Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4625
Peer-review started: October 25, 2021
First decision: December 17, 2021
Revised: December 24, 2021
Accepted: March 15, 2022
Article in press: March 15, 2022
Published online: May 16, 2022
Processing time: 199 Days and 19.4 Hours
Primary Sjogren's syndrome (pSS) is an autoimmune disease, and renal involvement has been considered to be one of the systemic complications of pSS. Patients who have sjogren's syndrome with renal disease as the first manifestation and no exocrine gland involvement or autoantibodies can be missed clinically.
We here in report an unusual case of a primary Sjogren's syndrome in a 43-year-old female who had minimal lesion nephropathy as the initial presentation, and the patient was negative for serum anti-SSA and anti-SSB antibodies and did not have signs of exocrine gland involvement. The patient’s Sjogren's syndrome was confirmed by a minor salivary gland biopsy (MSGB) and a filter paper test. the patient’s oedema subsided, and the patient’s urinary protein resolved, showing that the treatment was effective.
MSGB should be considered if pSS is suspected in patients who do not have the typical pSS symptoms or who are positive for the specific autoantibodies.
Core Tip: Cases of serum-negative Sjogren's syndrome, when the patient has no symptoms of exocrine gland involvement and no hyperglobulinemia, where kidney disease is the first manifestation, and when minimal lesion nephropathy is seen in renal pathology, are rare. Therefore, minor salivary gland biopsy (MSGB) should be considered if pSS is suspected in patients who do not have the typical pSS symptoms or specific autoantibodies. MSGS has a primary value in the diagnosis of these patients.
- Citation: Li CY, Li YM, Tian M. Serum-negative Sjogren's syndrome with minimal lesion nephropathy as the initial presentation: A case report. World J Clin Cases 2022; 10(14): 4625-4631
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4625.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4625
Primary Sjogren's syndrome (pSS) is a chronic autoimmune disease that is characterized by the lymphocytic infiltration of exocrine glands, resulting in the significant loss of the secretory function and the development of inflammatory and destructive lesions in the salivary glands and lacrimal glands. The most common clinical manifestations are dryness in the mouth and eyes. Extraglandular involvement may also occur, including in the kidneys (3%-5%); The main renal lesions are the infiltration of inflammatory cells into the renal tubules and interstitium, and the glomerular involvement often manifests as the development of a membranous nephropathy[1]. Herein, We report a case of Sjogren's syndrome with micropathic nephropathy as the primary presentation, and this patient was negative for anti-SSA and anti-SSB antibodies and had no symptoms of exocrine gland involvement.
A 43-year-old female patient was admitted due to recurrent systemic oedema for 5 months.
The patient’s lower oedema appeared five months prior to presentation, and the oedema was obvious at night and disappeared in the morning. Then, the patient’s oedema of the extremities worsened and had extended to the face, and this was accompanied by oliguria (approximately 500-800 mL/day) and the presence of foamy urine. The patient did not have a dry mouth, and the patient had no need to consume water with the ingestion of dry food, did not have dry eyes, and had flaky tooth loss, parotid gland enlargement, joint pain, hair loss, light allergy, or oral ulcers.
The prior history of the patient was nonspecific.
The patient’s personal and family history was nonspecific.
The physical examination showed that the patient had oedema in her lower extremities, which was not specific.
The results of the patient’s laboratory examinations are shown in Table 1.
| Items | Before treatment | After treatment | Reference range |
| WBC (× 109/L) | 4.64 | 6.35 | 3.5-9.5 |
| HB (g/L) | 126 | 109 | 130-175 |
| PLT (×109/L) | 205 | 124 | 100-300 |
| IgG (g/L) | 8.35 | 6.05 | 7.51-15.6 |
| C3 (g/L) | 0.76 | 0.78 | 0.79-1.52 |
| C4 (g/L) | 0.176 | 0.179 | 0.16-0.38 |
| ALT (U/L) | 19 | 16 | 7-40 |
| AST (U/L) | 57 | 22 | 13-35 |
| Alb (g/L) | 24.3 | 41.5 | 40-55 |
| Urinary occult blood test | +++ | - | - |
| 24-hour urinary protein quantity (g/24 h) | 3.8 | 0.091 | 0-0.15 |
| RF (IU/mL) | < 20 | < 20 | 20 |
| Anti-RNP | - | - | - |
| Anti-dsDNA | - | - | - |
| Anti-Sm | - | - | - |
| Anti-SCL-70 | - | - | - |
| Anti-SSA | - | - | - |
| Anti-Jo-1 | - | - | - |
| Anti-SSB | - | - | - |
| Anti-A-fordrin | - | - | - |
| Anti-ACA | - | - | - |
| Anti-RO-52 | - | - | - |
| AnuA | - | - | - |
| AHA | - | - | - |
| ARPA/Rib-P | - | - | - |
| ANA (nuclear particle type) | 1:1000 | 1:1000 | - |
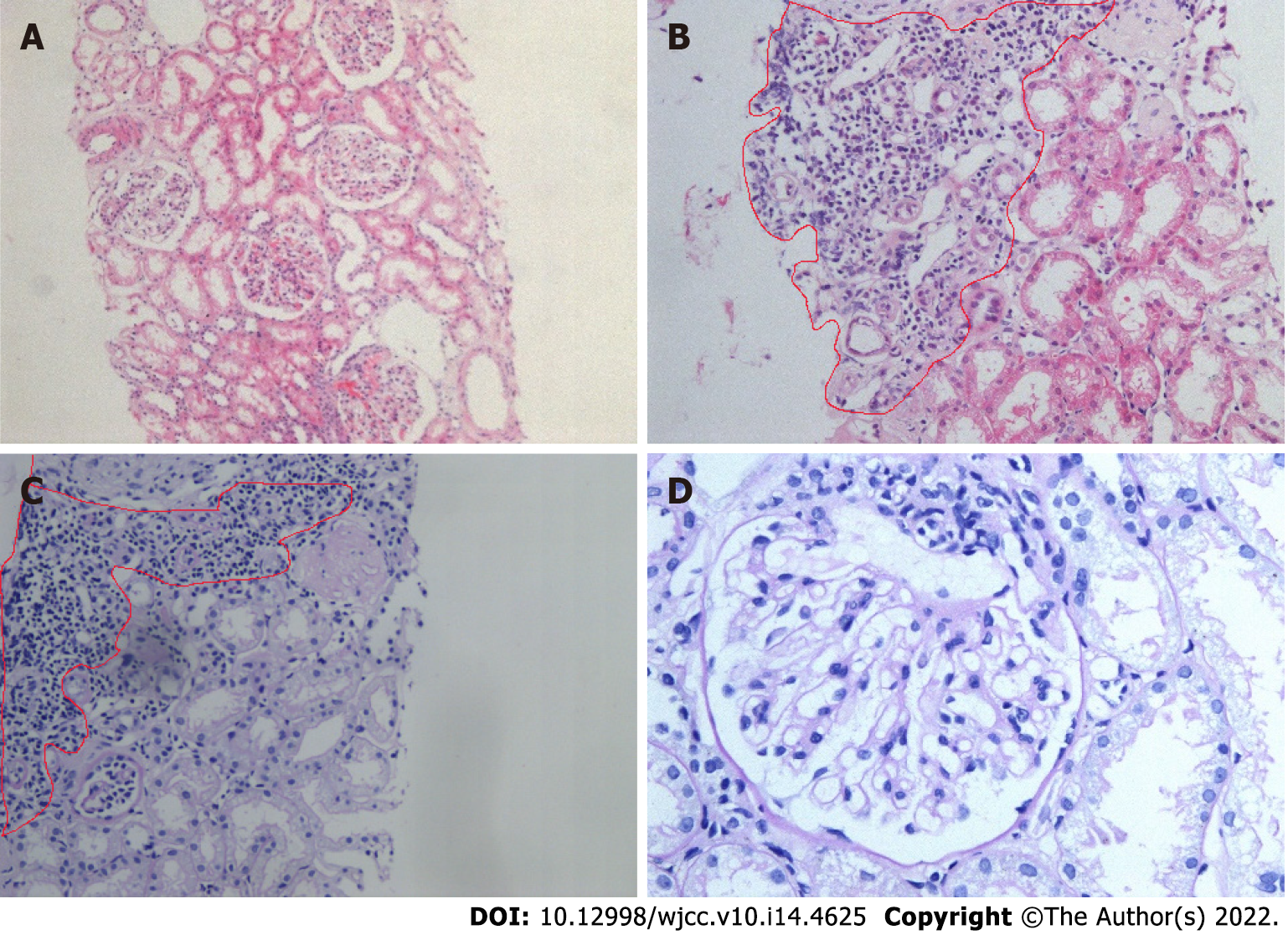
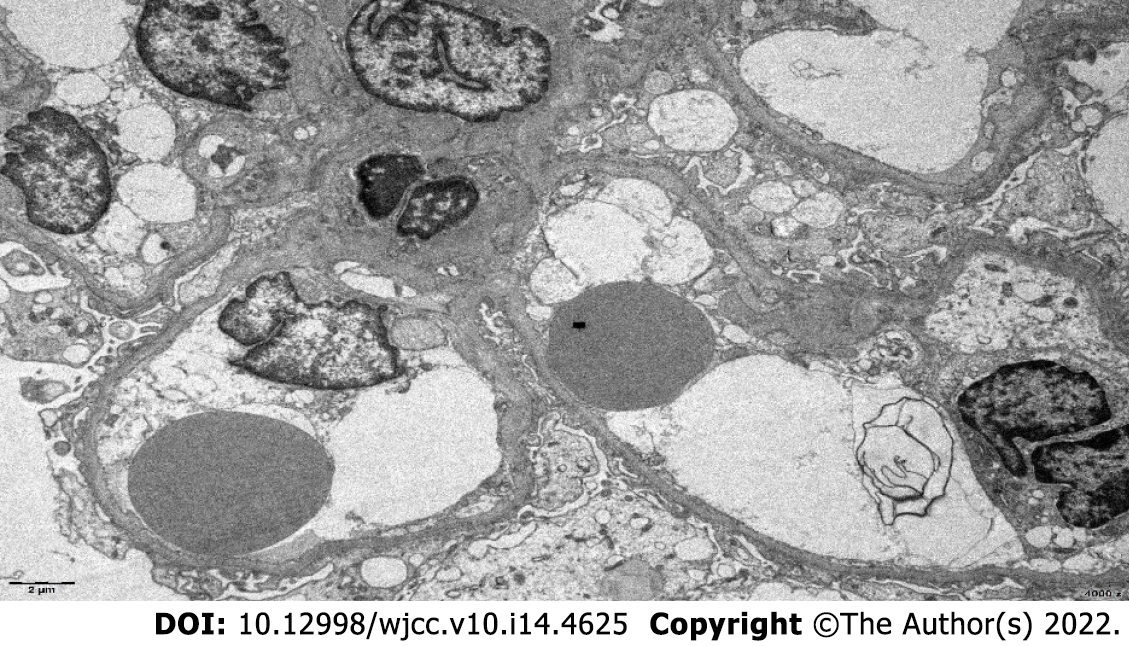
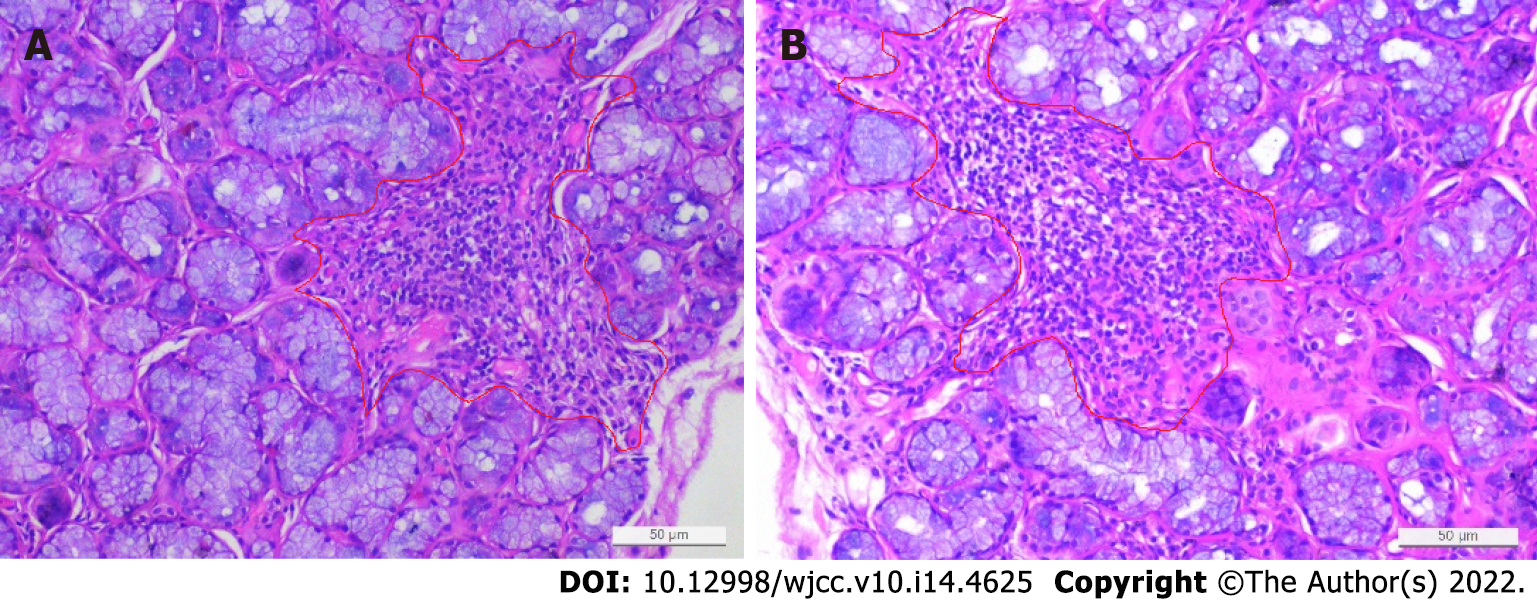
The patient underwent a renal biopsy on December 1, 2020 under ultrasonography guidance to assess the etiology of the nephrotic syndrome. The light microscopic analysis of the kidney biopsy samples identified thirteen glomeruli, and no abnormalities were observed. Tubular atrophy, interstitial fibrosis, focal vacuolar degeneration in the tubular epithelial cells, and focal aggregation of interstitial inflammatory cells were occasionally present (Figure 1). An immunofluorescence analysis of the kidney biopsy sample revealed that the kidney samples were negative for IgG, IgM, IgA, C1q and C3. An electron microscopic analysis of the kidney biopsy sample revealed that there was a diffuse effacement of the foot processes. Electron-dense deposits were not observed (Figure 2). An ophthalmic examination revealed a Schirmer test score of 3 mm/5 min and a tear breakup time of 4 s. An analysis of a labial gland biopsy sample analysis identified the presence of level IV lesions (Figure 3).
The final diagnosis was pSS with nephrotic syndrome.
The patient was given intravenous prednisolone (PSL) 40 mg/d for one week. The patient was discharged from the hospital on December 09, 2020. Then the patient was prescribed prednisone 40 mg/d po.
After 3 weeks of treatment, the patient’s systemic oedema had disappeared. A re-examination of the routine urine test revealed the following: urinary protein (-), serum albumin: 41.9 g/L, and urinary protein quantitation: 0.091 g/24 h (Table 1). Therefore, the treatment was effective.
Currently, there is no gold standard for diagnosing pSS. The diagnosis of pSS has been based on the presence of typical clinical symptoms such as dry mouth, dry eyes, the results of the minor salivary gland biopsy (MSGB) and autoantibodies the presence of autoantibodies. According to the 2016 ACR/EULAR pSS classification criteria, the following are used for a diagnosis: (1) Focal lymph infiltration of labial glands with a focal index ≥ 1 Lesion /4 mm2); and (2) A filter paper test (Schirmer test ≤ 5mm/5 min); pSS is diagnosed when the total score is ≥ 4[2]. The final diagnosis in this patient was pSS with nephrotic syndrome. Li et al[3] summarized 161 pSS patients and found that only 86 patients (53.42%) were positive for both anti-SSA and anti-SSB antibodies. Another study found that 37 (35.6%) of 104 pSS patients were negative for both anti-SSA and anti-SSB antibodies[4]. This suggests that pSS cases with negative anti-SSA and SSB antibodies are common in the clinic. Serologically negative SS-A and SS-B antibodies were more common in patients with late-onset pSS (age > 35 years) than in patients with early-onset pSS who had significant clinical manifestations and enhanced cellular immune system activation[5]. According to the classification, the patient in this case report had a late-onset, therefore, MSGB should be considered if pSS is suspected in the patients without the typical pSS symptoms or without the presence of the specific autoantibodies[6]. The value of MSGS is important in the pSS diagnosis. In addition, the detection of autoantibodies in this patient indicated a high titer positive anti-ANA antibody titre, indicating the possibility of an autoimmune diseases, and a positive anti-ANA titre is important for the diagnosis and differentiation of rheumatoid diseases. However, it should be noted that a positive ANA test is also common in healthy individuals[7].
Kidney involvement is an important extraglandular complication of pSS and can lead to chronic kidney disease and even death. At present, it is not clear the renal involvement in patients with pSS is caused by the SS disease itself or if it coexists with other diseases. Bossini et al[8] and Tu et al[9] reported that some pSS-related renal diseases may occur before the subjective symptoms of dry mouth/eyes and joint pain. In a previous report, renal biopsies were obtained from 103 pSS patients who had renal involvement complications in order to investigate the renal pathological types. Among these types, renal tubule and interstitial involvement was the main pathological type, which was identified in 53 of the patients (51.5%), and glomerulonephritis (GN) was the main pathological type in the other 50 patients (48.5%). The pathological types of the GN lesions included membranous nephropathy (37, 35.9%), membranoproliferative glomerulonephritis (6, 5.8%), IgA nephropathy (3, 2.9%), minimal change disease (4, 3.9%), and focal segmental glomerulosclerosis (3, 2.9%). Compared with pSS patients without complications, the patients who had pSS complications, especially renal involvement, developed atypical dry mouth and dry eyes (P < 0.05)[10]. In addition, the incidence of interstitial lung disease, leukopenia and elevated IgG levels were significantly lower (P < 0.05) in the pSS patients who had complications[5]. A number of previous studies that have evaluated the pathological characteristics of renal biopsy samples have also showed that tubulointerstitial nephritis (TIN) is the main pathological type of renal involvement in pSS patients. The pathophysiology of acute and chronic TIN involves the infiltration of T cells, B cells, and plasma cells (among other cell types) into the renal interstitium and renal tubules, which promotes interstitial fibrosis and can lead to CKD[11-14]. In SS patients, glomerular involvement is far less common than renal tubulointerstitial involvement, which may manifest as haematuria, proteinuria or even nephrotic syndrome. The deposition of circulating immune complexes in the glomerulus and the triggering of mesangial proliferation and extracellular matrix protein synthesis are considered to be key factors in the development of glomerular diseases[8]. The pathogenesis of renal involvement in pSS is unclear. Studies have been conducted to identify the factors that are associated with the renal involvement of pSS. A meta-analysis showed that the presence of anti-SSB antibodies was closely associated with arthralgia and the renal injury secondary to pSS and concluded that the presence of SSB antibodies was positively correlated with the development of renal involvement[15]. The most common glomerular disease associated with SS is membranous proliferative glomerulonephritis (MPGN), which is associated with the activation of polyclonal B lymphocytes and can cause cryoglobulinemia, and MPGN is the second most common renal manifestation in pSS[16,17]. Many studies have reported that steroids and immunosuppressants (such as cyclophosphamide, plasma exchange therapy, rituximab, azathioprine or mycophenolate) in the treatment of MPGN can significantly improve the renal function in patients with CKD[18]. Magali et al analysed 95 SS patients who had renal involvement confirmed by renal biopsy and found that TIN occurred in 93 of the SS nephritis patients (97.9%) and that glomerular lesions occurred in 22 patients (23.2%). Eighty-one patients (85.3%) received treatment, of whom 80 were treated with CSs (98.8%) and 21 were treated with immunosuppressants (primarily rituximab) (25.9%). Although significant interstitial fibrosis was found in the initial biopsy samples, the renal function of the patients improved significantly within 12 months after diagnosis (final eGFR 49.9 and 39 mL/min/1.73 m2 for those treated with CSs and immunosuppressants, respectively; the baseline eGFR was 8 mL/min/1.73 m2, P < 0.001). In this study, no additional benefit was found when using immunosuppressant therapy compared to steroid therapy alone[1].
In this case, renal tubular atrophy and interstitial fibrosis were occasionally observed in the samples under light microscopy. Interstitial inflammatory cell focal aggregation is a common pathological manifestation of the renal damage in pSS patients. In addition, pathological manifestations similar to lupus nephritis combined with secondary membranous nephropathy can be seen in pSS patients. Also, several immune complex deposits can be seen in the mesangial glomerulus and capillary walls, but this was not the case in this patient[19]. On the basis of these findings and the patient's clinical manifestations and laboratory examination results, atypical lupus nephritis was excluded. The electron microscopic analysis of the kidney biopsy samples from this patient revealed diffuse foot process effacement, and the patient was diagnosed with minimal lesion nephropathy.
Cases of Serum antibody-negative Sjogren's syndrome, where there are no symptoms of exocrine gland involvement and no hyperglobulinemia, where kidney is the first manifestation and where renal pathology is seen as minimal lesion nephropathy, are clinically rare. Therefore, the case analysis here aims to remind clinicians to consider the possibility of pSS in patients with a high titre positive antinuclear antibody combined with the presence of interstitial inflammatory cell focal aggregation in the kidney, even if the patients do not have the typical serological changes. MSGB plays an important role in the diagnosis of atypical pSS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Su CC, Taiwan Tsou HK, Taiwan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Jasiek M, Karras A, Le Guern V, Krastinova E, Mesbah R, Faguer S, Jourde-Chiche N, Fauchais AL, Chiche L, Dernis E, Moulis G, Fraison JB, Lazaro E, Jullien P, Hachulla E, Le Quellec A, Rémy P, Hummel A, Costedoat-Chalumeau N, Ronco P, Vanhille P, Meas-Yedid V, Cordonnier C, Ferlicot S, Daniel L, Seror R, Mariette X, Thervet E, François H, Terrier B. A multicentre study of 95 biopsy-proven cases of renal disease in primary Sjögren's syndrome. Rheumatology (Oxford). 2017;56:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, Mariette X; International Sjögren's Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 879] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 3. | Li YW, Li ZF, Han YM. Clinical data analysis of primary Sjogren's syndrome patients with positive anti-SSA and SSB antibodies. Chinese Journal of Internal Medicine. 2010;49:792-793. [DOI] [Full Text] |
| 4. | Guo J, Yan HM, Luo KK, Wang H, Wang YF. Characteristics of patients with primary Sjogren's syndrome with negative anti-SSA and SSB antibodies in serum[J]. Journal of Peking University Health Sciences 2013, 45: 316-318. [DOI] [Full Text] |
| 5. | Wei L, Zhifei X, Xiaoran N, Meilu L, Yang L, Yixuan L, Xiuying R, Yashuang S, Jingjing C, Shaoying G, Liu Y, Lijun S, Fengxiao Z, Wen Z. Patients with early-onset primary Sjögren's syndrome have distinctive clinical manifestations and circulating lymphocyte profiles. Rheumatology (Oxford). 2022;61:597-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Li X, Xu B, Ma Y, Li X, Cheng Q, Wang X, Wang G, Qian L, Wei L. Clinical and laboratory profiles of primary Sjogren's syndrome in a Chinese population: A retrospective analysis of 315 patients. Int J Rheum Dis. 2015;18:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Li QZ, Karp DR, Quan J, Branch VK, Zhou J, Lian Y, Chong BF, Wakeland EK, Olsen NJ. Risk factors for ANA positivity in healthy persons. Arthritis Res Ther. 2011;13:R38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Bossini N, Savoldi S, Franceschini F, Mombelloni S, Baronio M, Cavazzana I, Viola BF, Valzorio B, Mazzucchelli C, Cattaneo R, Scolari F, Maiorca R. Clinical and morphological features of kidney involvement in primary Sjögren's syndrome. Nephrol Dial Transplant. 2001;16:2328-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Tu WH, Shearn MA, Lee JC, Hopper J Jr. Interstitial nephritis in Sjögren's syndrome. Ann Intern Med. 1968;69:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Yang HX, Wang J, Wen YB, Fei YY, Jiang MD, Zhou MY, Zhang W, Li H, Li XM, Zhang FC, Li XW, Zhang X, Chen LM. Renal involvement in primary Sjögren's syndrome: A retrospective study of 103 biopsy-proven cases from a single center in China. Int J Rheum Dis. 2018;21:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Kim YK, Song HC, Kim WY, Yoon HE, Choi YJ, Ki CS, Park CW, Yang CW, Kim J, Kim YS, Choi EJ, Bang BK. Acquired Gitelman syndrome in a patient with primary Sjögren syndrome. Am J Kidney Dis. 2008;52:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Cohen EP, Bastani B, Cohen MR, Kolner S, Hemken P, Gluck SL. Absence of H(+)-ATPase in cortical collecting tubules of a patient with Sjogren's syndrome and distal renal tubular acidosis. J Am Soc Nephrol. 1992;3:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Ichikawa K, Konta T, Sato H, Ueda Y, Yokoyama H. The clinical and pathological characteristics of nephropathies in connective tissue diseases in the Japan Renal Biopsy Registry (J-RBR). Clin Exp Nephrol. 2017;21:1024-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Huang X, Wang X, Yu D. Development and Validation of a Nomogram for Renal Involvement in Primary Sjögren Syndrome Patients: A Retrospective Analysis. Mod Rheumatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Hong R, Xu D, Hsieh E, Xiang Y, Zhao J, Wang Q, Tian X, Li M, Zhao Y, Zeng X. Factors Associated With Renal Involvement in Primary Sjögren's Syndrome: A Meta-Analysis. Front Med (Lausanne). 2020;7:614482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Matignon M, Cacoub P, Colombat M, Saadoun D, Brocheriou I, Mougenot B, Roudot-Thoraval F, Vanhille P, Moranne O, Hachulla E, Hatron PY, Fermand JP, Fakhouri F, Ronco P, Plaisier E, Grimbert P. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Medicine (Baltimore). 2009;88:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Terrier B, Krastinova E, Marie I, Launay D, Lacraz A, Belenotti P, de Saint-Martin L, Quemeneur T, Huart A, Bonnet F, Le Guenno G, Kahn JE, Hinschberger O, Rullier P, Diot E, Lazaro E, Bridoux F, Zénone T, Carrat F, Hermine O, Léger JM, Mariette X, Senet P, Plaisier E, Cacoub P. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood. 2012;119:5996-6004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Khan S, Longhurst H. Epigenetic alterations on C1-inhibitor expression may influence hereditary angioedema attack frequency and C4 levels: Comment on: Karagianni P, Goules AV, Tzioufas AG. Epigenetic alterations in Sjogren's syndrome patient saliva. Clin Exp Immunol. 2020;202:144-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Zou Y, Ling G, Tian J, Chen J, Ge Y. [Research progress in renal injury relevant to primary Sjögren's syndrome]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |