Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4110
Peer-review started: September 1, 2021
First decision: November 11, 2021
Revised: November 23, 2021
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 6, 2022
Processing time: 241 Days and 4.9 Hours
Cardiopulmonary bypass (CPB) is an essential procedure for maintaining the blood supply to vital organs in patients undergoing cardiac surgery. However, perioperative cardiac injury related to CPB remains a severe complication in these patients. Cardiac protection is important for patients undergoing CPB.
To evaluate the potential cardioprotective efficacy of the Chinese medicine preparation Xuebijing injection (XBJ) in patients undergoing CPB.
Sixty patients undergoing cardiac surgery with CPB were randomly allocated to the XBJ and control groups (saline). XBJ was administered intravenously three times: 12 h prior to surgery, at the beginning of the surgery, and 12 h after the second injection. Cardiac function was evaluated by echocardiography 48 h after surgery. Circulating inflammation- and oxidative-stress-related markers were measured. Clinical outcomes related to intensive care unit (ICU) stay were recorded.
Compared to control treatment, XBJ was associated with improved PaO2/FiO2 and cardiac systolic function, but reduced troponin I and creatine kinase fraction after surgery (all P < 0.05). The circulating concentrations of tumor necrosis factor-α, interleukin (IL)-1β and IL-8 in the XBJ group were significantly lower than those in the control group (all P < 0.05), whereas the circulating concentration of IL-10 was significantly higher in the XBJ group (P < 0.05). In addition, the lengths of ICU stay and hospitalization after surgery tended to be shorter in the XBJ group than in the control group, although the differences were not significant.
Perioperative administration of XBJ was associated with attenuated cardiac injury during CPB, likely via anti-inflammatory and antioxidative mechanisms.
Core Tip: Xuebijing injection (XBJ) significantly reduced myocardial injury in patients undergoing cardiopulmonary bypass (CPB), as demonstrated by significantly lower levels of myocardial injury markers including troponin I and creatine kinase-MB, and the preserved cardiac ejection fraction in patients in the XBJ group compared with those in the control group. The benefit of XBJ against myocardial injury was accompanied by reduced serum concentrations of inflammatory markers, including tumor necrosis factor-α, interleukin (IL)-1β and IL-8 and an increase in anti-inflammatory factor IL-10. These results suggest that perioperative XBJ is associated with attenuated cardiac injury during CPB, likely via anti-inflammatory and antioxidative mechanisms.
- Citation: Jin ZH, Zhao XQ, Sun HB, Zhu JL, Gao W. Effect of Xuebijing injection on myocardium during cardiopulmonary bypass: A prospective, randomized, double blind trial. World J Clin Cases 2022; 10(13): 4110-4118
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4110.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4110
Cardiopulmonary bypass (CPB) is an essential procedure for maintaining the blood supply to vital organs in patients undergoing cardiac surgery[1]. However, perioperative cardiac injury related to CPB remains a severe complication in these patients[2]. Clinically, there are multiple risk factors for CPB-related cardiac injury, including type of surgery, pattern of CPB induction, and application of cardioplegia and hypothermia[3]. Pathophysiologically, ischemia–reperfusion injury (IRI) and inflammatory injury induced by CPB have been recognized as the most important mechanisms underlying the pathogenesis of CPB-related cardiac injury[1,4]. Patients who experience cardiac injury during CPB have a poor prognosis[5]. Therefore, identification of novel prevention and treatment strategies for CPB-related cardiac injury is of significant importance in clinical practice.
Xuebijing injection (XBJ), a traditional Chinese drug injection, has been applied for many critical clinical conditions related to the inflammatory response, such as pneumonia, sepsis, inflammatory lung injury, and disseminated intravascular coagulation due to its anti-inflammatory and antioxidative actions[6,7]. Moreover, clinical observations have confirmed the protective role of XBJ for multiple organs during inflammatory injury, including acute sepsis-related lung injury and liver injury[8-10]. In view of the important role of inflammation and oxidative stress in the process of CPB-related cardiac injury, we hypothesized that XBJ may attenuate myocardial injury during the CPB process. We aimed to evaluate the potential efficacy of XBJ on cardiac injury during CPB in these patients in a prospective, randomized, double blinded trial.
The protocol for the present study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University before the performance of the study. A predefined study protocol was registered with the Chinese Clinical Trial Registry (ChiCTR-TRC-14004628). Written informed consent forms were signed by all of the included patients before enrollment.
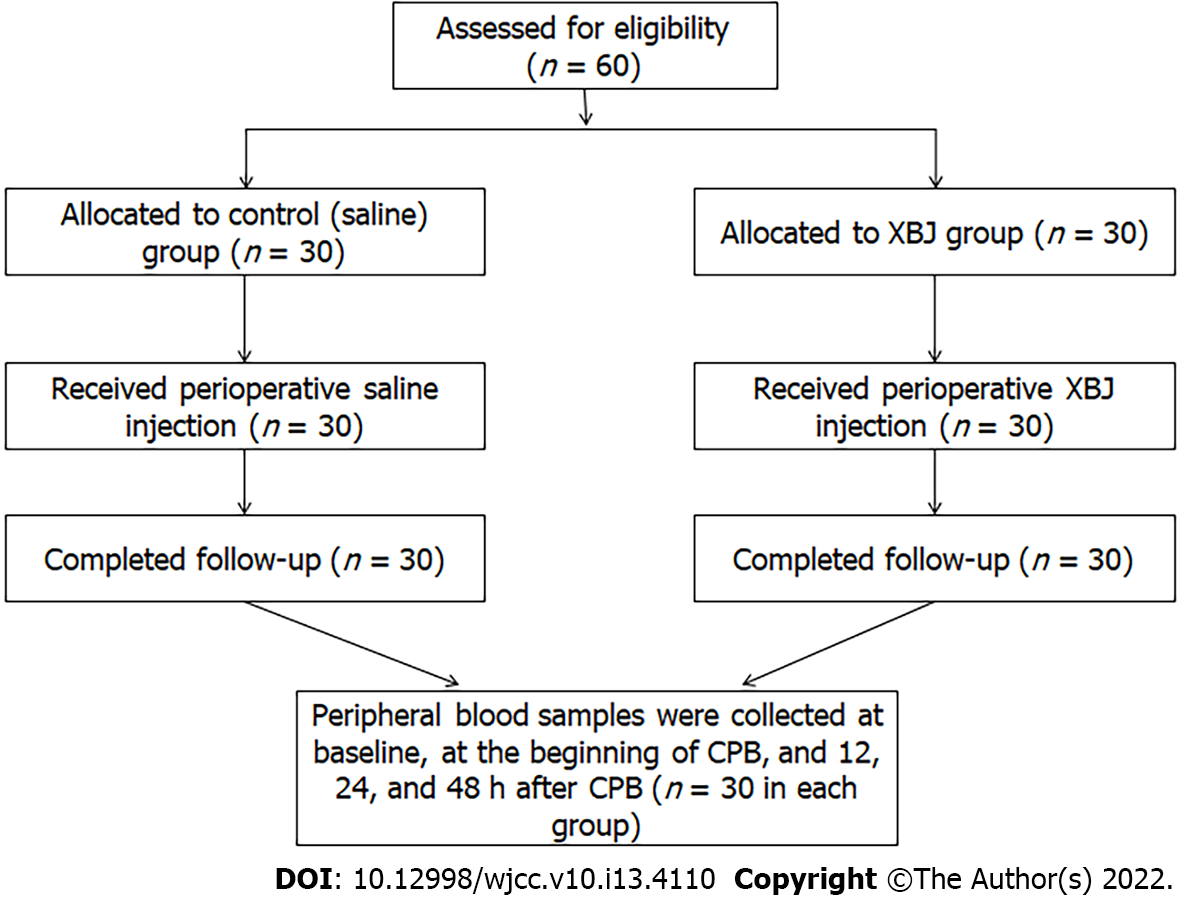
Sixty patients who underwent cardiac surgery with CPB in our center between November 1, 2018 and February 1, 2019 were included. Patients were randomized to the XBJ group or the control group via a protocol similar to that reported in a previous study[11]. A total of 60 balls, which were marked 0 or 1, were kept in a black box. When the patient was prepared for surgery, the anesthetist performed the randomization by choosing a random ball from the box, with 0 and 1 indicating saline and XBJ treatments, respectively. Patients who had severe lung dysfunction or infection, pulmonary hypertension, hypoproteinemia, hepatic or renal dysfunction, or a coagulation disorder were excluded from this study. For the 30 patients allocated to the XBJ group, XBJ for injection via photophobic infusion (100 mL, Tianjin Chase Sun Pharmaceutical Group, Tianjin, China)11 was administered 12 h before surgery, at the beginning of the surgery and 12 h after the second injection. For the other 30 patients allocated to the control group, intravenous infusions of saline (100 mL) were applied at the same time points, both via photophobic infusion (Qiaopai Group, Zibo, Shandong, China). An anesthesiologist who was not aware of the allocation performed the anesthesia for all patients. To minimize the influence of the operation on the outcomes in this study, all surgeries were performed by the same surgical team (Figure 1).
The standardized anesthesia and operative procedures were administered for all patients. Angiotensin-converting enzyme inhibitors and angiotensin II antagonists were discontinued before surgery, while the other preoperative cardiac medications were administered until the morning of surgery. Midazolam (0.2 mg/kg) was orally administered to the patients 30 min before the operation. In the operating room, a peripheral vein was cannulated, and a mixture of crystal and colloidal solution (1:2) was injected at 8 mL/kg/h. A radial artery of each patient was cannulated to monitor blood pressure and for arterial blood gas analysis. Anesthesia was induced with combined application of lidocaine (1 mg/kg), fentanyl (10 μg/kg), pipecuronium (0.05 mg/kg), and etomidate (0.2 mg/kg). After intubation, the right internal carotid artery was cannulated to monitor the central venous pressure (CVP) and for cardiac drug infusion. Volume-controlled ventilation (fraction of inspired oxygen 80% ± 5%, tidal volume 8 mL/kg, respiratory rate 10–12 /min, inspiratory to expiratory ratio 1:2) was applied as the model of mechanical ventilation before CPB. The anesthesia was maintained with sevoflurane (1.5%) and fentanyl (10 μg/kg/h) and changed to propofol (5 mg/kg/h) and fentanyl during CPB before being returned to sevoflurane and fentanyl. The surgical procedure was performed with the pH, arterial CO2 tension (PaCO2), and arterial oxygen tension (PaO2) maintained within ranges of 7.35-7.45, 35-40 mmHg, and > 150 mmHg, respectively.
After surgery, all of the patients were observed in the intensive care unit (ICU), where the third injection of XBJ or saline was applied. The standard cardiovascular medications and mechanical ventilation support were applied as needed. Weaning from the ventilator was attempted if the patients were considered hemodynamically and respiratorily stable according to the following parameters: PaO2/FiO2 ratio > 200 mmHg, acid–base balance, chest drainage < 80 mL/h, and body temperature ≥ 36°C.
Arterial blood gas analysis was performed before the first injection of XBJ (baseline) and 0, 12, 24 and 48 h after the surgery. The PaO2/FiO2 ratio was calculated. Peripheral blood samples were collected at the same time points.
Troponin (Tn)I and creatine kinase fraction MB (CKMB) concentrations in serum were determined using ELISA (Boster, Wuhan, China). Markers of inflammation, including serum concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-8, and IL-10 were also measured with ELISA (Sigma–Aldrich, St. Louis, MO, United States). We also determined the circulating levels of 8-isoprostane (Cayman Chemicals, Ann Arbor, MI, United States) and malondialdehyde (MDA) (Nanjing Jiancheng, Nanjing, China) as markers of oxidative stress using commercial kits.
The primary outcome of the study was the change in TnI value from the beginning of anesthesia to 48 h after surgery. In addition, the incidences of clinical outcomes including myocardial infarction, cardiac stunning, acute respiratory distress syndrome (ARDS), and acute kidney injury (AKI) were recorded.
To detect a 2 ng/mL reduction in TnI within 48 h after surgery between two groups, a total of 50 patients are needed with a power of 80% and an a level of 0.05. We included 30 patients in each group (rather than 25) after assuming the potential rate of loss during follow-up.
Continuous data that were normally distributed were presented as mean ± SD and compared with the independent t test. Data for categorical variables were presented as numbers and proportions, and these data were compared via Chi-square analysis. For continuous variables that were measured at multiple time points, repeated-measures analysis of variance was applied with Bonferroni’s correction as post hoc analysis. P < 0.05 was considered statistically significant. We used SPSS software (Chicago, IL, United States) for the above statistical analyses.
In the present study, a total 60 patients were enrolled, and no patient was excluded. All the patients were successfully weaned from CPB and transferred to the ICU. There were no significant differences in the demographic data between the XBJ and control groups (Table 1). In addition, no significant differences were found regarding the characteristics of CPB and surgery, including CPB time, aortic cross clamp time, surgical duration, and quantity of potassium cardioplegic solution used. Patients from the XBJ and control groups received a similar volume of perioperative fluid infusion, had similar urine volumes, and consumed similar amounts of vasoactive medications during the surgery (P > 0.05, Table 1).
| Saline group (n = 30) | XBJ group (n = 30) | P value | |
| Age (yr) | 57.4 ± 5.3 | 56.8 ± 6.2 | 0.69 |
| Male | 18 | 20 | |
| Weight (kg) | 63.6 ± 5.8 | 65.4 ± 5.6 | 0.22 |
| Diabetes mellitus (n) | 5 | 7 | 0.44 |
| Preoperative LVEF (%) | 51.7 ± 8.5 | 52.5 ± 7.6 | 0.71 |
| Ejection fraction | 50.2 ± 3.2 | 50.3 ± 3.8 | 0.91 |
| Preoperative medications (n) | |||
| β-blockers | 8 | 6 | 0.54 |
| Calcium channel blockers | 5 | 7 | 0.52 |
| Renin angiotensin system inhibitors | 5 | 3 | 0.44 |
| Statin | 4 | 2 | 0.39 |
| Digoxin | 6 | 8 | 0.54 |
| Diuretics | 16 | 19 | 0.43 |
| Surgical procedure (n) | |||
| Aortic valve replacement | 8 | 11 | 0.40 |
| Mitral valve replacement | 10 | 9 | 0.78 |
| Tricuspid annuloplasty | 12 | 10 | 0.59 |
| Pulmonary function test | |||
| FVC; % predicted | 82.8 ± 7.4 | 81.9 ± 8.1 | 0.65 |
| Operation time (min) | 257 ± 38 | 271 ± 41 | 0.17 |
| CPB time (min) | 104 ± 31 | 111 ± 27 | 0.35 |
| Intraoperative fluid balance (mL) | 2138 ± 348 | 2215 ± 387 | 0.42 |
Although the serum concentrations of TnI and CK-MB were comparable between patients in the two groups at baseline (P > 0.05), the serum concentrations of both biomarkers of cardiac injury were elevated in both groups after surgery. However, the serum concentrations of TnI and CK-MB were significantly lower in patients in the XBJ group than in those of the saline group after surgery (P < 0.05).
The perioperative hemodynamic variables, including mean arterial pressure (MAP) and CVP, were similar between the two groups. There also were no significant differences in the results of arterial blood analysis between the two groups except for PaO2/FiO2, which was increased significantly from 12 to 72 h after surgery in the XBJ group compared with the levels in the control group (P < 0.05, Table 2). Although the baseline ejection fraction in patients from both groups was similar, the ejection fraction after surgery was significantly higher in the XBJ group than in the control group (P < 0.05, Table 2). In addition, treatment with XBJ was associated with a significantly shorter length of ICU stay and time from operation to discharge compared with those in the saline group (both P < 0.05, Table 2).
| Saline group (n = 30) | XBJ group (n = 30) | P value | |
| Ejection fraction (day 2) | 42.2 ± 2.7 | 46.3 ± 3.7 | 0.001 |
| Mechanical ventilation time (h) | 10.5 ± 2.6 | 8.9 ± 1.7 | 0.006 |
| Length of ICU stay (h) | 31.6 ± 5.2 | 28.9 ± 4.4 | 0.034 |
| Time from surgery to discharge (d) | 12.5 ± 3.8 | 10.7 ± 2.6 | 0.032 |
| ARDS (n) | 3 | 4 | 0.687 |
Compared with those at baseline, all serum biomarker levels were increased significantly in the two groups. The circulating concentrations of TNF-α, IL-1β and IL-8 in the XBJ group were significantly lower than those in the control group (all P < 0.05, Table 3), whereas the circulating concentration of IL-10 was significantly greater in the XBJ group (P < 0.05, Table 3). No severe complications such as myocardial infarction, acute kidney failure, cerebrovascular accidents, or mortality were found during ICU stay.
| Group | Baseline | 0 h | 12 h | 24 h | 48 h | |
| PaO2/FiO2 | Saline | 398.40 ± 22.66 | 361.92 ± 48.62 | 258.93 ± 39.03 | 320.64 ± 46.43 | 341.44 ± 35.28 |
| XBJ | 387.12 ± 35.77 | 358.48 ± 39.21 | 289.40 ± 44.07a | 353.34 ± 31.54a | 379.08 ± 22.54a | |
| CK-MB | Saline | 0.26 ± 0.07 | 0.71 ± 0.12 | 2.74 ± 0.48 | 4.25 ± 0.83 | 1.95 ± 0.62 |
| XBJ | 0.26 ± 0.08 | 0.58 ± 0.12 | 2.27 ± 0.33a | 3.02 ± 0.44a | 1.09 ± 0.37a | |
| TnI | Saline | 0.02 ± 0.01 | 1.45 ± 0.28 | 8.01 ± 1.13 | 13.03 ± 2.56 | 4.03 ± 0.92 |
| XBJ | 0.02 ± 0.01 | 1.38 ± 0.93 | 4.77 ± 0.76a | 8.29 ± 1.73a | 3.16 ± 0.43a | |
| TNF-α | Saline | 13.51 ± 4.19 | 71.18 ± 29.21 | 147.36 ± 43.84 | 226.31 ± 60.22 | 122.48 ± 55.18 |
| XBJ | 15.61 ± 4.51 | 70.69 ± 27.24 | 125.64 ± 34.44a | 173.61 ± 40.03a | 99.96 ± 33.37a | |
| IL-1β | Saline | 16.98 ± 4.14 | 32.95 ± 9.05 | 92.31 ± 18.45 | 78.43 ± 17.54 | 62.71 ± 15.40 |
| XBJ | 13.13 ± 4.22 | 28.23 ± 6.24 | 77.25 ± 9.16a | 66.15 ± 12.18a | 43.60 ± 13.33a | |
| IL-8 | Saline | 831.36 ± 136.86 | 1002.73 ± 180.26 | 1530.90 ± 235.96 | 1400.83 ± 268.85 | 1134.86 ± 211.41 |
| XBJ | 767.30 ± 155.86 | 960.43 ± 187.49 | 1369.93 ± 244.21a | 1251.76 ± 240.81a | 984.86 ± 184.43a | |
| IL-10 | Saline | 90.60 ± 15.82 | 121.96 ± 18.15 | 167.41 ± 18.54 | 152.53 ± 33.94 | 121.26 ± 20.73 |
| XBJ | 87.27 ± 20.83 | 123.30 ± 18.31 | 176.72 ± 27.91 | 166.97 ± 21.94a | 131.87 ± 19.52a | |
| MDA | Saline | 0.11 ± 0.08 | 0.49 ± 0.19 | 0.14 ± 0.09 | 0.11 ± 0.08 | 0.11 ± 0.10 |
| XBJ | 0.09 ± 0.04 | 0.31 ± 0.12a | 0.13 ± 0.11 | 0.10 ± 0.06 | 0.10 ± 0.04 | |
| 8-Isoprostane | Saline | 1.84 ± 0.46 | 8.31 ± 0.76 | 6.29 ± 0.75 | 3.25 ± 0.52 | 1.83 ± 0.36 |
| XBJ | 2.00 ± 0.55 | 6.63 ± 0.88a | 6.29 ± 0.75 | 3.24 ± 0.51 | 1.84 ± 0.53 | |
In the present study, we found that XBJ significantly reduced myocardial injury in patients undergoing CPB, as demonstrated by the significantly lower levels of markers of myocardial injury, including TnI and CK-MB, as well as the preserved cardiac ejection fraction in patients in the XBJ group as compared with those in the control group. The benefit of XBJ against myocardial injury was accompanied by reduced serum concentrations of inflammatory markers, including TNF-α, IL-1β and IL-8, and an increase in the concentration of the anti-inflammatory factor IL-10. These results suggest that perioperative administration of XBJ is associated with attenuated cardiac injury during the CPB process, likely via anti-inflammatory and antioxidative mechanisms.
During the process of CPB and cardiac surgery, systemic inflammation and ischemia/reperfusion induced by these procedures contribute to cardiac injury[12,13]. Previous studies showed that myocardial injury is an independent risk factor for poor prognosis among patients who undergo cardiac surgery[5,14]. Although some agents have been proposed to exert a cardiac protective role in CPB, novel preventive agents targeting multiple potential mechanisms of CPB-related myocardial injury remain to be developed[15,16]. XBJ had been shown to exert a therapeutic effect against systemic inflammation in the treatment of pneumonia and sepsis[6,7]. Our previous clinical study demonstrated that XBJ attenuates local and systemic inflammation and improves the postoperative respiratory function in patients undergoing CPB[11]. These results confirmed the anti-inflammatory activity of XBJ in patients undergoing CPB. Because inflammation plays a key role in the pathogenesis of cardiac injury during CPB, we hypothesized that perioperative application of XBJ could at least attenuate cardiac injury after CPB. This hypothesis was confirmed by the present findings of significantly lower levels of markers of myocardial injury, including TnI and CK-MB, and preserved cardiac ejection fraction in patients in the XBJ group compared with those in the control group.
In addition to CBP-induced systematic inflammation, the cardiac surgery itself may also directly cause myocardial injury, which also can be represented by elevated serum levels of TnI and CK-MB[17-19]. In the present study, we found that XBJ significantly decreased the postoperative serum TnI and CK-MB levels. Moreover, XBJ also preserved the cardiac output postoperatively. These results indicate that XBJ ameliorated cardiac injury and improved cardiac function after CPB. Previous studies showed that CPB-induced inflammation, in which neutrophils, macrophages and monocytes secrete proinflammatory factors such as TNF-α, IL-1, IL-6, and IL-8, influences postoperative morbidity and mortality[1]. TNF-α and IL-1 are early mediators of inflammation as they initiate the local inflammatory response[20], while other inflammatory factors such as IL-6 and IL-8 are believed to contribute to systemic inflammation after CPB. A strong association between inflammatory cytokine levels and adverse clinical outcomes after CPB has been observed[21]. Increased levels of IL-6 and IL-8 have been proven to be positively correlated with TnI level, myocardial injury and mortality after CPB[21,22]. In the present study, we found that XBJ significantly reduced the levels of proinflammatory factors after CPB, which is consistent with our previous studies[7,9,11]. We also found that XBJ increased the level of the anti-inflammatory factor IL-10. A previous study showed that XBJ may regulate the inflammatory response via its inhibition of nuclear factor (NF)-κB[23]. However, the exact mechanisms underlying the potential regulatory effects of XBJ on the inflammatory response remain to be determined.
In addition to inflammation, the production of reactive oxygen species (ROS) induced by IRI has been considered an important contributor to cardiac injury during CPB[4,24]. Previous studies suggested that the cardiac injury resulted from ROS initiation of local cardiac inflammation, which then led to further oxidative-stress-mediated heart injury[25,26]. It has been confirmed that the circulating level of 8-isoprostane, the final product of lipid peroxidation, correlates directly with the tissue damage caused by ROS. The circulating level of 8-isoprostane has been shown to be a reliable indicator of cardiac oxidative stress in myocardial infarction[27] as well as coronary reperfusion[26], and this relationship is also associated with the elevation of TnI[28]. In the present study, we found the XBJ significantly decreased the serum levels of 8-isoprostane and MDA, and these results were consistent with previous studies[29,30], which demonstrated the potential antioxidative efficacy of XBJ. The mechanisms underlying the antioxidative efficacy of XBJ were proposed to be related to its regulation of the inducible nitric oxide synthase, superoxide dismutase[29] and oxidized glutathione activity[29].
Our study had some limitations. We have not observed long-term results, and we have not discussed in depth the mechanism of action of the drug.
The results of our study indicate that perioperative administration of XBJ is associated with attenuated cardiac injury during CPB, and this effect is likely the result of the anti-inflammatory and antioxidative activities of XBJ.
Cardiopulmonary bypass (CPB) provides good perioperative protection for patients undergoing cardiac surgery. However, perioperative cardiac injury related to CPB remains a severe complication in these patients and affects their prognosis.
Inflammation and oxidative stress play an important role in the CPB-related heart injury, and clinical observations have confirmed the protective effect of Xuebijing injection (XBJ) on multiple organs against inflammatory damage.
To evaluate the potential efficacy of XBJ on cardiac injury during CPB.
Sixty patients who underwent cardiac surgery with CPB in our center between November 1, 2018 and February 1, 2019 were included. XBJ was injected intravenously 3 times: 12 h before the operation, at the beginning of the operation, and 12 h after the second injection. The heart function was assessed by echocardiography 48 h after the operation. Markers related to circulatory inflammation and oxidative stress were measured and clinical results at intensive care unit (ICU) were recorded.
All the 60 patients were successfully weaned from CPB and transferred to the ICU. Compared to the control group, XBJ improved PaO2/FiO2 and cardiac systolic function, but reduced troponin I and creatine kinase fraction after surgery (all P < 0.05). Moreover, the circulating concentrations of TNF-α, IL-1β and IL-8 in the XBJ group were significantly lower than those in the control group (all P < 0.05), whereas the circulating concentration of IL-10 was significantly higher in the XBJ group (P < 0.05). In addition, the lengths of ICU stay and hospitalization after surgery tended to be shorter in the XBJ group than in the control group, although the differences were not statistically significant.
Perioperative administration of XBJ was associated with attenuated cardiac injury during the CPB surgery, likely via anti-inflammation– and anti-oxidation–related mechanisms.
Further research is required with more meaningful data to accurately evaluate the efficacy of XBJ on cardiac injury during CPB, and clarify the mechanism of action of XBJ.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Glumac S, Croatia; Jabbarpour Z, Iran S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 619] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 2. | Biccard BM. Detection and management of perioperative myocardial ischemia. Curr Opin Anaesthesiol. 2014;27:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Ngaage DL, Cowen ME, Cale AR. Cardiopulmonary bypass and left ventricular systolic dysfunction impacts operative mortality differently in elderly and young patients. Eur J Cardiothorac Surg. 2009;35:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Mentzer RM Jr. Myocardial protection in heart surgery. J Cardiovasc Pharmacol Ther. 2011;16:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Paparella D, Guida P, Caparrotti S, Fanelli V, Martinelli G, Mazzei V, Zaccaria S, Bisceglia L, Scrascia G. Myocardial damage influences short- and mid-term survival after valve surgery: a prospective multicenter study. J Thorac Cardiovasc Surg. 2014;148:2373-2379.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Yin Q, Li C. Treatment effects of xuebijing injection in severe septic patients with disseminated intravascular coagulation. Evid Based Complement Alternat Med. 2014;2014:949254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Qi F, Liang ZX, She DY, Yan GT, Chen LA. A clinical study on the effects and mechanism of xuebijing injection in severe pneumonia patients. J Tradit Chin Med. 2011;31:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | He XD, Wang Y, Wu Q, Wang HX, Chen ZD, Zheng RS, Wang ZS, Wang JB, Yang Y. Xuebijing Protects Rats from Sepsis Challenged with Acinetobacter baumannii by Promoting Annexin A1 Expression and Inhibiting Proinflammatory Cytokines Secretion. Evid Based Complement Alternat Med. 2013;2013:804940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Sun J, Xue Q, Guo L, Cui L, Wang J. Xuebijing protects against lipopolysaccharide-induced lung injury in rabbits. Exp Lung Res. 2010;36:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Li D, Lu L, Zhang J, Wang X, Xing Y, Wu H, Yang X, Shi Z, Zhao M, Fan S, Meng A. Mitigating the effects of Xuebijing injection on hematopoietic cell injury induced by total body irradiation with γ rays by decreasing reactive oxygen species levels. Int J Mol Sci. 2014;15:10541-10553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Gao W, Li N, Cui XG. Efficacy of Xuebijing Injection () on Cardiopulmonary Bypass-Associated Pulmonary Injury: A Prospective, Single-center, Randomized, Double Blinded Trial. Chin J Integr Med. 2018;24:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Salerno TA. Myocardial protection era: from valve surgery to heart transplantation. Artif Organs. 2012;36:939-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009;102:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F, Sisillo E. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 15. | De Hert S, Moerman A. Myocardial injury and protection related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol. 2015;29:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Chambers DJ, Fallouh HB. Cardioplegia and cardiac surgery: pharmacological arrest and cardioprotection during global ischemia and reperfusion. Pharmacol Ther. 2010;127:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Maynard SJ, Menown IB, Adgey AA. Troponin T or troponin I as cardiac markers in ischaemic heart disease. Heart. 2000;83:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Kazmi KA, Iqbal SP, Bakr A, Iqbal MP. Admission creatine kinase as a prognostic marker in acute myocardial infarction. J Pak Med Assoc. 2009;59:819-822. [PubMed] |
| 19. | Robinson DJ, Christenson RH. Creatine kinase and its CK-MB isoenzyme: the conventional marker for the diagnosis of acute myocardial infarction. J Emerg Med. 1999;17:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Poon KS, Palanisamy K, Chang SS, Sun KT, Chen KB, Li PC, Lin TC, Li CY. Plasma exosomal miR-223 expression regulates inflammatory responses during cardiac surgery with cardiopulmonary bypass. Sci Rep. 2017;7:10807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Allan CK, Newburger JW, McGrath E, Elder J, Psoinos C, Laussen PC, del Nido PJ, Wypij D, McGowan FX Jr. The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg. 2010;111:1244-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Holmes JH 4th, Connolly NC, Paull DL, Hill ME, Guyton SW, Ziegler SF, Hall RA. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res. 2002;51:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Jiang M, Zhou M, Han Y, Xing L, Zhao H, Dong L, Bai G, Luo G. Identification of NF-κB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J Ethnopharmacol. 2013;147:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Zakkar M, Guida G, Suleiman MS, Angelini GD. Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longev. 2015;2015:189863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 25. | Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GG, Possati G, Gaudino M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: a review of evidence. Eur J Cardiothorac Surg. 2004;25:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Mehlhorn U, Krahwinkel A, Geissler HJ, LaRosee K, Fischer UM, Klass O, Suedkamp M, Hekmat K, Tossios P, Bloch W. Nitrotyrosine and 8-isoprostane formation indicate free radical-mediated injury in hearts of patients subjected to cardioplegia. J Thorac Cardiovasc Surg. 2003;125:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Mehrabi MR, Serbecic N, Ekmekcioglu C, Tamaddon F, Ullrich R, Sinzinger H, Glogar HD. The isoprostane 8-epi-PGF(2alpha) is a valuable indicator of oxidative injury in human heart valves. Cardiovasc Pathol. 2001;10:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Caputo M, Mokhtari A, Rogers CA, Panayiotou N, Chen Q, Ghorbel MT, Angelini GD, Parry AJ. The effects of normoxic versus hyperoxic cardiopulmonary bypass on oxidative stress and inflammatory response in cyanotic pediatric patients undergoing open cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2009;138:206-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Chen Y, Tong H, Pan Z, Jiang D, Zhang X, Qiu J, Su L, Zhang M. Xuebijing injection attenuates pulmonary injury by reducing oxidative stress and proinflammatory damage in rats with heat stroke. Exp Ther Med. 2017;13:3408-3416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Zuo L, Zhou L, Xu T, Li Z, Liu L, Shi Y, Kang J, Gao G, Du S, Sun Z, Zhang X. Antiseptic Activity of Ethnomedicinal Xuebijing Revealed by the Metabolomics Analysis Using UHPLC-Q-Orbitrap HRMS. Front Pharmacol. 2018;9:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |