Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.5082
Peer-review started: December 16, 2021
First decision: February 14, 2022
Revised: February 23, 2022
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: May 26, 2022
Processing time: 159 Days and 7.1 Hours
Mycobacterium abscessus (M. abscessus) is a rapidly growing mycobacterium and ubiquitous in the environment, which infrequently causes disease in humans. However, it can cause cutaneous or respiratory infections among immunocompromised hosts. Due to the resistance to most antibiotics, the pathogen is formidable and difficult-to-treat.
Here, we present a case of catheter-related M. abscessus infections in a patient with motor neurone disease. Catheter and peripheral blood cultures of the patient showed positive results during Gram staining and acid-fast staining. The alarm time of catheter blood culture was 10.6 h earlier than that of peripheral blood. After removal of the peripherally inserted central catheter, secretion and catheter blood culture were positive. M. abscessus was identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry and 16S rDNA sequencing.
For catheter-related M. abscessus infection, rapid diagnosis and timely and adequate antimicrobial therapy are crucial.
Core Tip: Mycobacterium abscessus (M. abscessus) is a rapidly growing mycobacterium and ubiquitous in the environment, which infrequently causes disease in humans. However, it can cause cutaneous or respiratory infections among immunocompromised hosts. Due to the resistance to most antibiotics, the pathogen is formidable and difficult-to-treat. Here, we present a case of catheter-related M. abscessus infections in a patient with motor neurone disease. M. abscessus was identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry and 16S rDNA sequencing. For catheter-related M. abscessus infection, rapid diagnosis and timely and adequate antimicrobial therapy are crucial.
- Citation: Pan SF, Zhang YY, Wang XZ, Sun JJ, Song SL, Tang YR, Wang JL. Catheter-related infections caused by Mycobacterium abscessus in a patient with motor neurone disease: A case report. World J Clin Cases 2022; 10(15): 5082-5087
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/5082.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.5082
Mycobacterium abscessus (M. abscessus) is a rapidly growing non-tuberculous mycobacterium and is ubiquitous in the environment[1]. Recent gene sequence analysis has shown that M. abscessus can be divided into three different subspecies (M. abscessus subsp. bolletii, M. abscessus subsp. abscessus, and M. abscessus subsp. massiliense)[2]. M. abscessus can cause a variety of clinical manifestations including cutaneous infections, catheter-related infections, post-surgical soft tissue infections, and respiratory diseases[3]. Besides, the incidence of pulmonary non-tuberculous mycobacteria infection has been increasing[4]. Of all the rapidly growing mycobacteria, M. abscessus is the most common cause of pulmonary infections. M. abscessus is also one of the mycobacteria that are most often isolated from patients with cystic fibrosis[5]. However, M. abscessus is resistant to most antibiotics in vitro, and thus is a formidable and difficult-to-treat pathogen[6]. At present, little is known regarding the diagnosis and management of catheter-related M. abscessus infections due to only a limited number of cases that have been reported[7,8]. Here, we present a case of catheter-related M. abscessus infections in a patient with motor neurone disease.
On February 6, 2019, a 62-year-old Chinese man presented with mild skin edema at the site of the peripherally inserted central venous catheter (PICC, median cubital vein) which had been inserted for 30 d, without skin redness and inflammatory exudation.
He presented with a two-day history of dyspnea and was admitted to our emergency intensive care unit with a diagnosis of motor neurone disease on January 7, 2019. The patient received comprehensive symptomatic treatment including invasive ventilator-assisted ventilation, pulse oxygen saturation measurement, expectorant, and nutritional supplement.
There was no history of past illness.
There was no personal and family history.
Physical examination revealed that he was stuporous with a Glasgow coma scale of E1VTM1. His body temperature was 35.0 °C, heart rate 66 beats per minute, blood pressure 90/54 mmHg, and respiratory rate 23 breaths per minute.
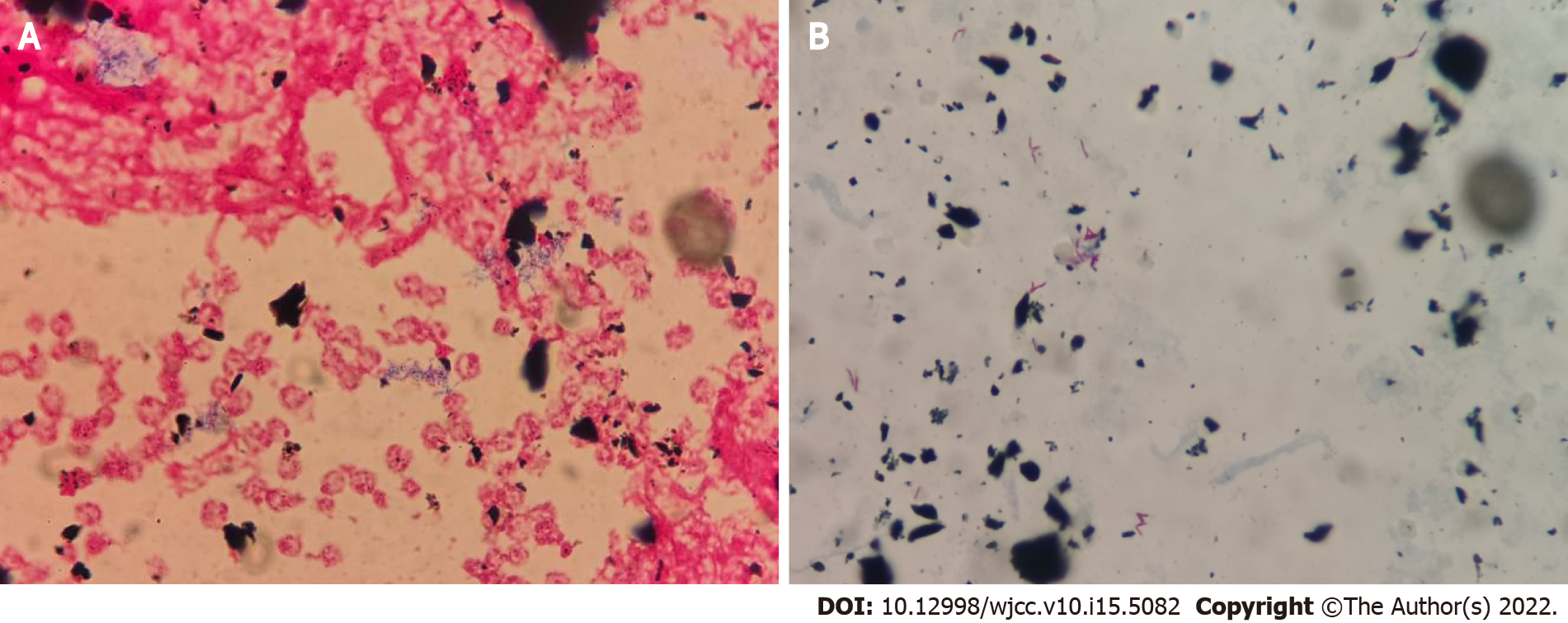
Routine blood tests revealed 3.7 × 109 cells/L white blood cells (reference range, 4.0-10.0 × 109 cells/L) and 67.2% neutrophils (reference range, 40%-75%). Catheter and peripheral blood cultures were performed on February 10, 2019. On February 11, 2019, he presented with elevated body temperature (37.1 °C), white blood cells (10.2 × 109 cells/L), and neutrophils (86.7%). The serum procalcitonin and C-reactive protein levels was 0.99 ng/mL (reference range, 0-0.05 ng/mL) and 184.9 mg/L (reference range, 0-5 mg/L), respectively. The patient began to receive anti-infective treatment with vancomycin. On February 19, 2019, the blood culture showed positive results during Gram staining (Figure 1A) and acid-fast staining (Figure 1B). The alarm time of catheter blood culture was 10.6 h earlier than that of peripheral blood culture. The treatment regimen of the patient was changed to combination therapy with vancomycin and amikacin.
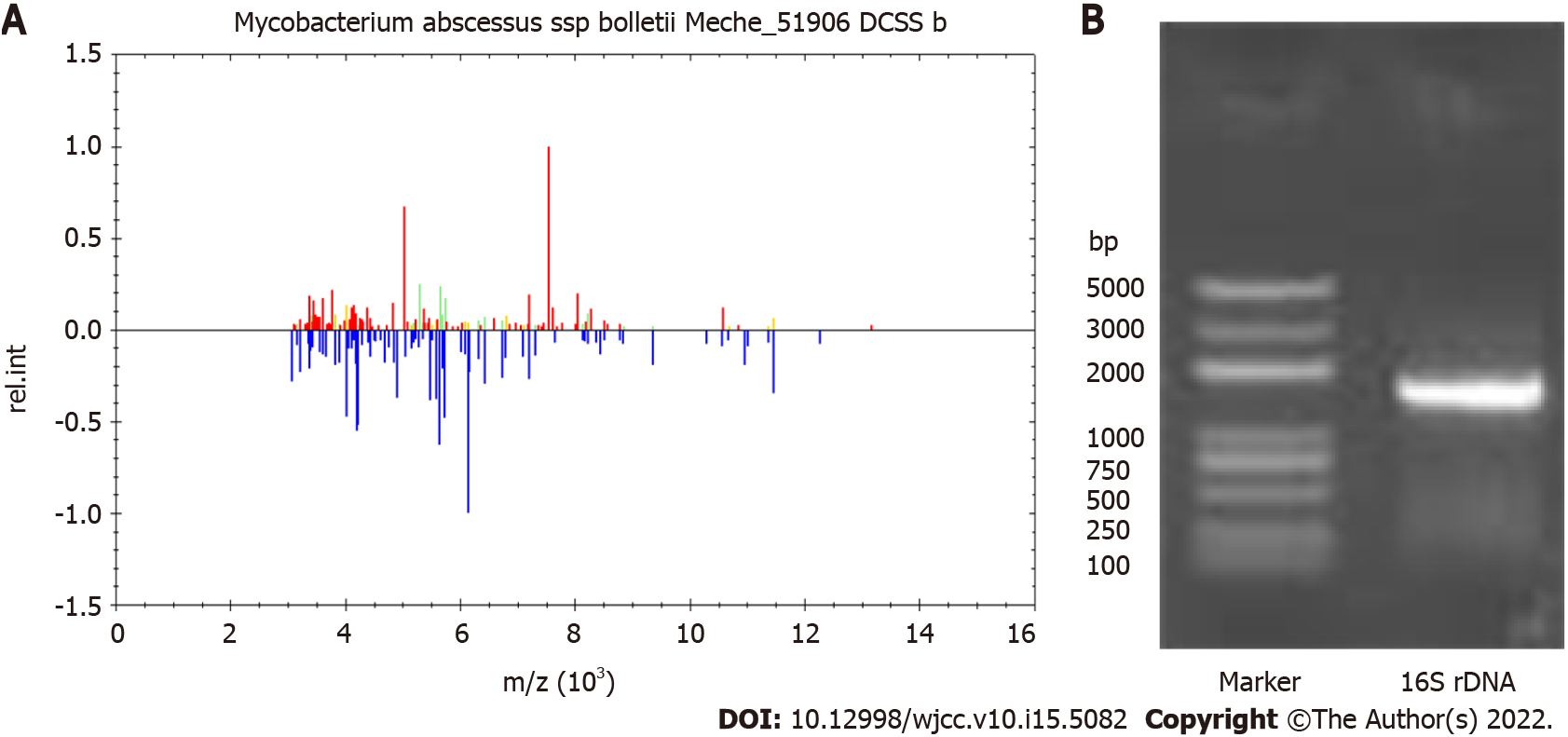
On February 23, 2019, repeat blood cultures showed positive acid-fast bacilli, and the treatment regimen was adjusted to amikacin plus clarithromycin. On the second day, his body temperature was 35.3 °C, his heart rate was 74 beats per minute, white blood cells 7.4 × 109 cells/L, and neutrophils 77.6%. After removal of the PICC on March 11, 2019, secretion culture and catheter blood culture were all positive. The blood culture isolate was identified as M. abscessus by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) (Figure 2A) and 16S rDNA sequencing (Figure 2B).
The patient was finally diagnosed with catheter-related infections caused by M. abscessus.
He continued to receive anti-infective treatment with amikacin plus clarithromycin.
His body temperature was maintained at a basic level (35-36.3 °C). Unfortunately, the patient gave up treatment due to an unsatisfactory response to respiratory failure and shock and was discharged on April 13, 2019.
M. abscessus was a terrible and difficult-to-treat mycobacterial pathogen, which is resistant to most antibiotics in vitro[9]. It was ubiquitous in the environment including soil, water, and dust, and survived extreme temperatures and nutritional deprivation[10]. It can cause soft tissue and skin infections after surgical procedures or trauma, pulmonary infections, and disseminated diseases among immunocompromised hosts[11]. Infections in immunocompetent patients tend to be more localized, and usually due to contamination of wounds or abrasions with soil, water, dust, or other materials[12]. While, infections in immunosuppressed patients are often deeper and more diffuse, involving subcutaneous tissue, and leading to the formation of an abscess. Catheter-related infections often occurred in the setting of central venous access devices[13]. In the present study, we present a case of catheter-related M. abscessus infections in a patient with motor neurone disease.
Catheter-related bloodstream infection (CRBSI) is the most common complication associated with the use of intravascular catheters[14]. Our patient was a 62-year-old man with motor neurone disease. He had been treated in our hospital many times due to respiratory failure, shock, and electrolyte disorder. During the hospitalization, the patient used PICC for blood controls, which was high-risk factor for CRBSI. No other obvious source of bloodstream infection was found, except for PICC. He presented with fever and mild skin edema at the site of PICC. Routine blood tests revealed elevated white blood cells and neutrophils, procalcitonin, and C-reactive protein. Catheter and peripheral blood cultures of the patient showed positive results during Gram staining and acid-fast staining. The alarm time of catheter blood culture was 10.6 h earlier than that of peripheral blood. He was diagnosed with catheter-related infections. Gram positive cocci (such as staphylococcus epidermidis, staphylococcus aureus, and enterococcus) have been historically the leading cause of CRBSI[15]. Catheter-related M. abscessus infection is relatively rare, with limited literature reports[7,8]. In the present study, M. abscessus was identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry and 16S rDNA sequencing. The patient was finally diagnosed with catheter-related infections caused by M. abscessus.
The main threat of M. abscessus is its antibiotic resistance. M. abscessus may be the most resistant species among pathogenic rapidly growing mycobacteria, and its antimicrobial therapy is still a challenge[8]. A previous study showed that the treatment strategy of M. abscessus infections was the combination of multiple antibiotics, including amikacin, ofloxacin, ciprofloxacin, clarithromycin, and doxycycline[16]. In vitro studies showed that clarithromycin was the most effective of these antibiotics[17]. In the present study, the patient received anti-infective treatment with amikacin plus clarithromycin. In addition, in the case of catheter-related M. abscessus infections, removal of the catheter is necessary due to the high incidence of relapsing or uncontrolled bacteremia[8]. After the removal of the PICC of our patient, he continued to receive anti-infective treatment with amikacin plus clarithromycin, and his body temperature was maintained at a basic level (35-36.3 °C). Unfortunately, the patient gave up treatment due to an unsatisfactory response to respiratory failure and shock, so the long-term treatment results were not obtained.
In conclusion, we reported a case of catheter-related M. abscessus infections in a patient with motor neurone disease. For catheter-related M. abscessus infection, rapid diagnosis, and timely and adequate antimicrobial therapy are crucial.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karavaş E, Turkey; Vagholkar K, India S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Rodríguez-García R, Espina Angulo MJ, Escudero Augusto D. Cutaneous infection with Mycobacterium abscessus. Intensive Care Med. 2018;44:2292-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Aziz DB, Low JL, Wu ML, Gengenbacher M, Teo JWP, Dartois V, Dick T. Rifabutin Is Active against Mycobacterium abscessus Complex. Antimicrob Agents Chemother. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Gutiérrez AV, Viljoen A, Ghigo E, Herrmann JL, Kremer L. Glycopeptidolipids, a Double-Edged Sword of the Mycobacterium abscessus Complex. Front Microbiol. 2018;9:1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Benwill JL, Wallace RJ Jr. Mycobacterium abscessus: challenges in diagnosis and treatment. Curr Opin Infect Dis. 2014;27:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Petrini B. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS. 2006;114:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 396] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 7. | Lee SA, Raad II, Adachi JA, Han XY. Catheter-related bloodstream infection caused by Mycobacterium brumae. J Clin Microbiol. 2004;42:5429-5431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Laurens C, Héry-Arnaud G, Chiron R, Oziol E, Jean-Pierre H, Bouzinbi N, Vande Perre P, Bañuls AL, Godreuil S. Sacroiliitis secondary to catheter-related bacteremia due to Mycobacterium abscessus (sensu stricto). Ann Clin Microbiol Antimicrob. 2014;13:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Griffith DE, Brown-Elliott BA, Benwill JL, Wallace RJ Jr. Mycobacterium abscessus. "Pleased to meet you, hope you guess my name...". Ann Am Thorac Soc. 2015;12:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Mooren VHJF, Bleeker MWP, van Ingen J, Hermans MHA, Wever PC. Disseminated Mycobacterium abscessus infection in a peritoneal dialysis patient. IDCases. 2017;9:6-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Bechara C, Macheras E, Heym B, Pages A, Auffret N. Mycobacterium abscessus skin infection after tattooing: first case report and review of the literature. Dermatology. 2010;221:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Drage LA, Ecker PM, Orenstein R, Phillips PK, Edson RS. An outbreak of Mycobacterium chelonae infections in tattoos. J Am Acad Dermatol. 2010;62:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Xie O, Khan S, Globan M, Lea K, Bajel A, Slavin M. Mycobacterium abscessus bloodstream infection: Unexpected catheter tunnel infection localized by PET/CT. Transpl Infect Dis. 2019;21:e13147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Ruiz-Ruigómez M, Fernández-Ruiz M, San-Juan R, López-Medrano F, Orellana MÁ, Corbella L, Rodríguez-Goncer I, Hernández Jiménez P, Aguado JM. Impact of duration of antibiotic therapy in central venous catheter-related bloodstream infection due to Gram-negative bacilli. J Antimicrob Chemother. 2020;75:3049-3055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Tarpatzi A, Avlamis A, Papaparaskevas J, Daikos GL, Stefanou I, Katsandri A, Vasilakopoulou A, Chatzigeorgiou KS, Petrikkos GL. Incidence and risk factors for central vascular catheter-related bloodstream infections in a tertiary care hospital. New Microbiol. 2012;35:429-437. [PubMed] |
| 16. | Kameyama H, Mori Y, Kimura T, Sugishita C, Adachi T, Sonomura K, Kusaba T, Tanda S, Kishimoto N, Okigaki M, Hatta T, Matsubara H. A case report of Mycobacterium abscessus peritonitis in a peritoneal dialysis patient. Ther Apher Dial. 2007;11:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Ellis EN, Schutze GE, Wheeler JG. Nontuberculous mycobacterial exit-site infection and abscess in a peritoneal dialysis patient. A case report and review of the literature. Pediatr Nephrol. 2005;20:1016-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |