Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4654
Peer-review started: November 24, 2021
First decision: January 22, 2022
Revised: February 5, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 16, 2022
Processing time: 170 Days and 5.9 Hours
Prostatic mucinous carcinoma (MC) and prostatic signet ring cell carcinoma are two variants of prostate cancer. MC has a higher overall survival time among all variants, while signet ring cell carcinoma is associated with lower survival time relative to other carcinomas. Only a small proportion of prostatic MC may contain signet ring cells. Over the last several decades there were only 12 patients that were documented in two studies.
We report on a 64-year-old man who was diagnosed with prostatic MC after he received a robotic-assisted laparoscopic radical prostatectomy in the West China Hospital. After robotic-assisted laparoscopic radical prostatectomy, the patient underwent three successive transurethral resections of bladder tumors. Path
This case report aimed to share the management experience, raise awareness, and highlight the importance of multidisciplinary cooperation of prostatic mucinous carcinoma with signet ring cells.
Core Tip: Prostatic mucinous carcinoma with signet ring cells (MCSRC) is a very rare morphologic variant of prostate cancer. Previously, only 12 patients have been reported, all of whom were diagnosed during the first prostate surgery. In this study, we report the first case of prostatic MCSRC that developed from the long course of prostatic mucinous carcinoma; the histological transformation was extremely uncommon. Because therapy of prostatic MCSRC is tricky and prognosis is worse, we share the management experience, raise awareness, and highlight the early and active treatment intervention for recurrent prostate mucinous carcinoma and prostatic MCSRC with multidisciplinary cooperation.
- Citation: Bai SJ, Ma L, Luo M, Xu H, Yang L. Management about intravesical histological transformation of prostatic mucinous carcinoma after radical prostatectomy: A case report. World J Clin Cases 2022; 10(14): 4654-4660
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4654.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4654
Prostatic mucinous carcinoma (MC) is defined pathologically through the presence of extracellular mucin lakes comprising at least 25% of the primary prostatic tumor. The incidence of this malignancy is low (approximately 0.2%) relative to other prostate cancer phenotypes[1,2]. Moreover, prostatic MC with signet ring cells (MCSRC), as defined by tumors that consist of > 25% extracellular mucin and < 25% signet ring cells, is a rarer morphologic variant of prostate cancer[3]. In the current study, we report on a patient who was diagnosed with prostatic MC after robotic-assisted laparoscopic radical prostatectomy (RARP). He suffered lower urinary tract irritation and subsequently received three successive transurethral resections of bladder tumors (TUR-BT) after the RARP. Additionally, total serum prostate-specific antigen (PSA) was maintained at < 0.003 ng/mL. Pathological examination of the three TUR-BT specimens showed a transformation of prostatic MC into prostatic MCSRC.
A systematic review of the literature was performed in June 2021 by searching PubMed, Embase, Cochrane Central Search Library, Web of Science, and China National Knowledge Infrastructure databases. Search MESH terms included “Adenocarcinoma, Mucinous,” “Prostate,” “Prostatic Neoplasms,” and “Carcinoma, Signet Ring Cell.” We reviewed all abstracts and articles on those topics and manually searched references of original studies.
In two previous studies, 12 prostatic MCSRC patients were recorded[4,5]. In those studies, the patients were diagnosed with MCSRC during the first surgery or biopsy. However, the transformation of prostatic MC into prostatic MCSRC, which recurred repeatedly in the urinary bladder, had not been investigated. As prostatic MCSRC is not associated with an elevation of PSA, its diagnosis can often be missed or is often misdiagnosed as another disorder. Thus, given the rarity of this disease and the lack of strong diagnostic markers, we aimed to share the therapy and management experience of a single patient treated at the West China Hospital.
In 2017, a 64-year-old man complaining of lower urinary tract irritation was referred to the urology department of West China Hospital.
The patient went to the urology department 9 mo prior for lower urinary tract irritation. The papillary neoplasm was found in the trigone with 0.5 cm diameter (cT1) and eminence lesion in the posterior wall of the bladder. Then he underwent the first TUR-BT; the postoperative pathological examination revealed prostatic MC. After the operation, the symptoms were relieved, and the patient was discharged from the hospital. However, he again suffered from lower urinary tract irritation for 5 d.
In 2015, the patient was referred to Mei Shan city hospital complaining of dysuria, increased urinary frequency, and urinary urgency for more than 1 year. The total serum PSA value was 3.600 ng/mL. The patient was diagnosed with benign prostatic hyperplasia and underwent transurethral resection of the prostate in December 2015. The patient was postoperatively diagnosed with prostatic MC. Postoperative positron emission tomography indicated that there was no evidence of metastatic malignancy except for the residual prostate.
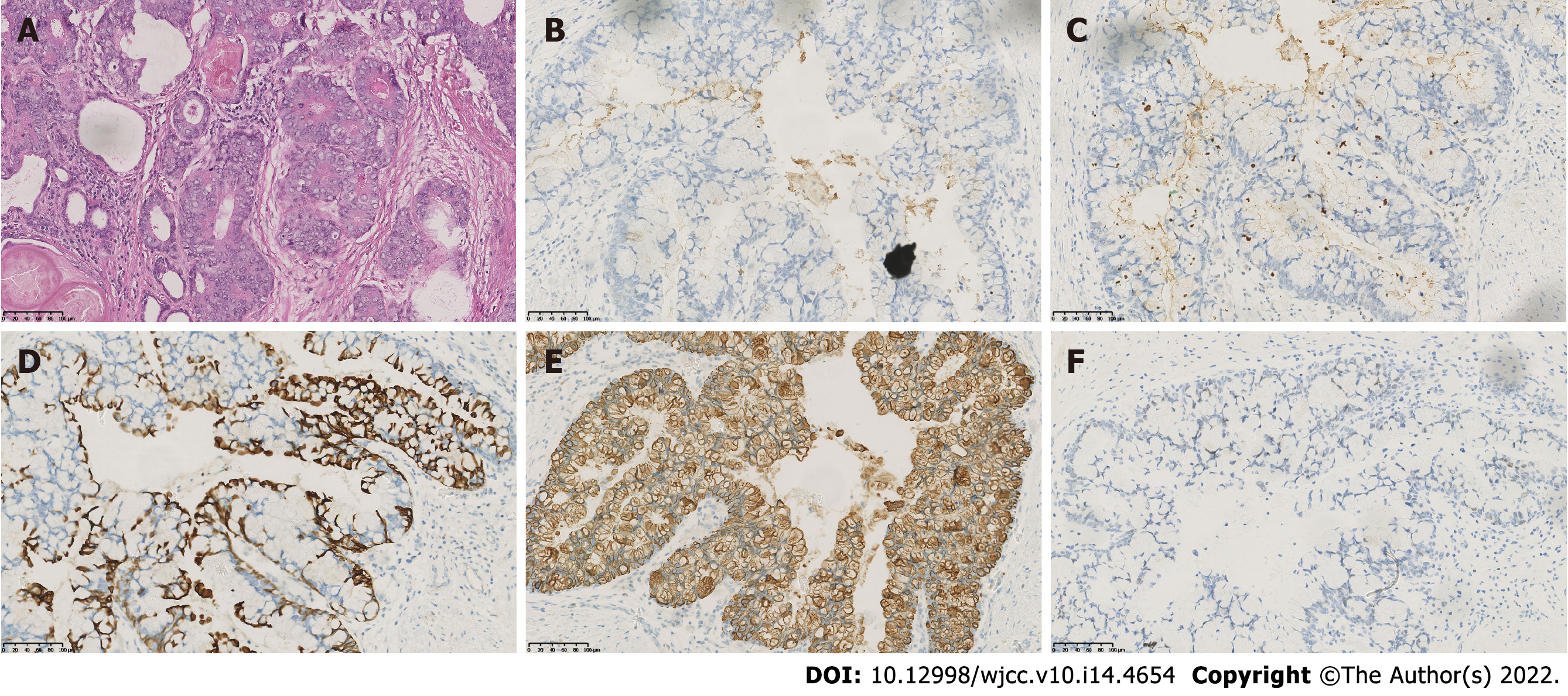
The patient was transferred to the Department of Urology at the West China Hospital of Sichuan University where he was thoroughly examined. A computed tomography scan of his abdomen and pelvic cavity showed no local metastasis. Additionally, the patient’s total and free serum PSA values were 0.186 ng/mL and 0.048 ng/mL, respectively. The patient then underwent RARP, and the pathological diagnosis of prostatic MC (pT2cN0M0) was made. Additionally, the surgical margin was negative. Immunohistochemical staining analyses of the specimen were positive for cell keratin 20 (CK20) and focally positive for PSA and prostatic serum acid phosphatase but negative for cell keratin 7 (CK7) and caudal-type homeobox transcription factor 2 (CDX-2) (Figure 1). The patient received regular clinical follow-up (once a month for 3 mo following RARP, and every 3 mo for 3-12 mo thereafter). Over those periods, the total serum PSA level was maintained at < 0.003 ng/mL without the need for adjuvant therapy.
The patient did not smoke or drink and had a free family history.
Physical examination was insignificant.
Urinalysis revealed a mild increase of 10/haptoglobin in urine red blood cells, with normal urine white blood cells. Blood analysis, prothrombin, and partial thromboplastin times as well as biochemistries were normal. Electrocardiogram and X-ray were also normal.
An initial imaging evaluation with Doppler ultrasound detected hypoechoic masses in the bladder and the blood flow signals in the masses. Then cystourethroscopy revealed rounded infiltrative neoplasms in the trigone and posterior wall of the urinary bladder (cT1), 0.5 cm and 0.8 cm in size, respectively.
The final diagnosis of the presented case was a recurrence of bladder tumors.
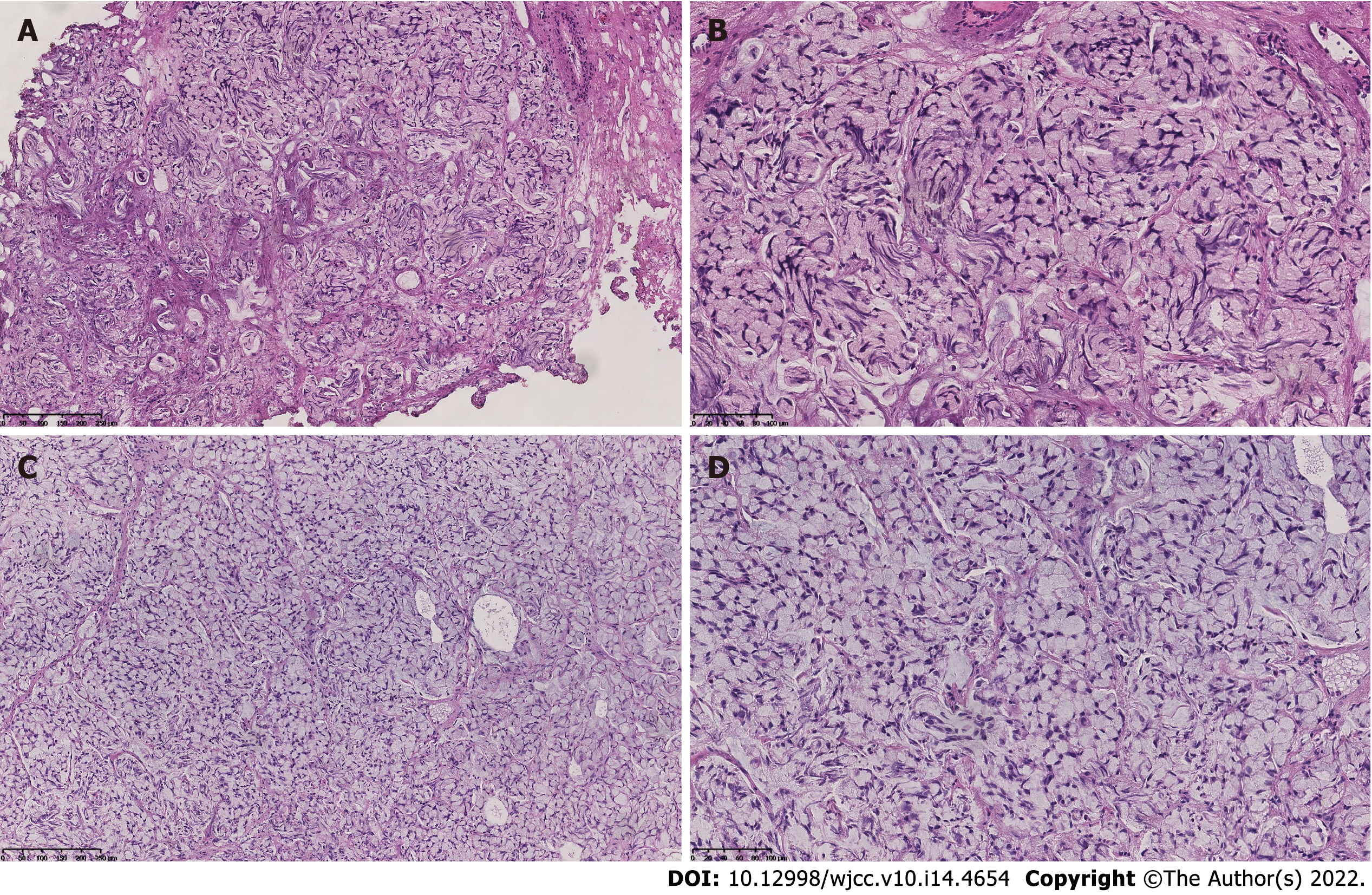
TUR-BT was performed, and pharmorubicin was prescribed for intravesical chemotherapy as the tissue origin had not been confirmed at that point. In contrast to the first TUR-BT (Figure 2A and B), hematoxylin and eosin staining and immunohistochemical examination of the specimen indicated the presence of prostatic MCSRC (Figure 2C and D). A multidisciplinary group of medical professionals from the departments of urology, oncology, radiology, and pathology recommended frequent follow-up examinations without any treatment. Regular reexamination (every 3 mo after TUR-BT) showed that the patient’s serum total PSA level was maintained at < 0.003 ng/mL, and follow-up cystourethroscopy revealed no suspicious lesions.
Nevertheless, due to the presence of lower urinary tract irritation, the cystourethroscopy revealed rounded infiltrative neoplasms again 9 mo after the previous operation, which located lesions of 0.5-1.0 cm in size in the trigone, posterior wall, and lateral wall of the bladder (cT1). The patient underwent the third TUR-BT. The samples supported the diagnosis of the second TUR-BT. The patient elected for follow-up at the Mei Shan City Hospital, a healthcare facility associated with West China Hospital, without postoperative adjunctive therapy.
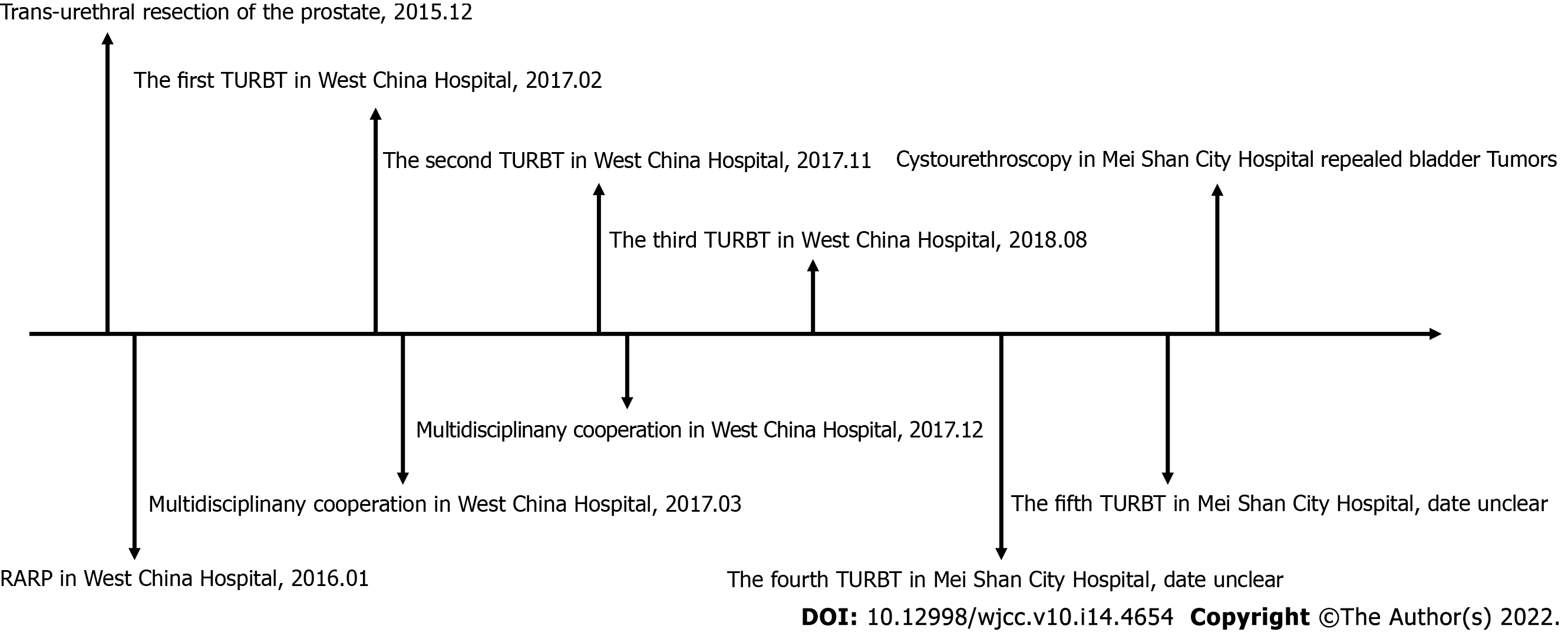
Since the patient left the Department of Urology, West China Hospital of Sichuan University, the patient underwent a fourth and fifth TUR-BT in the Mei Shan City Hospital. Cystourethroscopy in Mei Shan city hospital revealed the presence of bladder tumors. Radical cystectomy could not be implemented because the patient rejected urinary diversion. The patient is currently undergoing chemotherapy and using Chinese Herbs. The therapeutic effect of the treatment strategy and details about recurrent tumors cannot be evaluated here due to a lack of imaging data. A timeline of the patient’s medical care is provided (Figure 3).
Although the overall incidence of prostate cancer diagnosis following transurethral resection of the prostate has sharply decreased since the introduction of PSA screening[6], the median serum PSA levels of prostatic MC patients were reported as 6.7 ng/mL (interquartile range: 4.8-11.2), and an elevation of PSA was reported in 77.8% of cases of prostatic MC. On the other hand, patients with prostatic MC presented at the youngest age (median: 60, interquartile range: 54-67) among all prostate cancers[7], which indicated a fraction of prostatic MC were missed diagnoses or diagnosed following transurethral resection of the prostate[8,9]. The patient in the study had no elevation of PSA before the RARP. Therefore, radiographic evaluation should be considered in the postoperative follow-up strategy. But the urologist only chose PSA re-examination during the clinical follow-up and failed to make the perfect follow-up strategy before the multidisciplinary cooperation, which was a deficiency in the patient’s medical process.
Prostatic MC is a rare variant of prostate cancer. Its differential diagnosis includes prostatic adenocarcinoma with mucinous features and secondary infiltration from a colonic or bladder adenocarcinoma. The proportion of the extracellular mucin component (≥ 25%) could exclude the prostatic adenocarcinoma with mucinous features. On the other hand, combined immunostaining for CK7 and CK20 has been widely used in surgical pathology to help determine the origin of epithelial neoplasms. A CK7-/CK20+/CDX-2+ pattern is usually indicative of gastrointestinal adenocarcinoma, particularly colorectal carcinoma, and 100% expression of CDX-2 was found in primary adenocarcinoma of the bladder[10-12]. Furthermore, though primary adenocarcinoma of the bladder could stain a mixed pattern for CK7-/CK20+ (proportion of 29%, 5/17)[11], PSA and prostatic serum acid phosphatase are the most reliable immunohistochemical markers differentiating prostatic adenocarcinoma from primary adenocarcinoma of the bladder, which stain positive in most of the former and negative in the latter[13]. Positron emission tomography and a computed tomography scan of the abdomen and pelvic cavity indicated that there was no evidence of gastrointestinal tumors or bladder tumors. The absence of associated symptoms also excluded metastasis from adjacent organs.
In 2000, Sousa Escandón et al[14] tested criteria proposed by Elbadawi. Those authors demonstrated a more practical method to diagnose prostatic MC that was used in the current case study. Here, carcinoma specimens stained focally as PSA+/prostatic serum acid phosphatase+ and CK7-/CK20+/CDX-2- were considered as prostatic MC and not as a secondary carcinoma from surrounding organs or distant sites that metastasized to the urinary bladder.
Clinically, the treatment for prostatic MC is similar to typical acinar adenocarcinoma and includes androgen deprivation therapy, surgery, radiation therapy, and chemotherapy. In the past, it was controversial about the prognosis of prostatic MC. A study by Ro et al[1] comprising 12 patients found that prostatic MC had an aggressive biological behavior and a propensity to develop bone metastasis. However, it should be noted that the 12 patients were diagnosed as late-stage, and metastasis was found in lymph nodes, liver, lung, and bone. In contrast, some studies showed that prostatic MC did not implicate a poor prognosis relative to typical prostate acinar carcinoma (n = 60 and 12, respectively)[4,15]. Additionally, the proportion of the mucinous component (25%-49%, 50%-74%, and ≥ 75%) was not associated with the prognosis[16]. This observation has been supported by several recent studies.
Bronkema et al[7] revealed a similar estimated 10-year overall survival rate for a group of 1098 cases of prostatic MC compared with a group of 1340499 cases of typical adenocarcinoma (78.0% vs 71.1%, P = 0.002). Samaratunga et al[16] assessed parameters such as serum PSA, tumor volume, rate of extraprostatic extension, and 5-year biochemical recurrence rates and concluded a similar prognosis between the two groups of tumors. Zhao et al[17] came to a similar conclusion and indicated that prostatic MC patients who underwent radical prostatectomy are associated with significantly lower cancer-specific mortality relative to patients who did not receive surgery. Furthermore, patients who did not receive radiation therapy had similar cancer-specific mortality relative to radiation therapy patients. Together, these lines of evidence indicated the poor response to radiotherapy in prostatic MC patients.
In two studies, 12 prostatic MCSRC were described[4,5]. All 12 patients were at advanced stages of the disease and had a poor response to endocrine therapy as well as a poor prognosis compared to prostatic MC and typical adenocarcinoma. Unfortunately, in our case, the specimens collected during the second TUR-BT in West China Hospital of Sichuan University were positive for prostatic MCSRC. Signet ring cell carcinoma of the prostate was associated with forms of high-grade prostatic carcinoma (solid and comedonecrosis), which are graded as Gleason pattern 5 according to the 2014 International Society of Urological Pathology Consensus Conference. The grading of prostatic MC was Gleason pattern 4 or lower[18]. Thus, the poor prognosis observed in prostatic MCSRC was not unexpected.
The histological transformation of prostatic MC into prostatic MCSRC in the urinary bladder is extremely uncommon. However, this detrimental differentiation has been shown to result in a poor response to endocrine therapy and radiotherapy. There was no evidence to suggest that intravesical chemotherapy was useful in treating prostatic MC or MCSRC, and the reason and mechanism of this histological transformation were not clear. Therefore, the multidisciplinary cooperation suggested regular re-examination, but early active attempts may be useful to prevent this from occurring.
In the current study, we aimed to share our management experience as well as to raise awareness of prostatic MCSRC. Additionally, our observations highlight the benefits of multidisciplinary cooperation for the effective diagnosis and treatment of prostatic MCSRC. Furthermore, it is worth clarifying the molecular mechanism or cause by which this transformation occurs, which may be helpful to search for therapeutic targets and improve prognosis. Fundamental research is needed to eliminate the confusion.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ichihara K, Japan; Sharaf MM, Syria S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Ro JY, Grignon DJ, Ayala AG, Fernandez PL, Ordonez NG, Wishnow KI. Mucinous adenocarcinoma of the prostate: histochemical and immunohistochemical studies. Hum Pathol. 1990;21:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Marcus DM, Goodman M, Jani AB, Osunkoya AO, Rossi PJ. A comprehensive review of incidence and survival in patients with rare histological variants of prostate cancer in the United States from 1973 to 2008. Prostate Cancer Prostatic Dis. 2012;15:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Osunkoya AO. Mucinous and secondary tumors of the prostate. Mod Pathol. 2018;31:S80-S95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Saito S, Iwaki H. Mucin-producing carcinoma of the prostate: review of 88 cases. Urology. 1999;54:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Gumus E, Yilmaz B, Miroglu C. Prostate mucinous adenocarcinoma with signet ring cell. Int J Urol. 2003;10:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Tombal B, De Visccher L, Cosyns JP, Lorge F, Opsomer R, Wese FX, Van Cangh PJ. Assessing the risk of unsuspected prostate cancer in patients with benign prostatic hypertrophy: a 13-year retrospective study of the incidence and natural history of T1a-T1b prostate cancers. BJU Int. 1999;84:1015-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Bronkema C, Arora S, Sood A, Dalela D, Keeley J, Borchert A, Baumgarten L, Rogers CG, Peabody JO, Menon M, Abdollah F. Rare Histological Variants of Prostate Adenocarcinoma: A National Cancer Database Analysis. J Urol. 2020;204:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Enciu M, Aşchie M, Deacu M, Poinăreanu I. Morphological characteristics of a mucinous adenocarcinoma of the prostate: differential diagnosis considerations. Rom J Morphol Embryol. 2013;54:191-194. [PubMed] |
| 9. | Zhang L, Zhang L, Chen M, Fang Q. Incidental discovery of mucinous adenocarcinoma of the prostate following transurethral resection of the prostate: A report of two cases and a literature review. Mol Clin Oncol. 2018;9:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 641] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 11. | Wang HL, Lu DW, Yerian LM, Alsikafi N, Steinberg G, Hart J, Yang XJ. Immunohistochemical distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Am J Surg Pathol. 2001;25:1380-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 504] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 13. | Torenbeek R, Lagendijk JH, Van Diest PJ, Bril H, van de Molengraft FJ, Meijer CJ. Value of a panel of antibodies to identify the primary origin of adenocarcinomas presenting as bladder carcinoma. Histopathology. 1998;32:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Sousa Escandón A, Argüelles Pintos M, Picallo Sánchez J, Mateo Cambón L, González Uribarri C, Rico Morales M. Carcinoma mucinoso de la próstata: Revisión crítica de los criterios de elbadawi. Actas Urológicas Españolas. 2000;24:155-162. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Lane BR, Magi-Galluzzi C, Reuther AM, Levin HS, Zhou M, Klein EA. Mucinous adenocarcinoma of the prostate does not confer poor prognosis. Urology. 2006;68:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Samaratunga H, Delahunt B, Srigley JR, Yaxley J, Johannsen S, Coughlin G, Gianduzzo T, Kua B, Patterson I, Nacey JN, Egevad L. Mucinous adenocarcinoma of prostate and prostatic adenocarcinoma with mucinous components: a clinicopathological analysis of 143 cases. Histopathology. 2017;71:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Zhao F, Yu X, Xu M, Ye S, Zang S, Zhong W, Ren G, Chen X, Yan S. Mucinous Prostate Cancer Shows Similar Prognosis to Typical Prostate Acinar Carcinoma: A Large Population-Based and Propensity Score-Matched Study. Front Oncol. 2019;9:1467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2016;40:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1602] [Cited by in RCA: 2233] [Article Influence: 248.1] [Reference Citation Analysis (0)] |