Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4935
Peer-review started: September 27, 2021
First decision: January 11, 2022
Revised: February 21, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: May 26, 2022
Processing time: 239 Days and 12.9 Hours
Wernekink commissural syndrome (WCS) is a distinct midbrain syndrome that involves the caudal tegmentum of the midbrain and selectively damages the Wernekink commissure involved in the decussation of the superior cerebellar peduncle in midbrain. The aim of the study was to explore the clinical manifestations, imaging characteristics, and differential diagnosis of WCS in midbrain infarction to provide reference for clinicians in the diagnosis of WCS.
The clinical data of 4 patients with WCS with midbrain infarction were analyzed retrospectively. WCS is a rare syndrome that can be diagnosed based on its characteristic symptoms and imaging findings of magnetic resonance imaging.
Clinicians should look for this syndrome in cases of bilateral cerebellar dysfunction and eye movement disorders.
Core Tip: The aim of the study was to explore the clinical manifestations, imaging characteristics, and differential diagnosis of Wernekink commissural syndrome (WCS) in midbrain infarction and to provide reference for the clinicians in the diagnosis of WCS. WCS is a rare syndrome that can be diagnosed based on its characteristic symptoms and imaging findings of magnetic resonance imaging. Clinicians should look for this syndrome in cases of bilateral cerebellar dysfunction and eye movement disorders.
- Citation: Yang YZ, Hu WX, Zhai HJ. Special type of Wernekink syndrome in midbrain infarction: Four case reports. World J Clin Cases 2022; 10(15): 4935-4941
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4935.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4935
Wernekink commissural syndrome (WCS) is a distinct midbrain syndrome that involves the caudal tegmentum of the midbrain and selectively damages the cerebellar superior crural commissure (Wernekink commissure), resulting in characteristic clinical manifestations. Signs and symptoms associated with WCS include persistent bilateral cerebellar dysfunction, eye movement disorder, internuclear ophthalmoplegia, and, rarely, delayed palatine myoclonus. Due to the small number of cases, there are no data on the epidemiology of the disease and only a few case-reports in the literature describing the features of this syndrome[1,2]. Considering the rarity of the syndrome, we hereby present clinical manifestations and imaging results of 4 patients with WCS. Our report may improve clinicians' understanding of the syndrome and provide reference for clinical diagnosis.
Case 1: Dizziness for 1 wk.
Case 2: Sudden dizziness since 4 d.
Case 3: Sudden ptosis of left eyelid and dizziness since 2 d.
Case 4: Repeated chest tightness and shortness of breath since 2 d.
Specific signs and symptoms of WCS in our patients are presented in Table 1.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Ocular findings | Restricted adduction of eyeball | Ptosis of right eyelidRestricted adduction of eyeballPoor visual acuity in superior gaze | Ptosis of left eyelidLeft pupil was dilated Absence of pupillary reflexRestricted movement of the left globe in superior and downward gaze | Sluggish light reflexRestricted adduction of eyeball |
| Cerebellar findings | Abnormal left finger nose and left heel knee tibia testClosed eye sign positive | Dizziness | Dizziness | DizzinessAtaxia |
| Cranial nerve findings | Right side deviation of tongue | - | - | - |
| Other findings | Decreased tendon reflex of limbs | - | - | Nausea, vomiting, drowsinessReduced muscle power of lower limbs |
Case 1: A 55-year-old male patient reported to our institute with a main complaint of dizziness for 1 wk. On examination, the patient was conscious, was welloriented, and had a blood pressure of 190/110 mmHg. Bilateral pupils were circular, equal in size, and reactive to light. There was restricted adduction of the globe towards the right side. Bilateral nasolabial sulci were similar in depth. There was right side deviation of the tongue, normal muscle strength of both limbs, abnormal left finger nose and left heel knee tibia test, and normal right finger nose and heel knee tibia test. The closed eye sign was positive. The tendon reflex of limbs was decreased or even absent. Acupuncture sensation, tactile sensation, and joint position sensations were normal bilaterally but without pathological reflex and meningeal stimulation sign. Respiratory, cardiovascular, and abdominal examinations were normal.
WCS was diagnosed based on cranial magnetic resonance imaging (MRI), cervical vascular color Doppler ultrasound, and routine blood and biochemical investigations. Blood homocysteine levels were elevated to 20 mcmol/L, while blood counts, liver and kidney function tests, serum glucose, lipid and electrolyte levels were normal.
Case 2: A 77-year-old male patient was hospitalized in our institute due to sudden dizziness since 4 d. On admission, patient’s blood pressure was 140/85 mmHg, and the patient was conscious and well-oriented. There was drooping of right upper eyelid. On ocular examination, pupils were circular, equal in size, and reactive to light but with limited right eyeball adduction and poor visual acuity in superior gaze. The nasolabial sulci were symmetrical, and there was no deviation of tongue on protrusion. The muscle strength and tension of the limbs were normal with normal tendon reflexes. Neck was soft, and Kirschner's sign and Brinell's sign were negative. Respiratory, cardiovascular, and abdominal examinations were normal. There was no edema in both lower limbs. The National Institutes of Health Stroke Scale was 1 point. Blood investigation revealed increased total cholesterol (5.75 mmol/L), triglycerides (2.17 mmol/L), and blood glucose (6.19 mmol/L); decreased serum alanine; and increased serum homocysteine (18.95 mcmol/L). The percentage of neutrophils were decreased (48.9%), and monocytes were increased (11.4%).
Case 3: A 55-year-old male patient was hospitalized in our institute with complaints of sudden ptosis of left eyelid and dizziness since 2 d. On examination, the blood pressure was 140/90 mmHg. Patient was conscious and well-oriented, with normal speech. There was obvious ptosis of left eyelid. Left pupil was dilated with absence of pupillary reflex. There was restriction in the movement of the left globe in superior and downward gaze but not on abduction. Right pupil was normal. Bilateral nasolabial sulcus was symmetrical. The muscle strength and muscle tension of the limbs were normal with normal tendon reflexes. Neck was soft and Kirschner's and Brinell's sign were negative. Respiratory, cardiovascular, and abdominal examinations were normal. There was no edema in both lower limbs. Blood and stool routine test, liver and kidney function, and electrolyte, blood lipid, homocysteine, immune, and coagulation profile were all within normal limits.
Case 4: A 68-year-old female patient was hospitalized in our institute with complaints of repeated chest tightness and shortness of breath for 3 years that had aggravated since 2 d. On admission the patient was afebrile with blood pressure of 136/92 mmHg, conscious, and well-oriented. Bilateral pupils were circular, equal in size, and reactive to light. There was no enlargement of any superficial lymph nodes and thyroid gland. The trachea was centered, and jugular vein was dilated. Respiratory sounds were thick but without any rales. On percussion, cardiac boundary was expanded. Heart rate was 91 beats/min with grade 2/6 systolic murmur heard between the second intercostal space at the right edge of the sternum. Abdominal examination was normal. Bilateral swelling was seen in the lower limbs with prominent varicose veins. Tendon reflexes and muscle strength were normal. Patient was initially treated with antihypertensive, hypoglycemic, cardiotonic, and diuretic drugs to improve myocardial remodeling. However, in 2 d the patient developed dizziness, drowsiness, nausea, vomiting, and unclear speech. Pupils were normal is size but with sluggish light reflex. There was limited adduction of right eye. The patient also showed signs of ataxia. Muscle power of lower limbs was reduced. Right pulmonary arterial pressure sign was positive but with negative meningeal stimulation sign. On blood investigation, liver and kidney function, myocardial enzyme, coagulation function, blood routine, urine routine, and stool examination were normal. Troponin levels and B-type natriuretic peptide levels were increased while serum potassium was reduced.
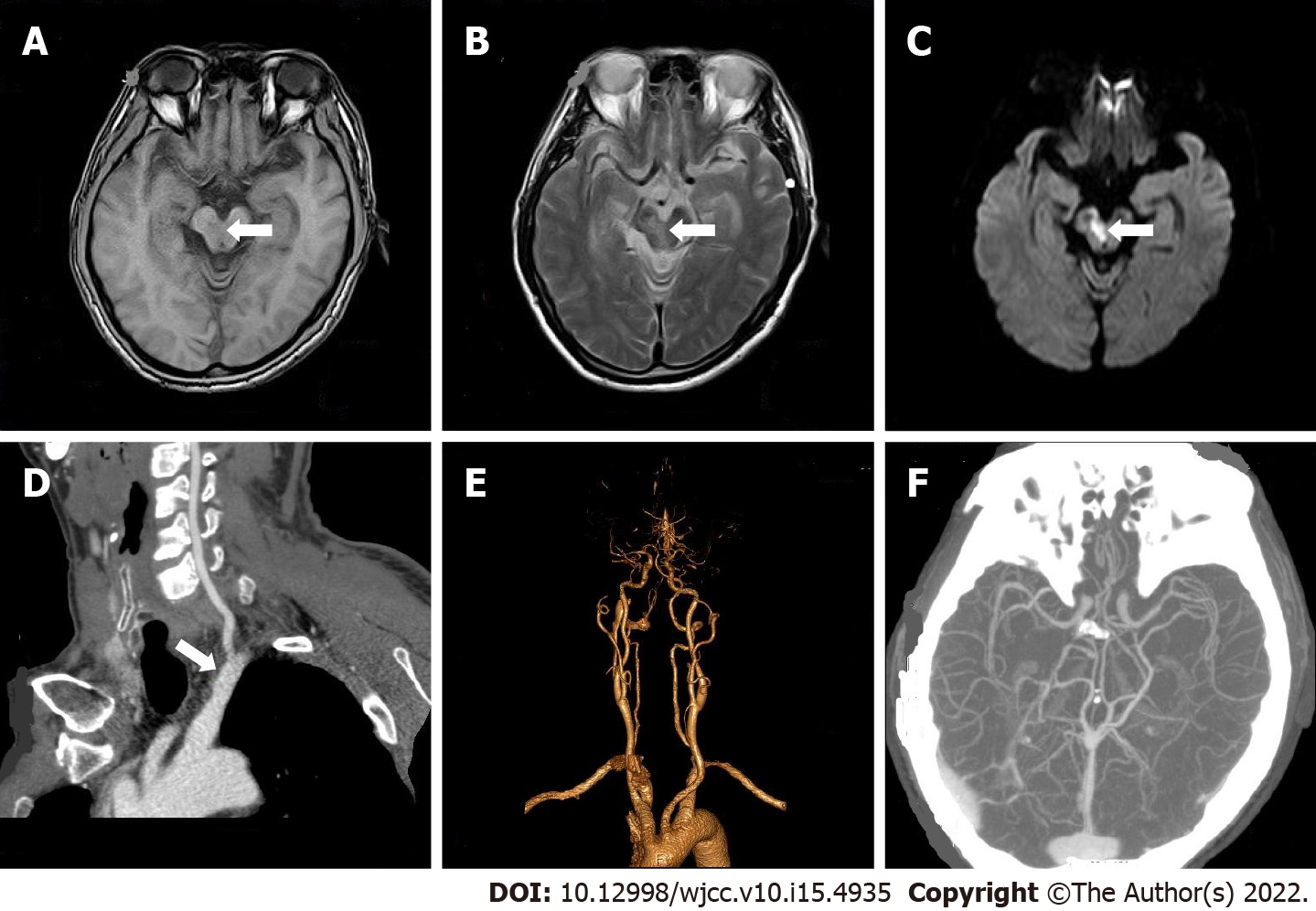
Case 1: Color Doppler ultrasound of carotid and vertebral arteries showed no obvious abnormalities. Brain MRI demonstrated multiple cerebral infarctions (fresh lesion on the right cerebral foot) and demyelination of cerebral white matter (Figure 1A-C). Electrocardiogram showed T-wave anomaly. Echocardiogram showed thickened left ventricular wall, decreased left ventricular diastolic function, mild mitral regurgitation, and mild tricuspid regurgitation. On computed tomography angiogram (CTA) of head and neck, there was mild atherosclerosis of bilateral carotid arteries and left subclavian artery (Figure 1D-F).
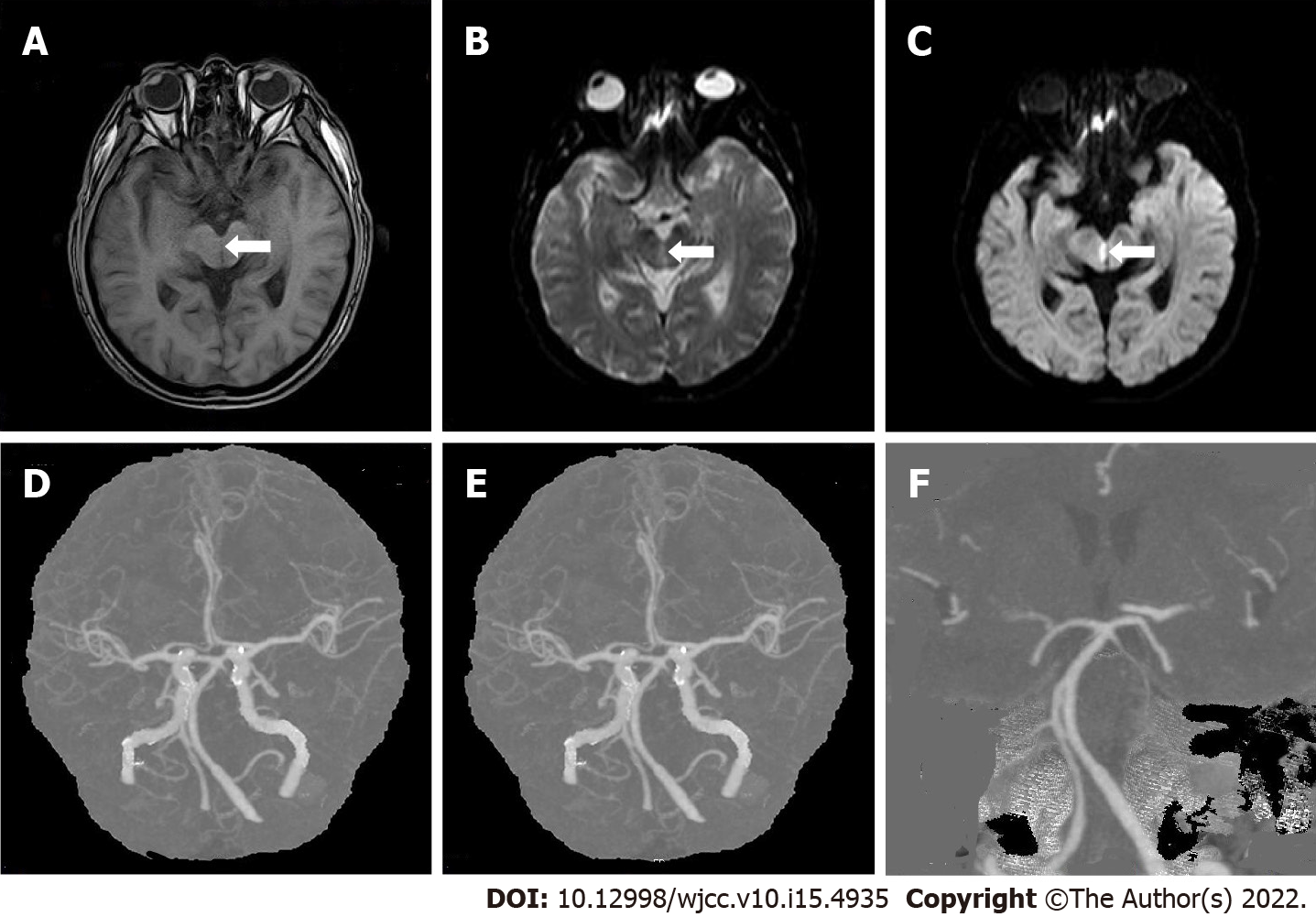
Case 2: Brain MRI showed multiple cerebral infarctions (fresh lesions in the right cerebral foot), demyelinating changes in white matter, and formation of local softening focus near the left ventricle (Figure 2A-C). Chest X-ray showed that the heart was enlarged. Cerebrovascular CTA revealed a few mixed plaques at the bifurcation of the right common carotid artery (Figure 2D-F).
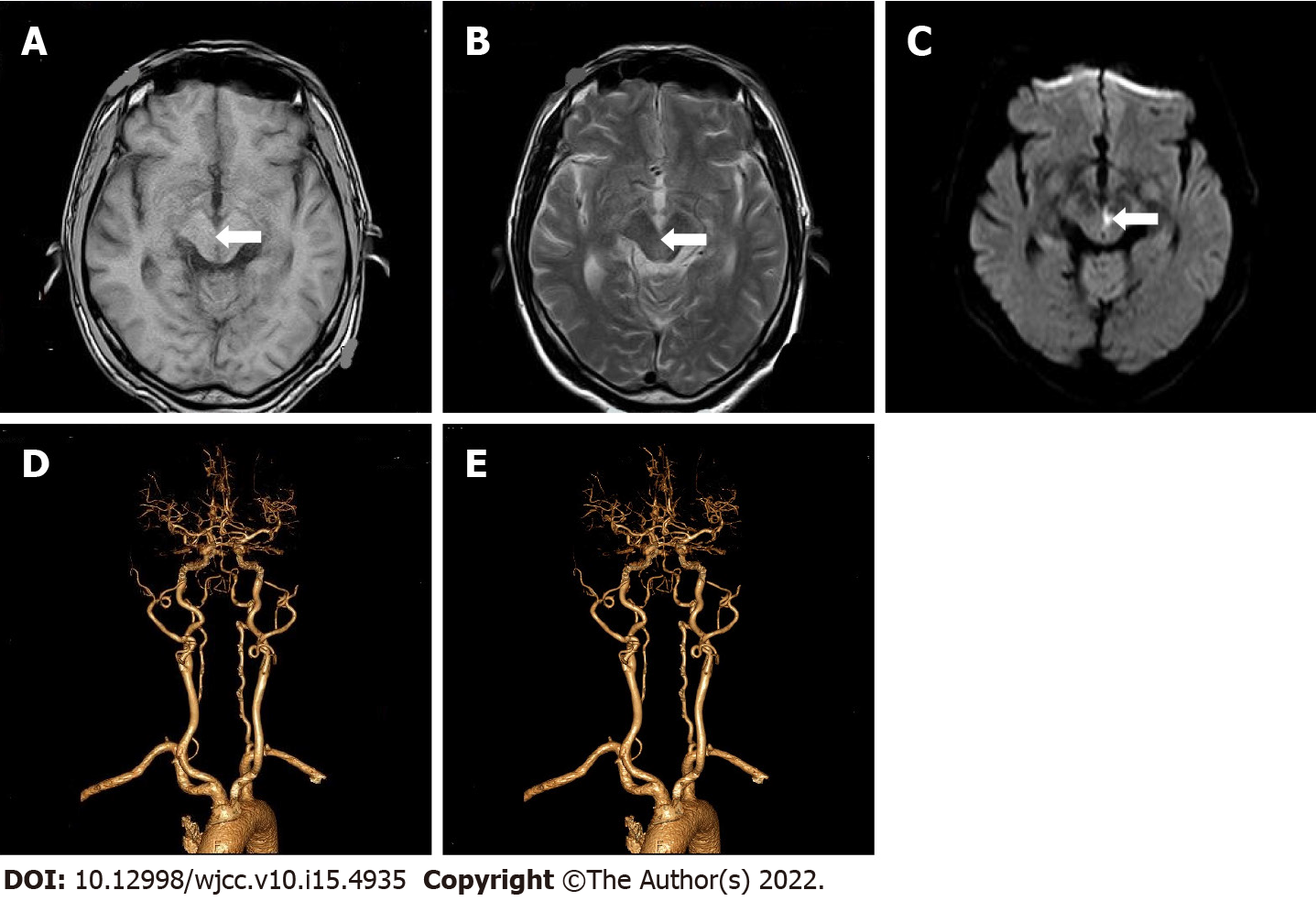
Case 3: Brain MRI showed multiple cerebral infarction (fresh brainstem lesions), suggesting the recovery of bilateral basal ganglia hemorrhage (Figure 3A-C). Color Doppler echocardiography showed left atrial enlargement, left ventricular hypertrophy, and decreased left ventricular diastolic function. Electrocardiogram, chest X-ray, and color Doppler ultrasound of neck blood vessels were normal. Head and neck CTA revealed multiple moderate stenoses of basilar artery and multiple mild to moderate localized stenoses of bilateral middle cerebral artery (Figure 3D and E).
Case 4: Chest X-ray showed enlarged heart. Cardiac ultrasound indicated moderate mitral stenosis with mild to moderate mitral regurgitation; mild aortic stenosis with mild aortic regurgitation; pulmonary hypertension with mild tricuspid regurgitation; distended left atrium, and no obvious left atrial mural thrombosis.
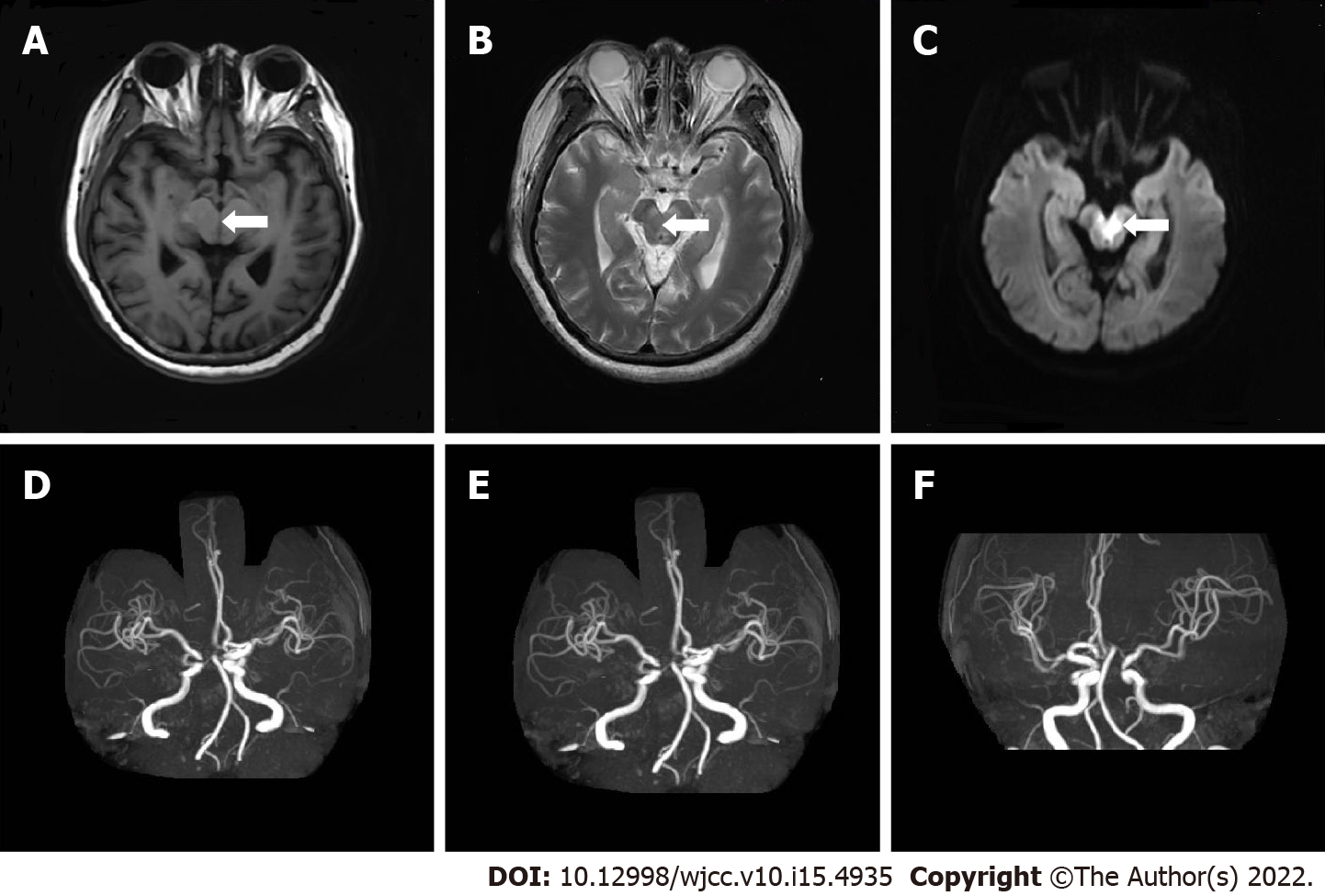
Brain MRI revealed multiple cerebral infarction and thin posterior cerebral arteries bilaterally (Figure 4A-C). CTA suggested right basal ganglia and left occipital lobe infarction (Figure 4D-F).
All 4 cases were WCS.
Case 1: Patient was started on anti-platelets and anti-plaque agents to protect brain function along with stabilization of blood pressure.
Case 2: Patient was started on anti-platelets, anti-arteriosclerosis drugs, free radical clearance, and symptomatic treatment.
Case 3: Patient was started on anti-platelets and anti-arteriosclerosis drugs, with regulation of blood sugar and blood pressure.
Case 4: Patient was started treatment on anti-platelets, anti-arteriosclerosis drugs, and free radical clearance.
Case 1: At the time of discharge, the patient's dizziness and visual acuity improved slightly; he could eat, but complained of poor sleep at night.
Case 2: At discharge, the patient had no dizziness with improvement in visual acuity.
Case 3: The patient’s condition improved steadily, but he still complained of unclear vision, which was better than initial presentation.
Case 4: Patient’s condition improved gradually, and she was subsequently discharged.
Due to the fact that the midbrain receives overlapping blood supply from the posterior cerebral artery, basilar artery, and superior cerebellar artery, the frequency of midbrain lacunar strokes is not high. The reported rate of midbrain infarction in literature varies from 0.6%-2.3% of all ischemic strokes[1,2]. While mesencephalic syndromes like Weber’s syndrome, Claude’s syndrome, and Benedikt’s syndrome are well-known amongst clinicians, WCS is a relatively uncommon mid-brain syndrome that was first described by Lhermitte[3] in 1958. It derives its name from the Wernekink commissure where it manifests (Supplementary Figure 1).
Initially called “the horseshoe-shaped commissure of Wernekink” based on the name of Friedrich Wernekink (a German anatomist), the commissure is a decussation of the brachium conjunctivum. It consists of the two major white matter tracts, namely the ascending dentato-rubro-thalamic tract and the descending dentato-rubro-olivary tract. The ascending tract joins the dentate nucleus of the cerebellum via the superior cerebellar peduncle to the contralateral red nucleus and thalamus. The descending tracts join the dentate and interposed nuclei of the cerebellum via the superior cerebellar peduncle and the inferior olivary nucleus in the medulla to the opposite red nucleus[4]. The Wernekink commissure is present just anterior to the aqueduct and is located at the paramedian area of upper brainstem. The anatomical areas abutting the commissure include medial longitudinal fascicle, reticular formation, and the trochlea and oculomotor nuclei.
Considering the anatomic location, any damage to the commissure results in bilateral cerebellar dysfunction along with dysarthric speech, truncal ataxia, and ataxic movements of all four limbs. Ophthalmoplegia of varying degree is also commonly seen with the syndrome and corresponds to the degree of damage[5]. Lesions at the Wernekink commissure seen on an MRI often lead to definitive diagnosis, as seen in our cases. Differential diagnosis for the syndrome can be acute cerebellar encephalitis, which, however, is seen in younger age groups.
To conclude, our case series highlights the signs and symptoms of WCS, which is a rare neurological disorder. Clinicians should include WCS in the differential diagnosis in case of patients presenting with bilateral cerebellar ataxia with or without ophthalmoplegia and palatal tremors. MRI is necessary for confirmatory diagnosis of WCS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anandan H, India; Miller N, United States S-Editor: Zhang H L-Editor: Filipodia CL P-Editor: Zhang H
| 1. | Bogousslavsky J, Maeder P, Regli F, Meuli R. Pure midbrain infarction: clinical syndromes, MRI, and etiologic patterns. Neurology. 1994;44:2032-2040. [PubMed] [DOI] [Full Text] |
| 2. | Kim JS, Kim J. Pure midbrain infarction: clinical, radiologic, and pathophysiologic findings. Neurology. 2005;64:1227-1232. [PubMed] [DOI] [Full Text] |
| 3. | Lhermitte F. [The cerebellar syndrome: anatomo-clinical study in the adult]. Rev Neurol (Paris). 1958;98:435-477. [PubMed] |
| 4. | Voogd J, van Baarsen K. The horseshoe-shaped commissure of Wernekinck or the decussation of the brachium conjunctivum methodological changes in the 1840s. Cerebellum. 2014;13:113-120. [PubMed] [DOI] [Full Text] |
| 5. | Liu H, Qiao L, He Z. Wernekink commissure syndrome: a rare midbrain syndrome. Neurol Sci. 2012;33:1419-1421. [PubMed] [DOI] [Full Text] |