Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4617
Peer-review started: October 20, 2021
First decision: February 14, 2022
Revised: February 23, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 16, 2022
Processing time: 204 Days and 17.7 Hours
Moyamoya disease is essentially an ischemic cerebrovascular disease. Here, we describe a case of acute recurrent cerebral infarction caused by moyamoya disease with concurrent adenomyosis which, to our knowledge, is the first in the literature. A literature review is also presented.
A 38-year-old female presented to the Research and Treatment Center of Moyamoya Disease in our hospital with "left limb weakness" as the main symptom. She was diagnosed with acute cerebral infarction and moyamoya disease through magnetic resonance imaging and digital subtraction angiography. Prior to this, she had experienced a prolonged menstrual period of one-month duration. This was investigated and adenomyosis was diagnosed. After passing the acute cerebral infarction phase, the patient underwent surgery for adenomyosis followed by combined cerebral revascularization. During the postoperative follow-up, improvements of the perfusion imaging stage and modified Rankin Scale were observed. A review of the literature showed only 16 reported cases of gynecological diseases complicated with stroke. The clinical characteristics, pathogenesis, therapeutic effects, and long-term prognosis of these cases have been studied and discussed.
In patients with moyamoya disease, early management of gynecological-related bleeding disorders is essential to prevent the complications of cerebral events.
Core Tip: For women with moyamoya disease, several factors may contribute further to the increased risk of stroke. Based on our report, abnormal uterine bleeding caused by gynecological diseases is a rare risk factor. In the literature to date, there is no case study on acute recurrent cerebral infarction caused by moyamoya disease combined with adenomyosis. Our case report represented the first, with detailed description of the sequence, safety, and long-term outcomes of the treatment.
- Citation: Zhang S, Zhao LM, Xue BQ, Liang H, Guo GC, Liu Y, Wu RY, Li CY. Acute recurrent cerebral infarction caused by moyamoya disease complicated with adenomyosis: A case report . World J Clin Cases 2022; 10(14): 4617-4624
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4617.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4617
Moyamoya disease, which is named after the smoke-like appearance on cerebral angiography depicting the abnormal vascular networks at the base of the skull[1,2], is a rare cerebrovascular disease. It is characterized by chronic progressive stenosis or occlusion at the ends of bilateral internal carotid arteries, anterior cerebral arteries, and the origins of the middle cerebral arteries. The main clinical manifestations of moyamoya disease are dizziness, headache, dyskinesia, language dysfunction, cognitive dysfunction, and epilepsy. Moyamoya disease has a high incidence in Japan, Korea, China, and other Asian countries with some degree of family aggregation, suggesting the involvement of genetic factors[3-5]. According to the national epidemiological survey in Japan in 2008, the total number of patients with moyamoya disease treated in the country in 2003 was 7700, with a male to female ratio of 1:1.8 and an annual incidence of 0.54/100000[3]. According to the national epidemiological survey in South Korea in 2014, 8164 patients were diagnosed with moyamoya disease between 2007 and 2011, including 2928 males and 5226 females, with 38.1 years for a 1:1.8 male to female ratio. The average age of the patients was 36.8 years, with an average age for male patients of 34.4 years and 38.1 years for females. The annual number of cases ranged from 848 to 1192, and the annual incidence was 1.7-2.3/100000[4]. A survey of the incidence and prevalence of moyamoya disease in urban China in 2021 showed that the estimated incidence and prevalence were 0.59/100000 and 1.01/100000, respectively. The prevalence of moyamoya disease in females was higher than that in males, and the age distribution showed a bimodal pattern, which was more obvious in females[5]. Adenomyosis is a common benign gynecological disease in women of reproductive age, characterized by the presence of ectopic endometrial glands and stroma in the myometrium resulting in diffuse growth of the myometrium[6]. The main clinical manifestations of adenomyosis are dysmenorrhea, menorrhagia, and infertility, among which abnormal uterine bleeding is the most common symptom of the disorder[7]. Higher levels of myometrial invasion are associated with increased frequencies and amounts of abnormal uterine bleeding[8], which is also a rare factor leading to stroke in patients with moyamoya disease.
Left limb weakness for 1 d.
A 38-year-old female presented to the Moyamoya Disease Research and Treatment Center of Henan Provincial People's Hospital with "left limb weakness" as the main symptom, in addition to difficulty in lifting the left arm and walking with dragging of the left leg. On further inquiry, her regular medication included the long-term consumption of aspirin (100 mg/d) for recurrent transient ischemic attack.
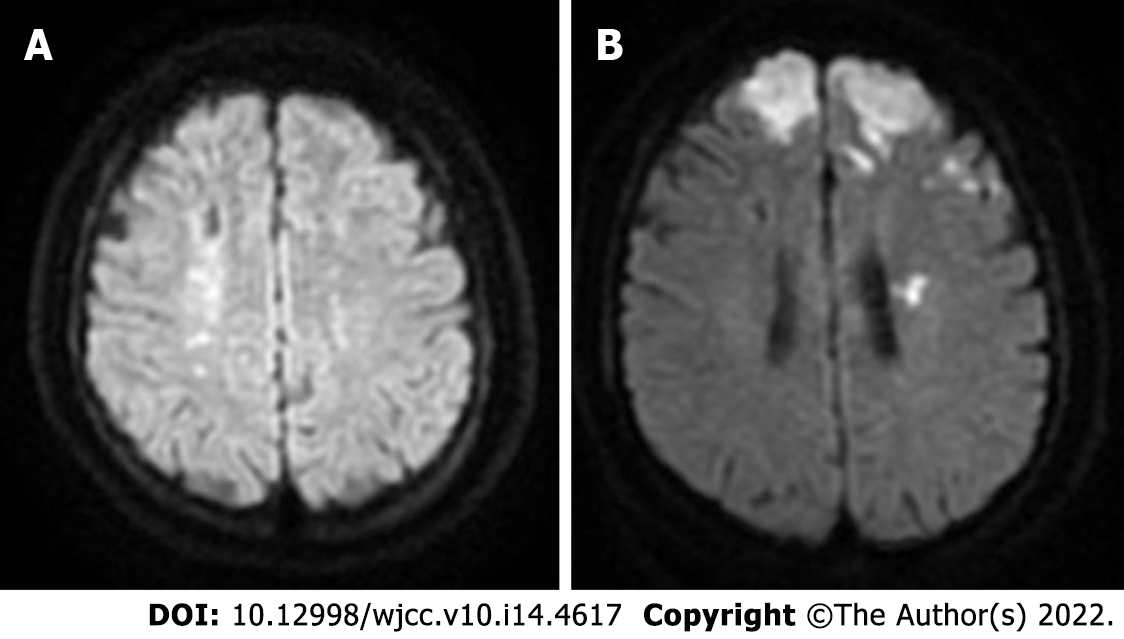
Four months previously, she had been diagnosed with an acute cerebral infarction by a brain MRI in our hospital following the complaints of "weakness of lower limbs" (Figure 1A). Additionally, she had also experienced abnormal uterine bleeding for a month, which was heavy at times.
There was no significant past personal history or any diseases running in the family. She was not a smoker and did not drink alcohol.
The patient was conscious but in poor spirits. The tongue appeared skewed to the left on extension. The left upper and lower limbs had muscle strengths of grade 3 and grade 4, respectively, with a positive Babinski sign on the left.
The blood investigation showed a low hemoglobin level of 61.0 (normal reference value: 115-150 g/L), RBC count 3.1 × 1012/L (reference value 3.5-5.0), platelet count 350 × 109/L (reference value 100-300), and abnormal thromboelastogram (residual platelet function after taking ADP drugs = 14.2 mm, normal reference value: 31-47 mm). The coagulation was slightly low.
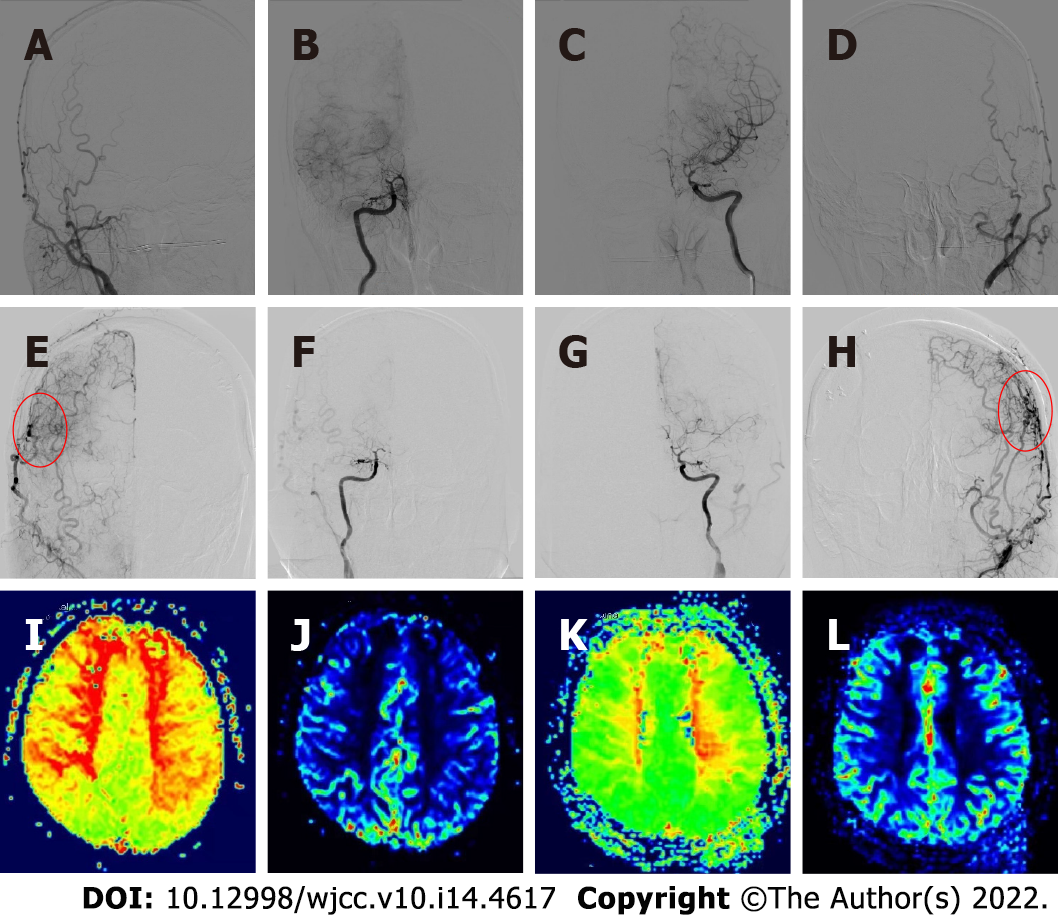
Upon admission, the first brain magnetic resonance imaging (MRI) (diffusion weighted imaging sequence) revealed acute cerebral infarction in the bilateral fronto-parietal lobes and posterior horn of the left ventricle (Figure 1B). Preoperatively, the digital subtraction angiography (DSA) demonstrated bilateral internal carotid artery occlusion (Figure 2A-D), with cerebral perfusion perfusion-weighted imaging (PWI) suggesting severe cerebral ischemia in both hemispheres (Figure 2I-J).
Moyamoya disease; Acute recurrent cerebral infarction; Adenomyosis; Severe anemia.
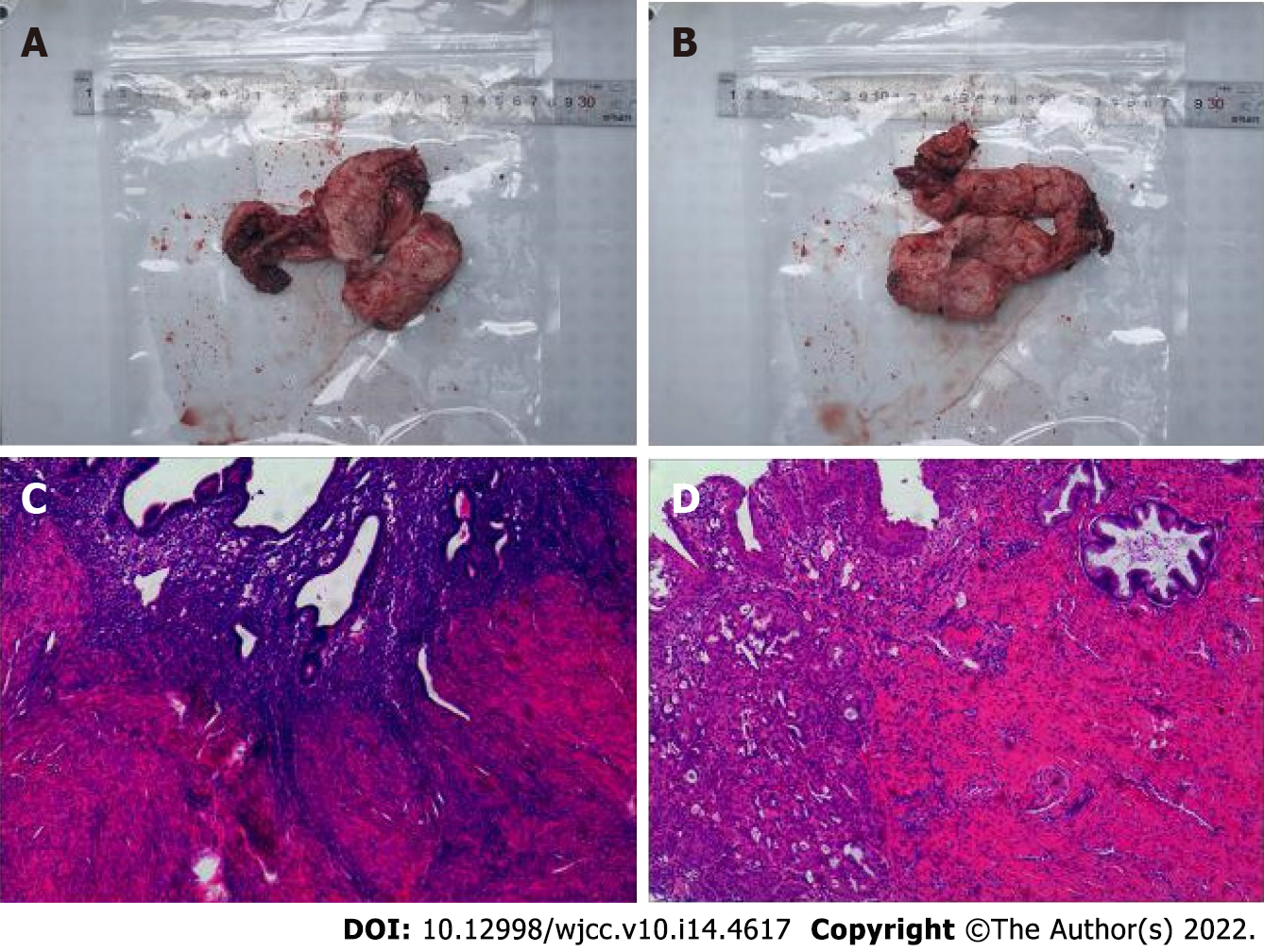
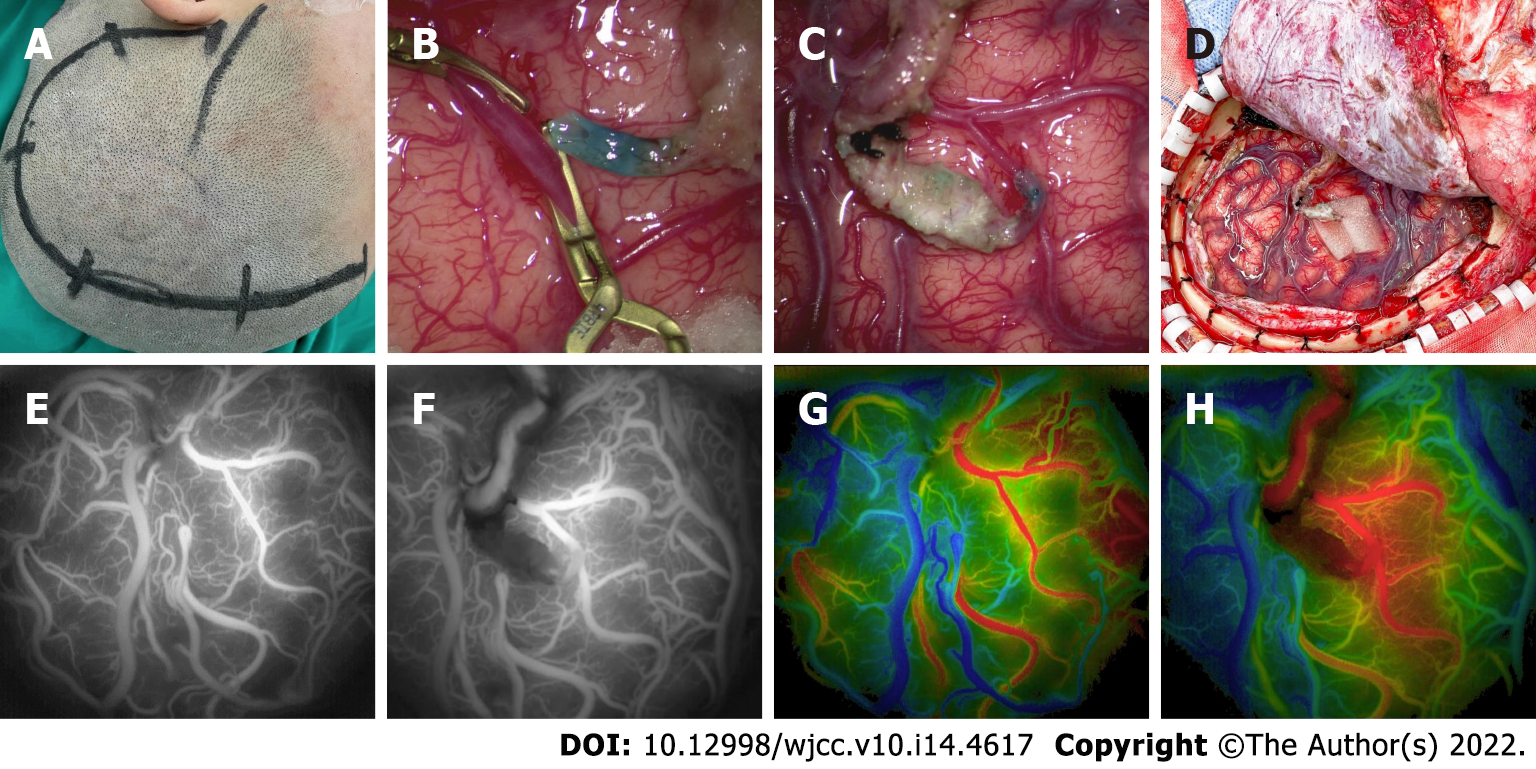
The patient was in the acute stage of cerebral infarction and surgery was contraindicated. The treatment included blood transfusion and improvement of the microcirculatory and trophic nerve function, using Edaravone injection Q12 h, argatroban anhydrous Q12 h, ganglioside QD, and long-term oral aspirin 100 mg/d antiplatelet therapy. After 2 wk, the patient's condition gradually stabilized. Given the persistent irregular per-vaginal bleeding, a pelvic ultrasound was performed which again revealed adenomyosis. Therefore, she underwent laparoscopic total hysterectomy and bilateral salpingectomy (Figure 3). At 2 and 9 mo after the recurrent acute cerebral infarction, the patient underwent a combined revascularization procedure for the left and right hemispheres. The preoperative blood tests on two occasions showed no anemia (hemoglobin: 117 g/L and 135 g/L). Given that the patient had more severe ischemia in the right hemisphere and the symptoms on the left side were more pronounced than those on the right, the first surgery was performed on the right hemisphere, and the left hemisphere surgery was performed 7 mo later. The revascularization surgery was conducted using superficial temporal artery (STA)-middle cerebral artery bypass combined with encephalo-duro-myo-synangiosis muscle adhesion (Figure 4A-D). With the modified pterional approach, the superficial temporal artery should be protected in the anterior auricular incision to avoid injury. Depending on the condition and preoperative angiography results, the parietal branch of the STA is dissociated from the flap to form a lipid vascular segment. In this case, the temporalis muscle was separated from the temporal bone and completely turned over to the base of the skull and protected. The skull was opened by temporal line milling resulting in a bone window of approximately 10 cm × 10 cm in size. The dura mater was cut along the trunk and branches of the middle meningeal artery, and the rest of the dura mater was radially cut. After hemostasis, the dura mater was folded and flattened under the bone window, so that the side of the dura mater originally facing the skull was applied to the surface of the cerebral cortex. Flow 800 angiography was performed after puncture of the pia meningeal membrane to release cerebrospinal fluid (Figure 4G). According to the angiography results and the preoperative plan, the superficial temporal artery-middle cerebral artery bypass was performed with 10-0 sutures using OPMI PENTERO 900 microscope (ZEISS) at 15 x magnification. Flow 800 fluorescence angiography was performed again after bypass. The blood flow patency and blood flow were determined, the dural flap at the trunk and branch of the middle meningeal artery was reversed, and the bone window margin was sutured and fixed. The separated temporalis muscle was applied to the surface of the brain, and its edge was sutured at the dural reflex. A curved bone flap was opened at the place where the bridging vessels passed to prevent vessel compression. Finally, the skin was sutured in layers. The patient’s basic blood pressure level was strictly maintained during surgery, and the PaCO2 was kept between 35-40 mmHg[9]. We perform ambulatory blood pressure monitoring 24 h before surgery in all patients, using the average blood pressure readings as the standard for maintaining the blood pressure levels during surgery to reduce perioperative complications. Postoperative microcirculation improvement and neurotrophic therapy were performed, and sodium valproate was used in the short term to prevent postoperative epilepsy.
At 6 mo after bilateral combined cerebral revascularization, the repeated DSA showed good bilateral neovascularization (Figure 2E-H), and the cerebral perfusion PWI (Figure 2K-L) demonstrated significant improvement. The patient's symptoms had improved and were significantly better than before surgery. The modified Rankin scale (mRS) score was 3 before surgery, while the postoperative score was 1.
To date, there are only 16 cases of gynecological diseases complicated with stroke reported in the literature, as outlined in Table 1. The age of the patients ranged from 34 to 59 (43.81 ± 6.05) years. Of these, 10 patients suffered a stroke during menstruation, and only 6 patients had a prior history of other cerebrovascular diseases., Moreover, 9 patients were anemic, of which 2 were mild (hemoglobin 90-110 g/L), 1 was moderate (hemoglobin 60-90 g/L), and 5 were severe (hemoglobin < 60 g/L).
| Ref. | Case No. | Age | During menstruation | Cerebrovascular disease | Infarction side | Hemoglobin (g/L) | D-Dimer (mg /L) | CA125 (U/mL) | CA199 (U/mL) |
| Present case | 38 | Yes | Yes | Bilateral | 61 | 0.26 | 14.07 | 2.97 | |
| Okazaki et al[12] | 1 | 42 | Yes | Yes | Left | – | 14 | 395 | – |
| 2 | 50 | No | Yes | Left | – | 37 | 143 | – | |
| Yin et al[13] | 3 | 34 | Yes | No | Bilateral | 134 | 1.05 | 937.1 | 462.5 |
| 4 | 37 | Yes | No | Right | 108 | 2.34 | 456.8 | 50.3 | |
| 5 | 46 | Yes | Yes | Bilateral | 121 | 12.04 | 546.5 | 1076.6 | |
| Zhao et al[14] | 6 | 34 | Yes | Yes | Right | 112 | 27.4 | 937.7 | – |
| Hijikata et al[15] | 7 | 59 | No | – | Bilateral | – | 7.0 | 334.8 | – |
| Aso et al[16] | 8 | 44 | Yes | Yes | Bilateral | 103 | 17.0 | 2115 | – |
| Aiura et al[17] | 9 | 48 | Yes | Yes | Bilateral | 82 | 79.3 | 3536 | 892 |
| Naito et al[20] | 10 | 42 | – | – | Bilateral | 53 | 2.0 | 185 | – |
| 11 | 42 | No | No | Left | 113 | 0.4 | – | – | |
| Yamashiro et al[21] | 12 | 45 | No | No | Bilateral | 84 | 1.1 | 159 | – |
| 13 | 44 | – | No | Right | 70 | – | – | – | |
| 14 | 50 | Yes | No | Left | 69 | 0.57 | 42.6 | – | |
| 15 | 42 | Yes | – | Bilateral | 86 | 6.0 | 1750 | – | |
| Akaishi et al[24] | 16 | 42 | Yes | – | Bilateral | 69 | 17.9 | 433 |
The histopathological features of adenomyosis reveal invasion of the myometrium by endometrial glands and stroma[10]. Severe adenomyosis may lead to increased intramural pressure, impaired venous drainage, impaired endometrial vasodilation, and abnormal uterine bleeding[11]. To our knowledge, there has been no previous report in the literature on moyamoya disease combined with adenomyosis leading to acute cerebral infarction.
Menstrual abnormalities caused by adenomyosis have commonly been associated with increased levels of D-dimer, CA125, and CA19-9, and an increased risk of systemic embolism, including multiple cerebral infarctions[12-17].In addition, the activation of the tissue factor coagulation pathway induced by menstruation may also lead to coagulation abnormalities in adenomyosis patients[18]. Consequently, patients with adenomyosis are at an increased risk of cerebral infarction during menstruation. Low peripheral blood volume, cerebral blood flow, and cerebral oxygen reserve contribute to stroke in patients with moyamoya disease[2]. Anemia has a clear association with cerebrovascular events given the presence of a direct link between the central nervous system, blood supply, and tissue oxygen delivery[19,20]. Yamashiro et al[21] have described in their case report that four middle-aged women with adenomyosis developed severe anemia (6.9-8.6 g/dL hemoglobin) followed by multiple cerebral infarcts, with two of the women suffering from multiple cerebral infarcts during menstruation. On the other hand, Tsai et al[22] have reported that for patients with intracranial vascular stenosis, acute anemia results in insufficient cerebral blood flow and reduced oxygen-carrying capacity, especially when the hemoglobin level is below the critical level, leading to ischemic injury to the brain tissue. In addition, studies have shown that hemoglobin concentration is an independent predictor of stroke recurrence and complex vascular events[23]. Although hypercoagulable blood may cause an acute cerebral infarction, the loss of systemic blood volume from prolonged irregular uterine bleeding leads to acute cerebral infarction in susceptible patients with moyamoya disease who are already in a state of chronic cerebral ischemia.
Our patient had been taking aspirin regularly. Although the thromboelastography confirmed that the aspirin had decreased the platelet function, our patient continued to suffer from multiple acute cerebral infarctions, suggesting the use of antiplatelet drugs in patients with chronic abnormal uterine bleeding does not prevent the occurrence of acute cerebral infarction[24]. To date, the medical treatment for moyamoya disease is limited, and surgical cerebral blood flow reconstruction remains a relatively effective treatment that can delay or even prevent the disease progression[1]. Our patient underwent gynecological surgery for adenomyosis prior to bypass surgery for moyamoya disease, and her anemia was resolved without recurrence of acute cerebral infarction. The subsequent combined revascularization surgery was beneficial as it significantly reduced her symptoms with improved mRS and PWI perfusion compared to preoperative examinations.
Adenomyosis is a rare driving factor to acute cerebral infarction in patients with moyamoya disease. Therefore, acute stroke may occur in patients with moyamoya disease during menstruation, resulting in delayed revasculopathy surgery and even a serious threat to patients' life and health. In these patients, it is essential to ensure the early management of the gynecological-related bleeding disorder to prevent complications of cerebral events, which then allows effective intervention for moyamoya disease in a timely manner.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gokce E, Turkey; Jugovic D, Germany; Shibata Y, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7:1056-1066. [PubMed] [DOI] [Full Text] |
| 2. | Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226-1237. [PubMed] [DOI] [Full Text] |
| 3. | Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, Tsuji I, Inaba Y, Yoshimoto T. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008;39:42-47. [PubMed] [DOI] [Full Text] |
| 4. | Ahn IM, Park DH, Hann HJ, Kim KH, Kim HJ, Ahn HS. Incidence, prevalence, and survival of moyamoya disease in Korea: a nationwide, population-based study. Stroke. 2014;45:1090-1095. [PubMed] [DOI] [Full Text] |
| 5. | Sun Y, Zhou G, Feng J, Chen L, Liu G, Wang J, Wang Q, Yu J, Yang X, Yang Z, Gao P, Wang S, Zhan S. Incidence and prevalence of moyamoya disease in urban China: a nationwide retrospective cohort study. Stroke Vasc Neurol. 2021;6:615-623. [PubMed] [DOI] [Full Text] |
| 6. | Bourdon M, Santulli P, Marcellin L, Maignien C, Maitrot-Mantelet L, Bordonne C, Plu Bureau G, Chapron C. Adenomyosis: An update regarding its diagnosis and clinical features. J Gynecol Obstet Hum Reprod. 2021;50:102228. [PubMed] [DOI] [Full Text] |
| 7. | Peric H, Fraser IS. The symptomatology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:547-555. [PubMed] [DOI] [Full Text] |
| 8. | Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691. [PubMed] [DOI] [Full Text] |
| 9. | Liming Z, Weiliang S, Jia J, Hao L, Yang L, Ludtka C, Jahromi BR, Goehre F, Zemmar A, Tianxiao L, Hernesniemi J, Andrade-Barazarte H, Chaoyue L. Impact of blood pressure changes in cerebral blood perfusion of patients with ischemic Moyamoya disease evaluated by SPECT. J Cereb Blood Flow Metab. 2021;41:1472-1480. [PubMed] [DOI] [Full Text] |
| 10. | Chapron C, Vannuccini S, Santulli P, Abrão MS, Carmona F, Fraser IS, Gordts S, Guo SW, Just PA, Noël JC, Pistofidis G, Van den Bosch T, Petraglia F. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26:392-411. [PubMed] [DOI] [Full Text] |
| 11. | Turner BM, Cramer SF, Heller DS. The pathogenesis of abnormal uterine bleeding in myopathic uteri. Ann Diagn Pathol. 2021;52:151726. [PubMed] [DOI] [Full Text] |
| 12. | Okazaki K, Oka F, Ishihara H, Suzuki M. Cerebral infarction associated with benign mucin-producing adenomyosis: report of two cases. BMC Neurol. 2018;18:166. [PubMed] [DOI] [Full Text] |
| 13. | Yin X, Wu J, Song S, Zhang B, Chen Y. Cerebral infarcts associated with adenomyosis: a rare risk factor for stroke in middle-aged women: a case series. BMC Neurol. 2018;18:213. [PubMed] [DOI] [Full Text] |
| 14. | Zhao Y, Zhang Y, Yang Y. Acute cerebral infarction with adenomyosis in a patient with fever: a case report. BMC Neurol. 2020;20:210. [PubMed] [DOI] [Full Text] |
| 15. | Hijikata N, Sakamoto Y, Nito C, Matsumoto N, Abe A, Nogami A, Sato T, Hokama H, Okubo S, Kimura K. Multiple Cerebral Infarctions in a Patient with Adenomyosis on Hormone Replacement Therapy: A Case Report. J Stroke Cerebrovasc Dis. 2016;25:e183-4. [PubMed] [DOI] [Full Text] |
| 16. | Aso Y, Chikazawa R, Kimura Y, Kimura N, Matsubara E. "Recurrent multiple cerebral infarctions related to the progression of adenomyosis: a case report". BMC Neurol. 2018;18:119. [PubMed] [DOI] [Full Text] |
| 17. | Aiura R, Nakayama S, Yamaga H, Kato Y, Fujishima H. Systemic thromboembolism including multiple cerebral infarctions with middle cerebral artery occlusion caused by the progression of adenomyosis with benign gynecological tumor: a case report. BMC Neurol. 2021;21:14. [PubMed] [DOI] [Full Text] |
| 18. | Nakamura Y, Kawamura N, Ishiko O, Ogita S. Acute disseminated intravascular coagulation developed during menstruation in an adenomyosis patient. Arch Gynecol Obstet. 2002;267:110-112. [PubMed] [DOI] [Full Text] |
| 19. | Dubyk MD, Card RT, Whiting SJ, Boyle CA, Zlotkin SH, Paterson PG. Iron deficiency anemia prevalence at first stroke or transient ischemic attack. Can J Neurol Sci. 2012;39:189-195. [PubMed] [DOI] [Full Text] |
| 20. | Naito H, Naka H, Kanaya Y, Yamazaki Y, Tokinobu H. Two cases of acute ischemic stroke associated with iron deficiency anemia due to bleeding from uterine fibroids in middle-aged women. Intern Med. 2014;53:2533-2537. [PubMed] [DOI] [Full Text] |
| 21. | Yamashiro K, Tanaka R, Nishioka K, Ueno Y, Shimura H, Okuma Y, Hattori N, Urabe T. Cerebral infarcts associated with adenomyosis among middle-aged women. J Stroke Cerebrovasc Dis. 2012;21:910.e1-910.e5. [PubMed] [DOI] [Full Text] |
| 22. | Tsai CF, Yip PK, Chen CC, Yeh SJ, Chung ST, Jeng JS. Cerebral infarction in acute anemia. J Neurol. 2010;257:2044-2051. [PubMed] [DOI] [Full Text] |
| 23. | Chang JY, Han MK. Response by Chang and Han to Letter Regarding Article, "Influence of Hemoglobin Concentration on Stroke Recurrence and Composite Vascular Events". Stroke. 2020;51:e152-e153. [PubMed] [DOI] [Full Text] |
| 24. | Akaishi T, Kuroda H, Tateyama M, Yoshida Y, Otsuki T, Watanabe M, Yaegashi N, Aoki M. Recurrent cerebral infarction synchronous with menorrhagia caused by endometrial stromal sarcoma. J Neurol Sci. 2015;358:509-511. [PubMed] [DOI] [Full Text] |