Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4608
Peer-review started: October 19, 2021
First decision: January 24, 2022
Revised: March 25, 2022
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 16, 2022
Processing time: 205 Days and 22.8 Hours
The pathological type of simple squamous carcinoma in colorectal malignancies is rare. Simple squamous cell carcinoma of the colorectum occurs most frequently in the rectum. The clinicopathological features and biological behaviors of squamous colorectal carcinoma are unclear, and its prognosis may be worse than that of simple adenocarcinoma. Studies on squamous colorectal cancer are currently limited to case reports, and there is no standard treatment protocol. Therefore, more case reports are required to fully understand squamous colorectal cancer.
We reported the case of a 56-year-old woman who complained of constipation for 2 years. Colonoscopy revealed a sigmoid colon tumor, and the pathological result of colonoscopy was squamous carcinoma. After completing the relevant assessment, the patient was clinically diagnosed with cT4aN0M0, stage IIB, and surgery was performed. Based on postoperative pathological results, the patient was diagnosed with pT4bN0M0, stage IIC. Six cycles of adjuvant chemotherapy were administered after surgery. Liver metastasis and abdominal wall mass were found more than 1 mo after the end of the last chemotherapy session. Targeted local treatment was not performed because the liver had multiple metastases, but I125 particle implantation of the abdominal wall mass was performed. Two cycles of first-line chemotherapy were administered after the surgery. The patient underwent 14 mo of treatment and eventually died from the tumor.
Squamous carcinoma of sigmoid colon is a rare tumor with unclear pathogenesis. Its clinicopathological diagnosis should be paid close attention.
Core Tip: Simple squamous cell carcinoma of colorectal cancer is very rare, especially when it occurs in the sigmoid colon. Squamous colon cancer appears to have a worse prognosis than adenocarcinoma and there are no standard treatment options. The case also had liver metastases, which may also have some impact on the prognosis. We need more reports to understand its prognosis and treatment.
- Citation: Li XY, Teng G, Zhao X, Zhu CM. Primary sigmoid squamous cell carcinoma with liver metastasis: A case report. World J Clin Cases 2022; 10(14): 4608-4616
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4608.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4608
Colorectal malignancies are among the most common malignancies worldwide[1], and their most common pathological type is adenocarcinoma[2], followed by adenosquamous carcinoma. Squamous carcinoma alone is rare, accounting for approximately 0.1 to 0.25 per 1000 of all colorectal cancers[3]. Squamous cell carcinoma (SCC) of the colon was first reported in the German literature published in 1919[4]. Ozuner et al[5] reported 11 cases of squamous carcinoma of the colon, of which eight were squamous carcinoma of the rectum, two were squamous carcinoma of the ascending colon, and only one was squamous carcinoma of the sigmoid colon. In 2019, researchers[6] compiled the Surveillance, Epidemiology, and End Results (SEER) database for the period 1998–2012 and analyzed its clinical staging and other characteristics. A total of 464 patients were diagnosed with squamous carcinoma of the colon, of which only 50 (10.8%) patients had squamous carcinoma of the colon. The pathogenesis of colonic squamous carcinoma is unclear, and its prognosis is worse than that of simple adenocarcinoma[7]. Most studies have been limited to case reports, and there are no standard treatment protocols.
Herein, we reported a case of primary SCC of the sigmoid colon with liver metastasis. This study aimed to analyze and study the characteristics of this case and summarize the clinical characteristics, pathological features, and treatment of squamous carcinoma of the colon to improve our understanding of such disease.
A 56-year-old woman presented to our gastrointestinal surgery clinic in March 2019 who complained of constipation for 2 years.
The patient complained of a history of constipation, with stools 3-5 d/once, requiring oral laxatives for 2 years.
The patient was previously physically fit.
Personal and family histories were not exceptional.
The patient had no positive signs.
Laboratory tests were unremarkable (Table 1).
| Project name | Result | Unit | Reference range |
| White blood cell count | 17.08 | 109/L | 3.5-9.5 |
| Red blood cell count | 4.00 | 1012/L | 3.8-5.1 |
| Hemoglobin | 118 | g/L | 115-150 |
| Platelet count | 390 | 109/L | 125-350 |
| Alanine aminotransferase | 18.8 | U/L | 0.00-50.00 |
| Aspartate aminotransferase | 14.54 | U/L | 0.00-40.00 |
| Total bilirubin | 10.96 | μmol/L | 3.00-22.00 |
| Urea nitrogen | 2.81 | mmol/L | 2.50-6.10 |
| Creatinine | 53.6 | μmol/L | 46.00-92.00 |
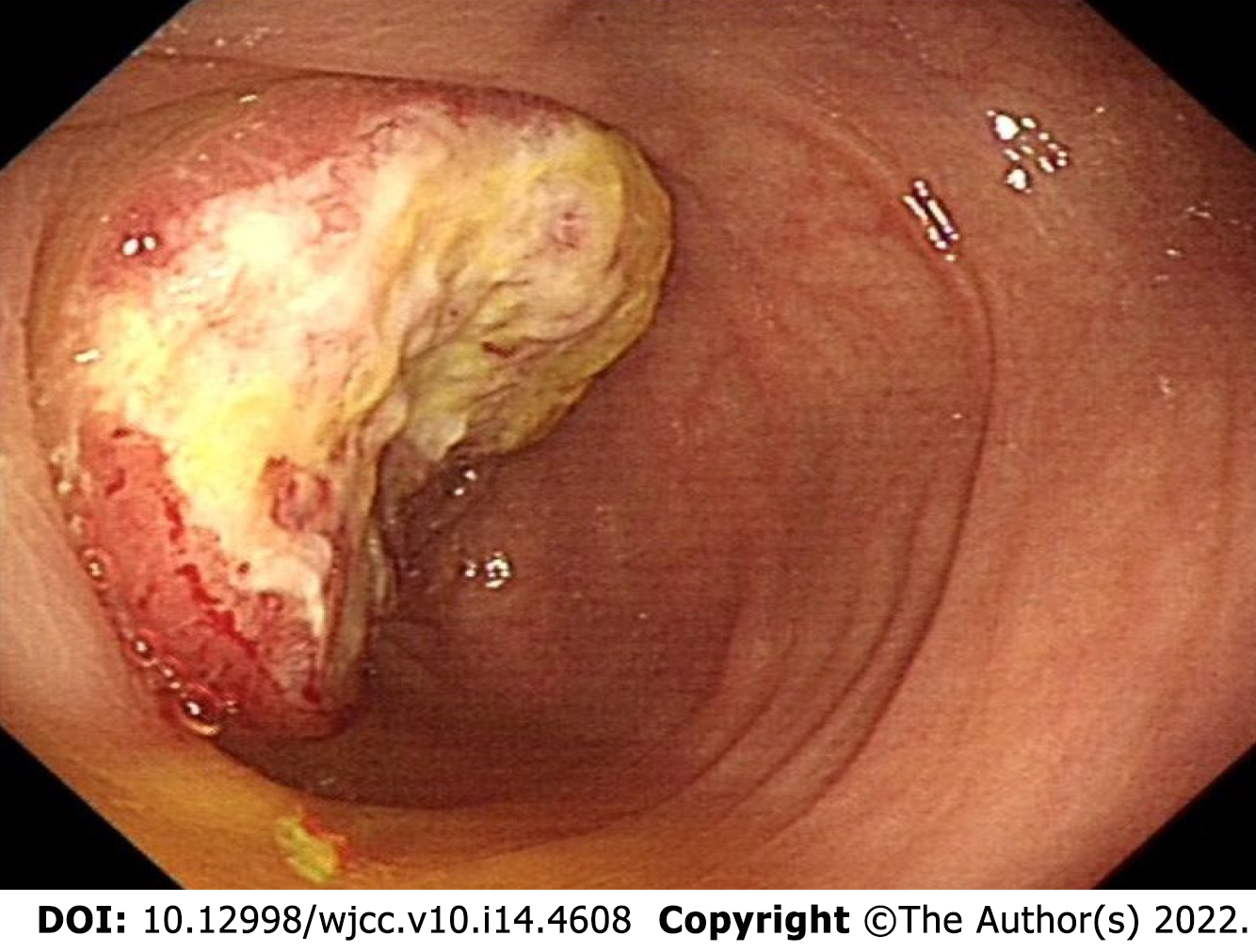
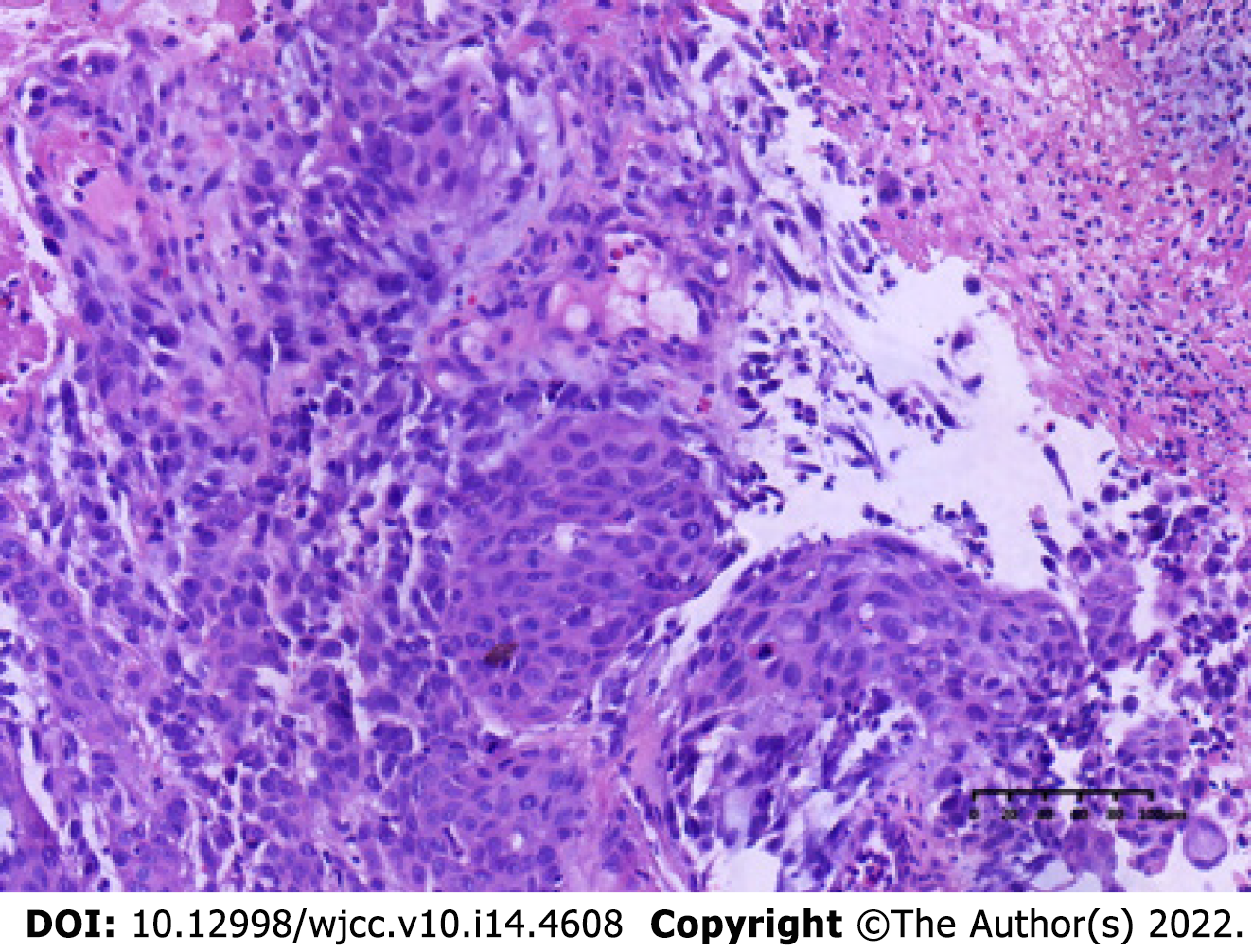
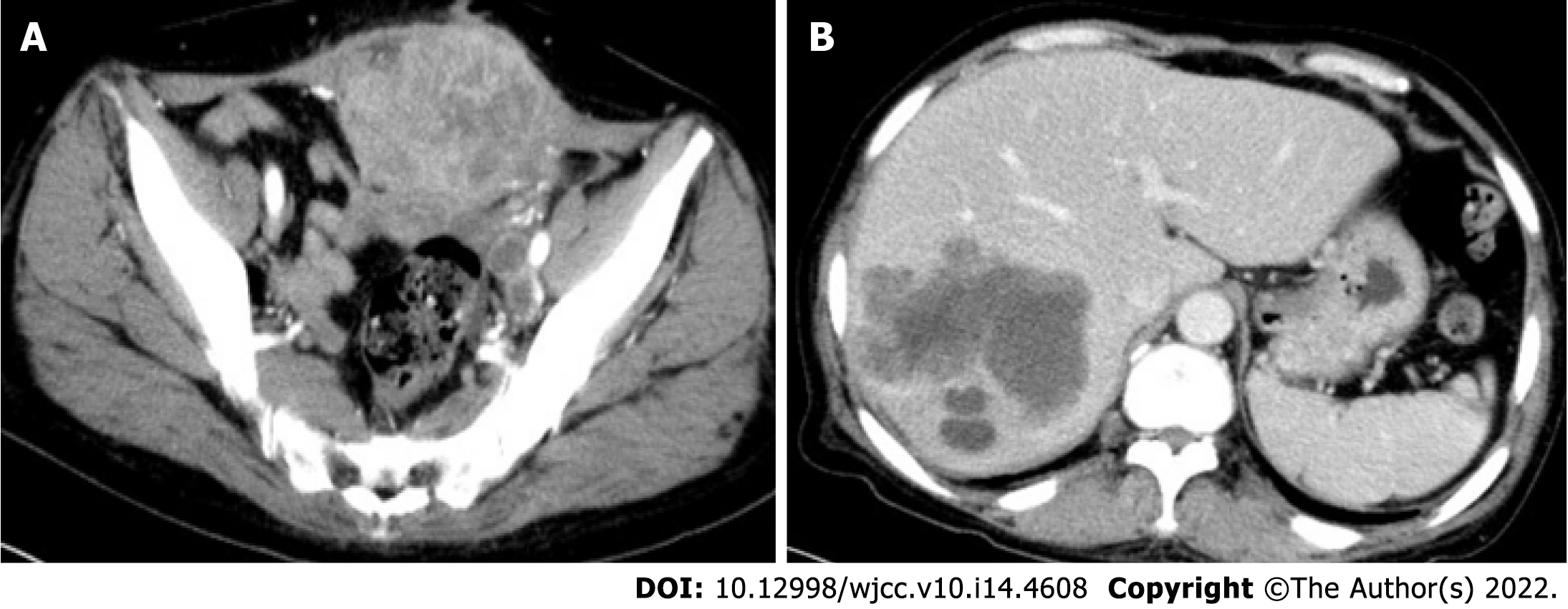
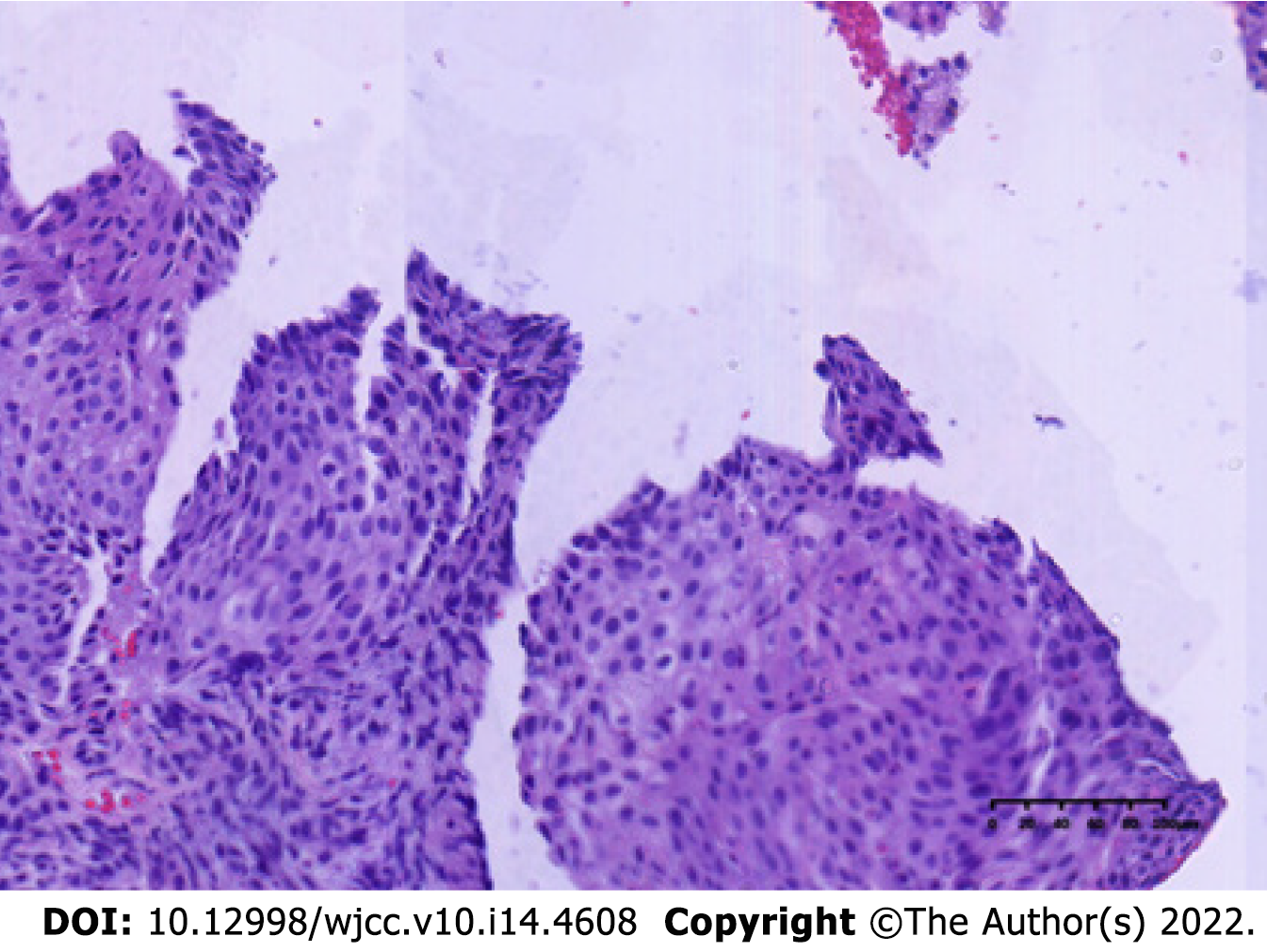
The patient underwent computed tomography examination, which confirmed the presence of an irregular soft tissue mass located in the left lower abdomen with unclear borders and nonuniform density (Figure 1A) and the absence of metastatic lesions in the liver (Figure 1B). Total colonoscopy was advised, which indicated the presence of an ulcerated mass with infiltrative growth in the colon approximately 30 cm from the anus (Figure 2). Pathological examination of the tissue obtained by colonoscopy revealed squamous carcinoma (Figure 3).
A biopsy was performed at colonoscopy, and histology showed squamous carcinoma.
The patient underwent combined partial ileal and anterior peritoneal resection for sigmoid colon cancer on March 28, 2019. The sigmoid colon was incised 12 cm from the proximal edge and 6 cm from the distal edge of the tumor. Intraoperatively, the mass invaded the anterior peritoneum and ileum approximately 40 cm from the ileocecal region and was resected.
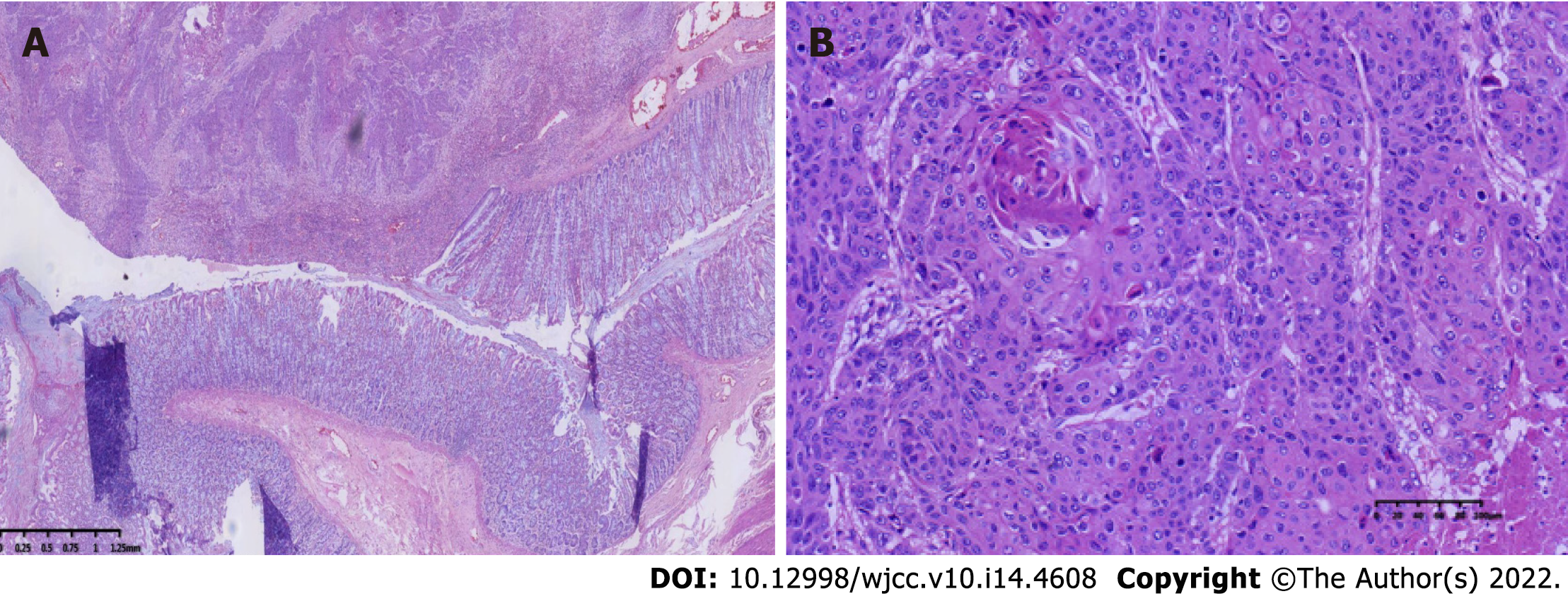
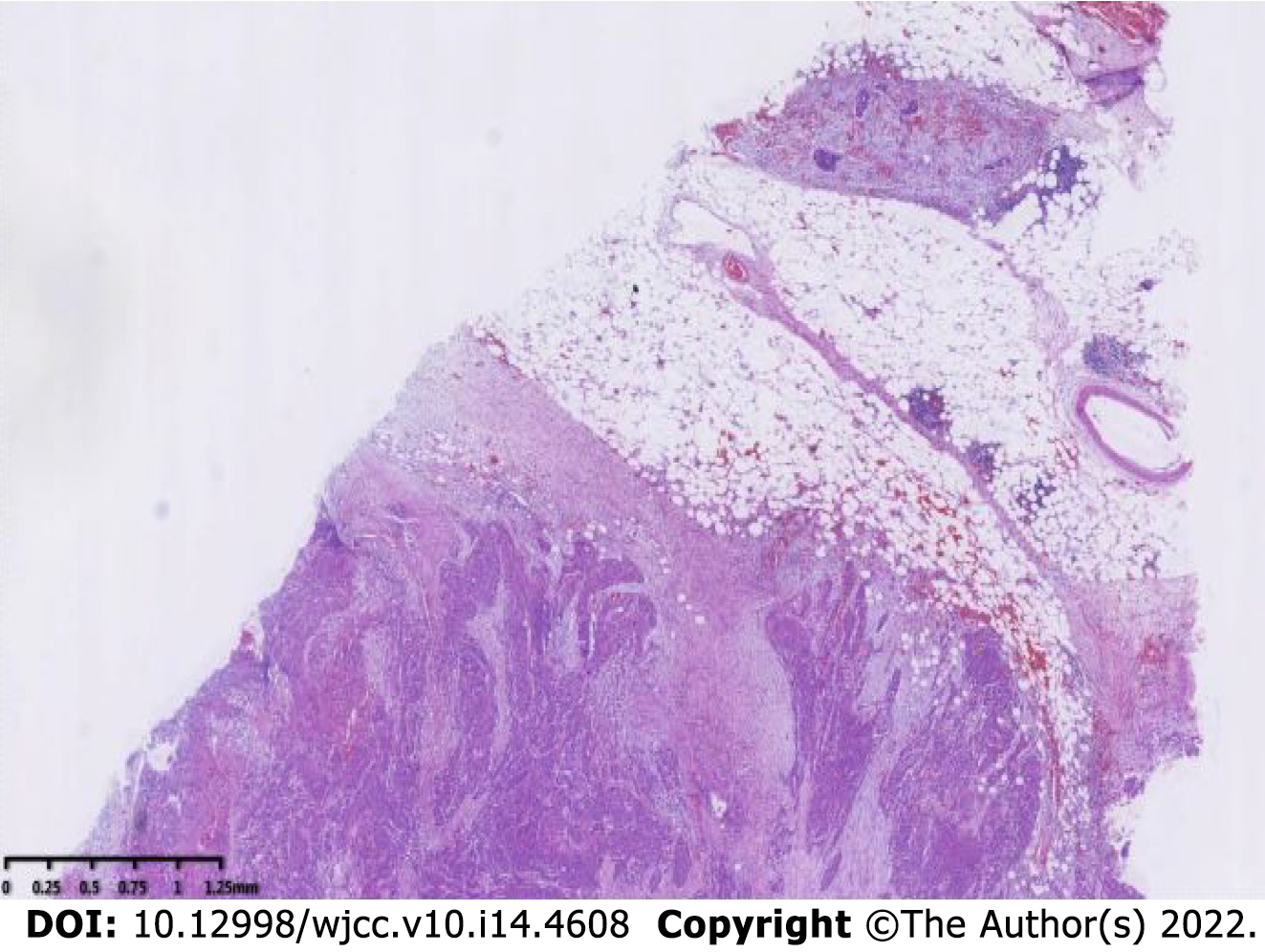
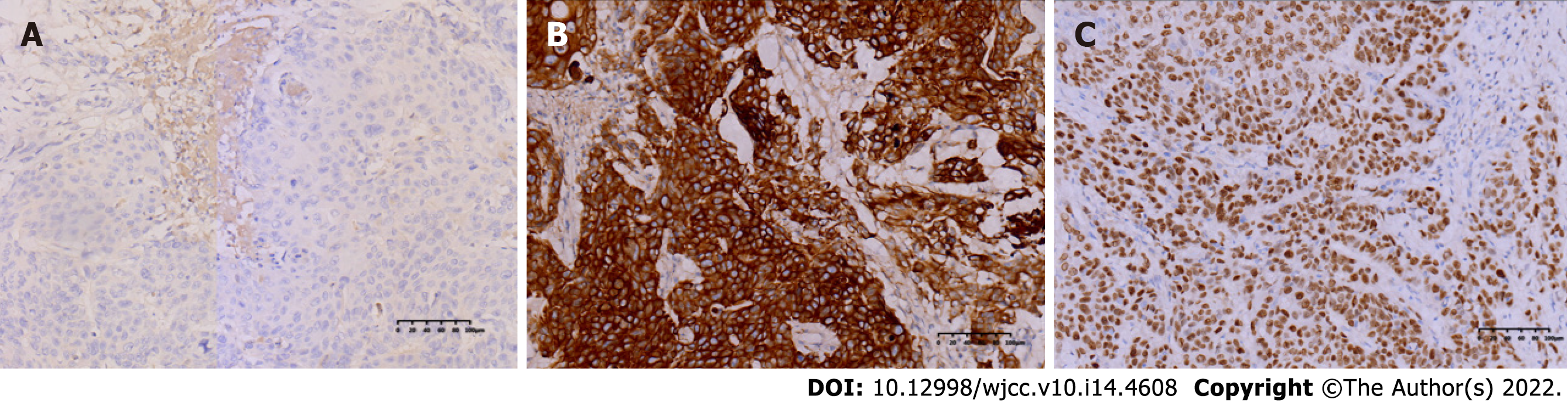
The specimen was embedded in paraffin and cut into 4-μm-thick sections. Hematoxylin and eosin staining was performed, and the sections were examined microscopically at the Pathology Department of the Affiliated Hospital of Chengde Medical College. Histologically, the tumor cells were arranged in irregular nest-like clusters, the tumor cells were ill-defined, the nucleoplasm ratio was disordered, the nuclei were large and densely stained, the polar direction disappeared, the nuclear membrane was clear, and the chromatin was concentrated, consistent with the diagnosis of intermediate-to-low differentiated squamous carcinoma (Figure 4A and B). The cancerous tissue had invaded the entire layer of the intestinal wall (Figure 5), and no residual cancer was observed on the upper and lower incision margins. Immunohistochemically, the cancer cells were negative for C-erbb-2, bcl-2, COX2, CDX2 (Figure 6A), PS2, and CK7. These cells were positive for MSH2, PMS2, MSH6, MLH1, P53, EGFR, P40 (Figure 6B), CK5/CK6 (Figure 6C), D2-40, CD34, and TOPII. The Ki-67 proliferation index was 70%.
Eight lymph nodes were examined, and no lymph node metastasis was observed on postoperative pathology. The results of the preoperative examination suggested no distant organ metastasis, such as liver, lung, or brain metastasis. Combining the above information on histopathological specimens, lymph nodes, and distant metastases, the stage of sigmoid squamous carcinoma was determined to be pT4bN0M0, stage IIC.
The patient was diagnosed with early-stage colon cancer, and surgical treatment was preferred, without prior systematic treatment. After surgical treatment, the patient received six cycles of postoperative adjuvant chemotherapy.
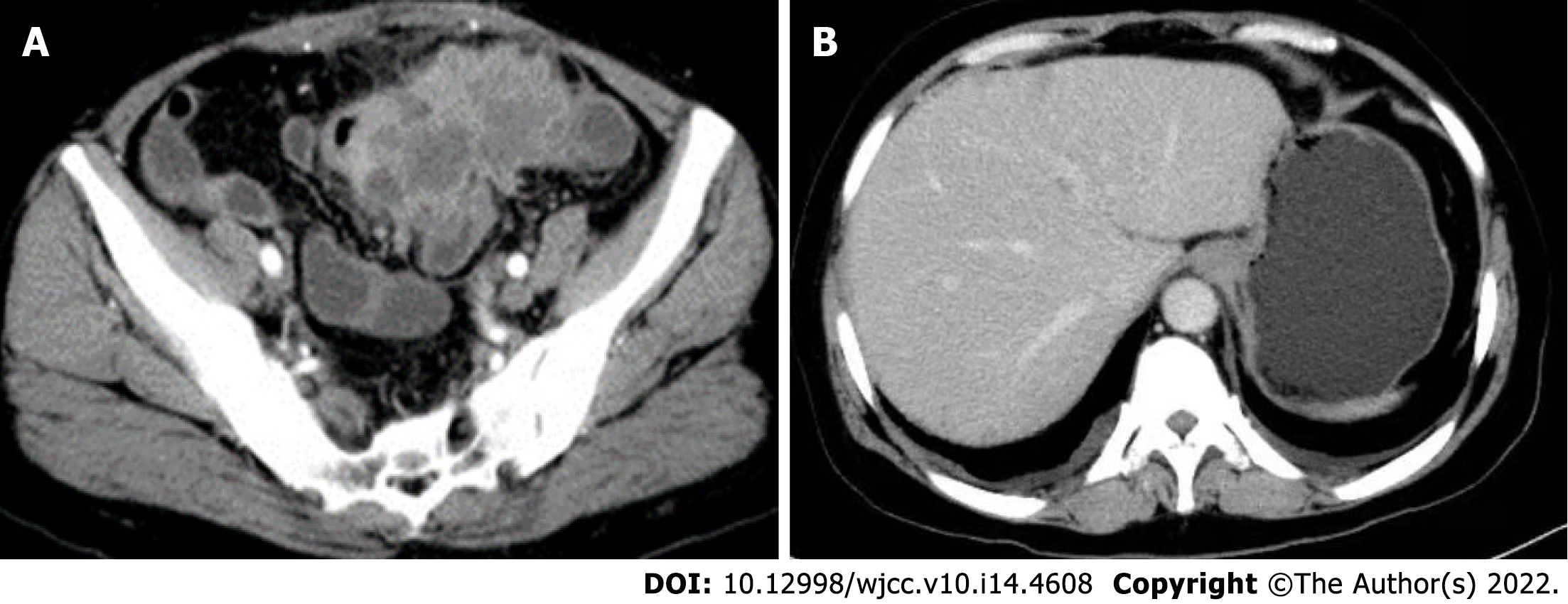
Postoperatively, the patient recovered well, without complications. Routine chemotherapy and regular follow-up were performed postoperatively. On November 10, 2019, multiple liver metastases (Figure 7A) and an anterior pelvic mass (Figure 7B) were observed. A liver biopsy was performed immediately after the discovery of liver metastases, and the pathological results showed that it was a metastatic SCC derived from the liver (Figure 8). Genetic testing revealed the presence of somatic mutations (Table 2). Unfortunately, the liver cannot be surgically removed because of multiple metastases. The patient did not undergo radiofrequency or ablation therapy. However, I125 seed implantation was performed on the abdominal wall mass. After seed implantation, two cycles of first-line chemotherapy were administered. By May 2020, the patient had died of the tumor. The patient’s overall survival rate was 14 mo.
| Gene name | Type of mutation | Exon number | Variant site | Mutation frequency |
| FBXW7 | SNV | Exon11 | p.S550R | 2.21% |
| TP53 | SNV | Exon7 | p.S241Y | 51.98% |
| KRAS | SNV | Exon2 | p.G12D | 55.95% |
| PTEN | SNV | Exon5 | p.G127E | 56.89% |
SCC occurs most often in areas covered by squamous cells; therefore, it rarely occurs in organs without squamous cell coverage. The colon is not covered by squamous epithelium and is dominated by glandular epithelium. Therefore, SCC in the primary colon is significantly rare, with an incidence of approximately 0.025-0.1 per 1000 colon cancers[8]. Regarding the high incidence age of colorectal squamous carcinoma, Donato-Brown et al[6] reported that the average age for the entire cohort in the SEER database was 60.7 ± 13.7 years, and the median age of onset of colonic SCC was 55-60 years according to the literature[9-15]. SCC can occur in any part of the colorectum[5,16]; the majority of them are located in the rectum (93.4%), followed by the right hemicolon (3.4%), and SCC in the left hemicolon is extremely rare. The first reported SCC of the colon in China also occurred in the ascending colon[17]. The clinical manifestations of patients with colorectal squamous carcinoma are similar to those of adenocarcinoma, mainly including abdominal pain, blood in the stool, and change in bowel habits, and a few may present with acute abdominal and intestinal obstructions[9]. We reported a case of simple squamous carcinoma of the sigmoid colon, the main clinical manifestation of which was chronic constipation without blood in the stool or abdominal pain.
The possible mechanisms of colonic squamous carcinoma pathogenesis are currently reported in the literature with the following possibilities. First, squamous metaplasia of the glandular epithelium is commonly caused by chronic inflammation due to radiation therapy[18], schistosomiasis infection[19], and inflammatory bowel disease[17], and squamous cell metaplasia is caused by continuous chronic stimulation of the glandular epithelium, which in turn leads to cell degeneration and carcinoma, as reported by Cheng et al[20] in a 51-year-old female patient with squamous colorectal cancer who had a history of ulcerative colitis for more than 10 years with a long history of hormonal therapy. Second is the pathological process of undifferentiated cell proliferation, squamous differentiation, and carcinogenesis. Comer et al[21] suggested that basal cells undergo proliferation and repair after colorectal epithelial damage, whereas repeated destructive effects cause basal cells to lose their normal differentiation ability and produce adenocarcinoma, squamous carcinoma, or adenosquamous carcinoma. Michelassi et al[22] proposed that SCC originates from undifferentiated basal cells after mucosal damage proliferation and malignant transformation. Third, adenoma or adenocarcinoma cells differentiate into squamous carcinoma, and squamous cells have been gradually recognized in colonic adenomas[23]. Cramer et al[24] reported three cases of colonic adenomas in which multipotential reserve cells were found to differentiate into squamous epithelium, and primary colonic SCC may originate from squamous cell differentiation and carcinoma of cells within the adenoma. Fourth, considering ectopic ectodermal cell nest carcinoma, Gooneratne et al[25] reported a case of SCC of the sigmoid colon originating from a malignant teratoma of the ovary. Fifth, residual implantation of squamous cell epithelial cells is more common due to trauma and surgery[26].
The following conditions should be met to establish the diagnosis of colorectal SCC[27]: first, consistent with the pathological features of SCC without adenoid differentiation; second, ruling out the possibility of metastasis or direct invasion of other tissues or organs by SCC; third, ruling out the possibility of upward extension of SCC of the anal canal to the lower rectum; and fourth, absence of long-term persistent squamous cell epithelium-lined fistula in the intestinal canal where the tumor is located.
In this case, the tumor was 30 cm away from the anal verge, excluding cloacogenic embryologic nests as origins of the SCC. The patient had no specific past medical history, no evidence of SCC on imaging, and no colonic fistula formation. After three pathological examinations, both the primary lesion and liver metastases showed simple SCC on hematoxylin and eosin staining. Positive AE1/AE3 suggests an epithelial origin tumor; positive p40 and CK5/CK6 suggest SCC, negative CDX2 rules out the possibility of adenocarcinoma; and negative CGA and Syn rule out neuroendocrine origin. Immunohistochemistry was consistent with the pathology of primary SCC.
Commonly used treatments for colorectal cancer include surgery, radiotherapy, and chemotherapy. Owing to its extremely low incidence rate, there is no clear standard treatment plan for SCC. Some case reports have suggested chemoradiotherapy as the initial treatment and surgery as the treatment in case of poor chemotherapy effect or tumor recurrence because chemoradiotherapy can effectively control rectal squamous carcinoma well[28,29]. Zhao et al[13] reported a patient with ascending colon squamous carcinoma combined with rectal adenocarcinoma who received two cycles of neoadjuvant chemotherapy based on gemcitabine, combined with oxaliplatin and capecitabine, and experienced disease stabilization. Surgery was performed in this patient. The original regimen was continued for six cycles of chemotherapy after surgery, and progression-free survival exceeded 10 mo after treatment.
SCC of the colon is rare; the sigmoid colon is even rarer, and its treatment is mostly referred to as adenocarcinoma of the colon. We reported the case of a patient with squamous carcinoma of the sigmoid colon who developed liver metastases and pelvic metastases 7 mo after surgery and postoperative chemotherapy treatment, which showed poor sensitivity to conventional chemotherapy regimens for colorectal adenocarcinoma. Therefore, summarizing the case characteristics and treatment experience of patients with colonic squamous carcinoma and determining a more suitable chemotherapy regimen for colonic squamous carcinoma to improve the survival time of such patients are the main issues to be explored in the future.
Colon SCC, especially sigmoid colon SCC, is one of the rare lesions encountered by surgeons and pathologists. Due to its rarity, it is easily misdiagnosed. Here, we reported the case of a patient with sigmoid SCC at stage IIC (pT4bN0M0). Colon SCC appears to have a worse prognosis and no standard treatment options; therefore, determining the accurate diagnosis and appropriate management of SCC is important.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Caputo D, Italy; Karaca CA, Turkey; Karamarkovic AR, Serbia; Lambrecht NW, United States; Taheri S, Iran; Tsimogiannis K, Sweden S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12991] [Article Influence: 1443.4] [Reference Citation Analysis (2)] |
| 2. | Bosman FT, Carnerio F, Hruban RH, Theise ND. WHO classification of tumors of the digestive system [M]. 4th ed. Lyon: IARC Press, 2010: 134-136. |
| 3. | Linardoutsos D, Frountzas M, Feakins RM, Patel NH, Simanskaite V, Patel H. Primary colonic squamous cell carcinoma: a case report and review of the literature. Ann R Coll Surg Engl. 2020;102:e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Schmidtmann M. Zur kenntnis seltener Krebsformen. Virch Arch Pathol. 1919;226:100-118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Ozuner G, Aytac E, Gorgun E, Bennett A. Colorectal squamous cell carcinoma: a rare tumor with poor prognosis. Int J Colorectal Dis. 2015;30:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Donato-Brown D, Antoniou A, Murphy J. Colorectal squamous cell carcinoma: A population-based study of rare tumor type. Dis Colon Rectum. 2019;6:e145. |
| 7. | Tîrcol SA, Ghidersa A, Negreanu L, Sajin M, Dumitru AV. Squamous metaplasia within a sigmoid adenoma. A rare feature. Rom J Morphol Embryol. 2020;61:235-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Juturi JV, Francis B, Koontz PW, Wilkes JD. Squamous-cell carcinoma of the colon responsive to combination chemotherapy: report of two cases and review of the literature. Dis Colon Rectum. 1999;42:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Dikshit V, Ali I, Patil C, Manerikar K, Mody P. Squamous Cell Carcinoma of Colon-an Etiopathological Surprise. J Gastrointest Cancer. 2019;50:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Wang XP, Du H, Li XB, Liu SS. Clinicopathologic analysis of primary colon adenosquamous carcinoma and squamous carcinoma. Guangzhou Yiyao. 2018;49:94-97. |
| 11. | Landau D, Garrett C, Chodkiewicz C. A case of primary squamous cell colon cancer. J Oncol Pharm Pract. 2007;13:47-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Samo S, Sherid M, Liu K, Kia L, Elliott GJ. Basaloid Squamous Cell Carcinoma of the Sigmoid Colon. ACG Case Rep J. 2015;2:161-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Zhao S, Guo J, Sun L, Lv J, Qiu W. Gemcitabine-based chemotherapy in colon squamous cell carcinoma: A case report and literature review. Mol Clin Oncol. 2017;6:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Lu ZJ, Sun XW. A case of primary squamous colon carcinoma. J Diag Pathol. 2016;23:978. |
| 15. | Dong Y, Wang J, Ma H, Zhou H, Lu G, Zhou X. Primary adenosquamous carcinoma of the colon: report of five cases. Surg Today. 2009;39:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Kang H, O'Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2007;22:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Shao YF, Pan GL, Zhou CN, Yu HT. [Squamous cell carcinoma of the ascending colon--a case report and review of literature]. Zhonghua Zhong Liu Za Zhi. 1987;9:315-316. [PubMed] |
| 18. | Leung KK, Heitzman J, Madan A. Squamous cell carcinoma of the rectum 21 years after radiotherapy for cervical carcinoma. Saudi J Gastroenterol. 2009;15:196-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Pittella JE, Torres AV. Primary squamous-cell carcinoma of the cecum and ascending colon: report of a case and review of the literature. Dis Colon Rectum. 1982;25:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Cheng H, Sitrin MD, Satchidanand SK, Novak JM. Colonic squamous cell carcinoma in ulcerative colitis: Report of a case and review of the literature. Can J Gastroenterol. 2007;21:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Comer TP, Beahrs OH, Dockerty MB. Primary squamous cell carcinoma and adenocanthoma of the colon. Cancer. 1971;28:1111-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Michelassi F, Mishlove LA, Stipa F, Block GE. Squamous-cell carcinoma of the colon. Experience at the University of Chicago, review of the literature, report of two cases. Dis Colon Rectum. 1988;31:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Neumann H, Vieth M. Squamous cell carcinoma of the sigmoid colon arising on base of a nonpolypoid tubulovillous adenoma. Endoscopy. 2014;46 Suppl 1 UCTN:E455-E456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Cramer SF, Velasco ME, Whitlatch SP, Graney MF. Squamous differentiation in colorectal adenomas. Literature review, histogenesis, and clinical significance. Dis Colon Rectum. 1986;29:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Gooneratne AT, James AO, Gupta J, Abdulaal Y. Squamous cell carcinoma arising in a mature cystic teratoma invading the sigmoid colon: a rare presentation. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Mondal SK. Primary squamous cell carcinoma of the cecum. J Cancer Res Ther. 2009;5:328-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Dyson T, Draganov PV. Squamous cell cancer of the rectum. World J Gastroenterol. 2009;15:4380-4386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Péron J, Bylicki O, Laude C, Martel-Lafay I, Carrie C, Racadot S. Nonoperative management of squamous-cell carcinoma of the rectum. Dis Colon Rectum. 2015;58:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Musio D, De Felice F, Manfrida S, Balducci M, Meldolesi E, Gravina GL, Tombolini V, Valentini V. Squamous cell carcinoma of the rectum: The treatment paradigm. Eur J Surg Oncol. 2015;41:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |