Maxillofac Surg. 2020;49:1566-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Pal US, Gupta C, Chellappa AA. Crouzon syndrome with primary optic nerve atrophy and normal brain functions: A case report. J Oral Biol Craniofac Res. 2012;2:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Torun GS, Akbulut A. Crouzon syndrome with multiple supernumerary teeth. Niger J Clin Pract. 2017;20:261-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Forte AJ, Lu X, Hashim PW, Steinbacher DM, Alperovich M, Persing JA, Alonso N. Analysis of Airway and Midface in Crouzon Syndromes. Ann Plast Surg. 2019;82:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Quintero-Rivera F, Martinez-Agosto JA. Bilateral exophthalmos: Report of a case and review of a fibroblast growth factor receptor 2 mutation associated with non-penetrant Crouzon syndrome. J Paediatr Child Health. 2010;46:693-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Stavropoulos D, Bartzela T, Tarnow P, Mohlin B, Kahnberg KE, Hagberg C. Dental agenesis patterns in Crouzon syndrome. Swed Dent J. 2011;35:195-201. [PubMed] |
| 16. | Reitsma JH, Balk-Leurs IH, Ongkosuwito EM, Wattel E, Prahl-Andersen B. Dental maturation in children with the syndrome of crouzon and apert. Cleft Palate Craniofac J. 2014;51:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Ko JM. Genetic Syndromes Associated with Craniosynostosis. J Korean Neurosurg Soc. 2016;59:187-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Elmi P, Reitsma JH, Buschang PH, Wolvius EB, Ongkosuwito EM. Mandibular asymmetry in patients with the crouzon or apert syndrome. Cleft Palate Craniofac J. 2015;52:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Agochukwu NB, Solomon BD, Doherty ES, Muenke M. Palatal and oral manifestations of Muenke syndrome (FGFR3-related craniosynostosis). J Craniofac Surg. 2012;23:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Hassona Y, Al-Hadidi A, Ghlassi TA, Dali HE, Scully C. Pfeiffer syndrome: oral healthcare management and description of new dental findings in a craniosynostosis. Spec Care Dentist. 2017;37:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | López-Estudillo AS, Rosales-Bérber MA, Ruiz-Rodríguez S, Pozos-Guillén A, Noyola-Frías MÁ, Garrocho-Rangel A. Dental approach for Apert syndrome in children: a systematic review. Med Oral Patol Oral Cir Bucal. 2017;22:e660-e668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Lu X, Sawh-Martinez R, Forte AJ, Wu R, Cabrejo R, Wilson A, Steinbacher DM, Alperovich M, Alonso N, Persing JA. Mandibular Spatial Reorientation and Morphological Alteration of Crouzon and Apert Syndrome. Ann Plast Surg. 2019;83:568-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ye X, Attaie AB. Genetic Basis of Nonsyndromic and Syndromic Tooth Agenesis. J Pediatr Genet. 2016;5:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Khominsky A, Yong R, Ranjitkar S, Townsend G, Anderson PJ. Extensive phenotyping of the orofacial and dental complex in Crouzon syndrome. Arch Oral Biol. 2018;86:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Nelson T. The continuum of behavior guidance. Dent Clin North Am. 2013;57:129-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Karekar P, Bijle MN, Walimbe H. Effect of Three Behavior Guidance Techniques on Anxiety Indicators of Children Undergoing Diagnosis and Preventive Dental Care. J Clin Pediatr Dent. 2019;43:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Ma HW, Lajeunie E, Le Merrer M, de Parseval N, Serville F, Weissenbach J, Munnich A, Renier D. No evidence of genetic heterogeneity in Crouzon craniofacial dysostosis. Hum Genet. 1995;96:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Nagase T, Nagase M, Hirose S, Ohmori K. Mutations in fibroblast growth factor receptor 2 gene and craniosynostotic syndromes in Japanese children. J Craniofac Surg. 1998;9:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Kress W, Collmann H, Büsse M, Halliger-Keller B, Mueller CR. Clustering of FGFR2 gene mutations inpatients with Pfeiffer and Crouzon syndromes (FGFR2-associated craniosynostoses). Cytogenet Cell Genet. 2000;91:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Taylor JA, Bartlett SP. What's New in Syndromic Craniosynostosis Surgery? Plast Reconstr Surg. 2017;140:82e-93e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Lloyd MS, Trost JG, Khechoyan DY, Hollier LH Jr, Buchanan EP. Identical Twins with Crouzon Syndrome: Eight-Year Follow-up, Genetic Considerations, and Operative Management. Craniomaxillofac Trauma Reconstr. 2017;10:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |