Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3615
Peer-review started: December 12, 2021
First decision: January 26, 2022
Revised: February 8, 2022
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: April 16, 2022
Processing time: 117 Days and 3 Hours
Synchronous combined hepatocellular-cholangiocarcinoma (CHC) and hepatocellular carcinoma (HCC) is very rare, with few literature reports and poor clinical outcomes associated with the disorder. Surgical resection is the main treatment, which makes the preoperative diagnosis very important. However, due to imaging manifestations overlapping with HCC, diagnosis of this type of synchronous cancer is challenging and it tends to be misdiagnosed as multiple HCC. Herein, we report the contrast-enhanced ultrasound (CEUS) manifestations of a case of synchronous CHC and HCC, aiming at adding to the understanding of this disease. CEUS displayed exquisite vascularity and tissue perfusion in real time with good spatial and temporal resolution and more accurately reflect tumor washin and washout times than contrast-enhanced computed tomography (CT) in this case.
The patient was a 69-year-old female with a 20-year history of chronic hepatitis B. Due to months of epigastric pain and anorexia, she reffered to our hospital for treatment. Five days before hospitalization, abdominal magnetic resonance imaging performed at another hospital detected a space-occupying lesion in the liver. After her hospitalization, laboratory tests showed elevated alpha-fetoprotein and carbohydrate antigen 19-9 level. Two suspicious liver lesions located in S4 and S6, respectively, were identified in a cirrhotic background by abdominal contrast-enhanced CT (CECT). Furthermore, the lesion in S4 and S6 were detected by CEUS and assigned to CEUS LI-RADS 5 and M categories, respectively. The patient underwent tumor radical resections. Post-operative pathology confirmed the S4 and S6 lesions to be HCC and CHC, respectively. A newly-found suspicious liver nodule with potential malignancy was detected in liver S1 by both CEUS and CECT 7 mo after operation.
The CEUS characteristics of CHC and HCC are different. CEUS features in combination with clinical information could help in effective diagnosis, clinical decision-making and better prognosis.
Core Tip: Synchronous hepatocellular-cholangiocarcinoma (CHC) and hepatocellular carcinoma (HCC) is rare and tend to be misdiagnosed as multiple HCC in clinical settings. Patients afflicted with this disorder generally have poor prognosis, moreover, preoperative imaging diagnosis is often challenging. This paper introduces contrast-enhanced ultrasound (CEUS) manifestations of a case of synchronous CHC and HCC, which showed different imaging features on CEUS images. Overall, the combination of CEUS characteristics with clinical information could help in effective diagnosis of synchronous CHC and HCC, as well as clinical decision-making and patients’ prognosis.
- Citation: Gao L, Huang JY, Lu ZJ, Lu Q. Contrast-enhanced ultrasound manifestations of synchronous combined hepatocellular-cholangiocarcinoma and hepatocellular carcinoma: A case report. World J Clin Cases 2022; 10(11): 3615-3623
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3615.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3615
Liver cancer is predicted to be the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide in 2018[1]. Approximately half of primary liver cancers exhibit multifocal origins[2]. According to different origins, multifocal liver cancer can be divided into primary liver cancer with intrahepatic metastasis and liver cancer with a multicentric origin[3,4]. Synchronous combined hepatocellular-cholangiocarcinoma (CHC) and hepatocellular carcinoma (HCC) is rare in multicentric liver cancer, and it is often misdiagnosed as multiple HCC and has a poor prognosis[5,6]. Moreover, the treatment strategies and outcomes of the two diseases are different. When CHC and HCC occur synchronously, due to the unique fiber components of intrahepatic cholangiocarcinoma (ICC) contained in CHC, treatments typically used for multiple HCC, e.g. transcatheterial arterial chemoembolization (TACE) and chemotherapy, provide limited benefits[6]. Therefore, an accurate preoperative diagnosis is very important.
A 69-year-old female referred to our hospital due to months of epigastric pain and anorexia.
Epigastric pain and anorexia were presented. No nausea, vomiting, acid regurgitation, belching, chills, fever, hematemesis, melena or jaundice (among other symptoms) were observed.
The patient had a 20-years history of chronic hepatitis B and did not receive standardized treatments.
The patient had a > 20-year history of alcoholism (approximately 50 mL liquor per day) and did not have a smoking history, nor did she have a travel history to pastoral areas or epidemic areas.
The patient's height and weight were 155 cm and 59 kg, respectively, with a body mass index of 24.6 kg/m². No swollen lymph nodes were found. The abdomen was soft without rebound tenderness, and the liver and spleen were not palpable under the ribs. There was no percussion pain in the liver area. Mobility dullness was negative, and bowel sounds were normal.
Platelet count: 93 × 109/L; white blood cell count: 2.65 × 109/L; red blood cell count: 3.99 × 1012/L; hemoglobin: 124 g/L; albumin: 42.2 g/L (normal range: 40.0-55.0 g/L); globulin: 23.1 g/L (normal range: 20.0-40.0 g/L); total bilirubin: 10.3 μmol/L (normal range: 5.0-28.0 μmol/L); direct bilirubin: 3.4 μmol/L (normal range: < 8.8 μmol/L); alanine aminotransferase (ALT): 50 IU/L (normal range: < 40.0 IU/L); aspartate aminotransferase (AST): 61 IU/L (normal range: < 35.0 IU/L); alkaline phosphatase (ALP): 126 IU/L (normal range: 50.0-135.0 IU/L); hepatitis B surface antigen (+); hepatitis B e antibody (+); hepatitis B core antibody (+); hepatitis C antibody (-); HBV DNA level: 6.32 × 105 IU/Ml (normal range: < 1.0 × 102 IU/mL); Serum bio-marker analysis showed elevated alpha-fetoprotein (AFP): 219.00 ng/mL (normal range: < 7 ng/mL), serum carbohydrate antigen 19-9 (CA19-9): 38.40 U/mL (normal range: < 30 U/mL) and protein induced by vitamin K absence or antagonist-II (PIVKA-II): 54.00 mAU/mL (normal range: 6.0-32.5 mAU/mL); carcinoembryonic antigen (CEA) and serum carbohydrate antigen 125 (CA-125) were both normal.
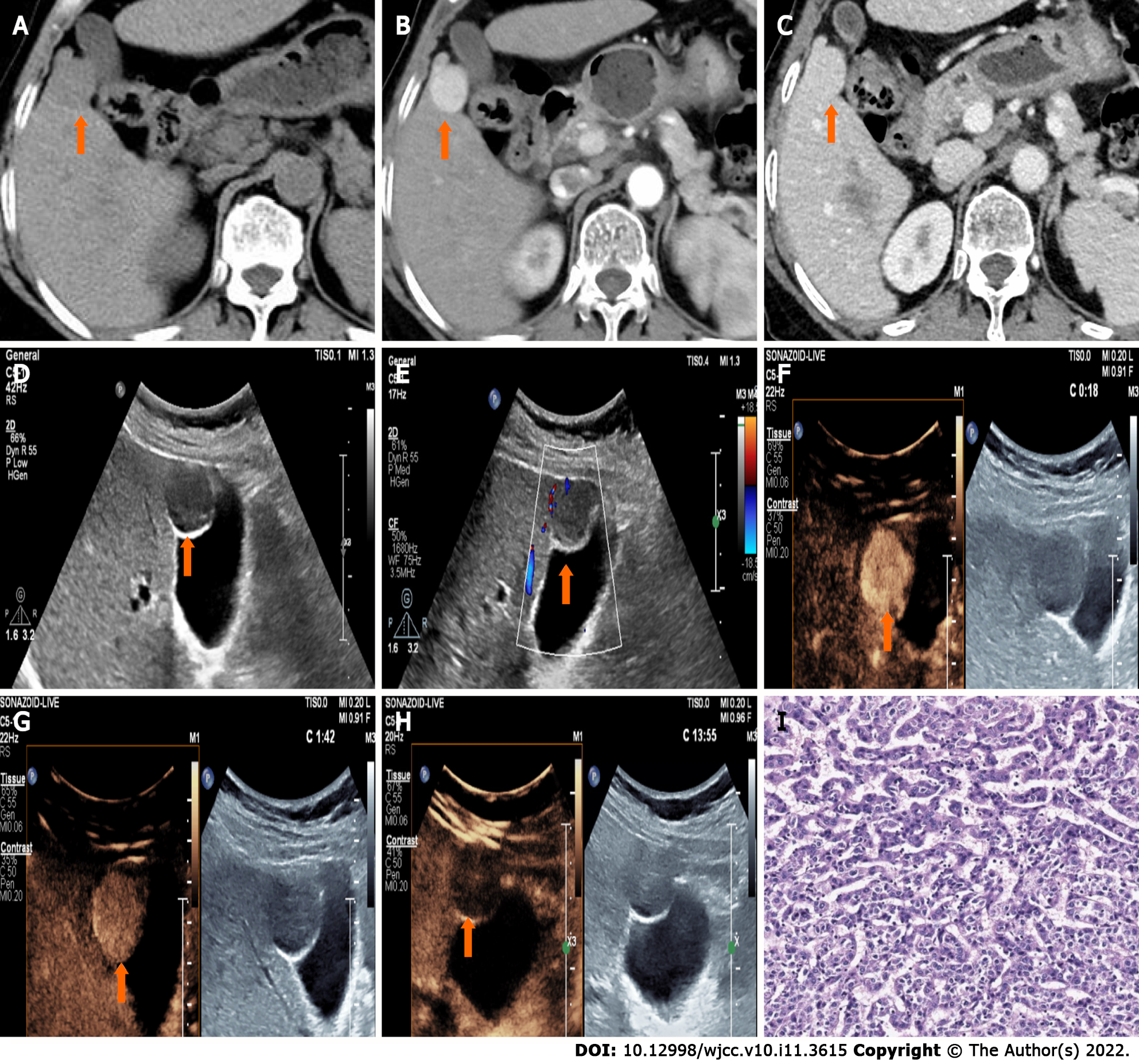
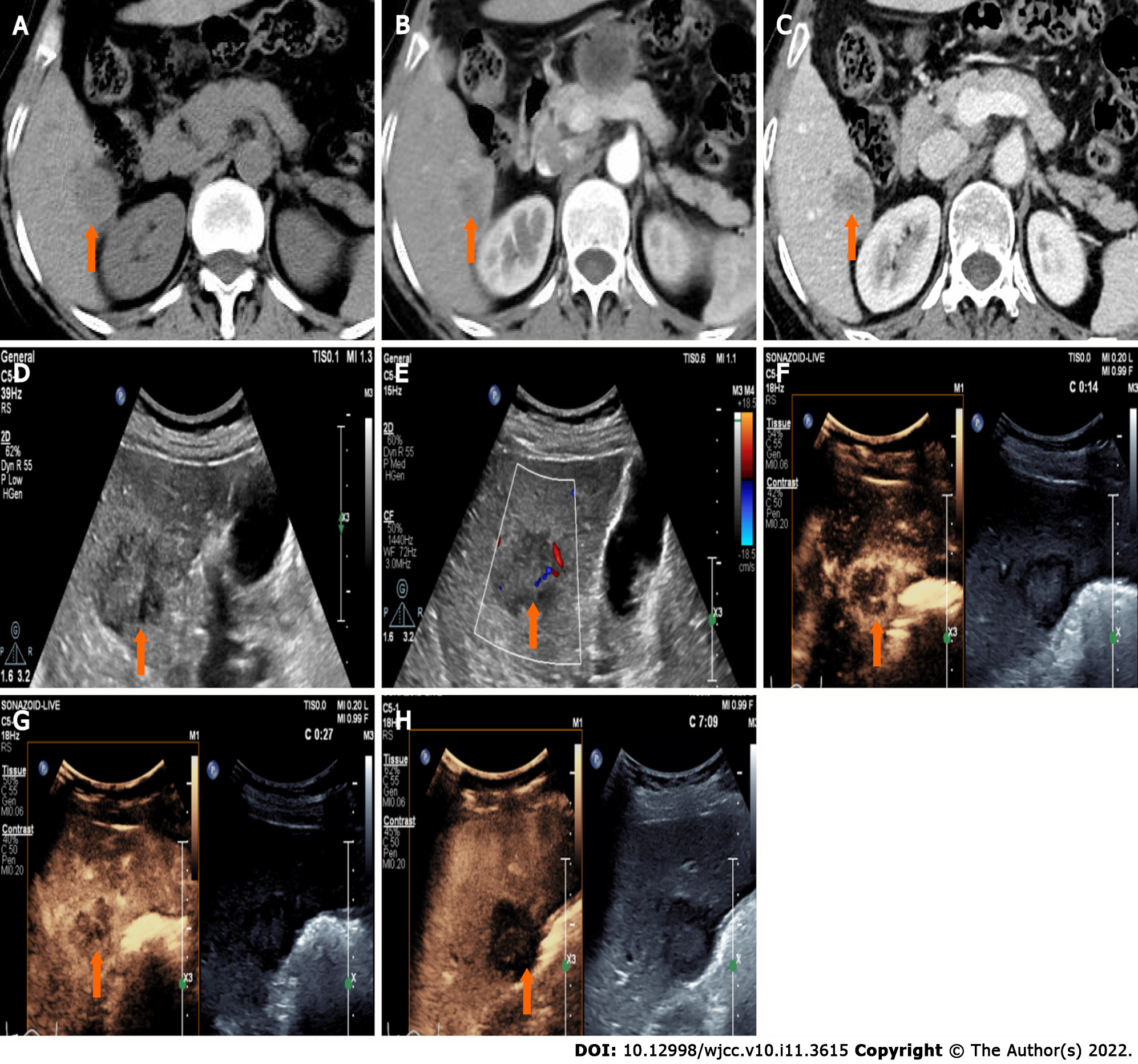
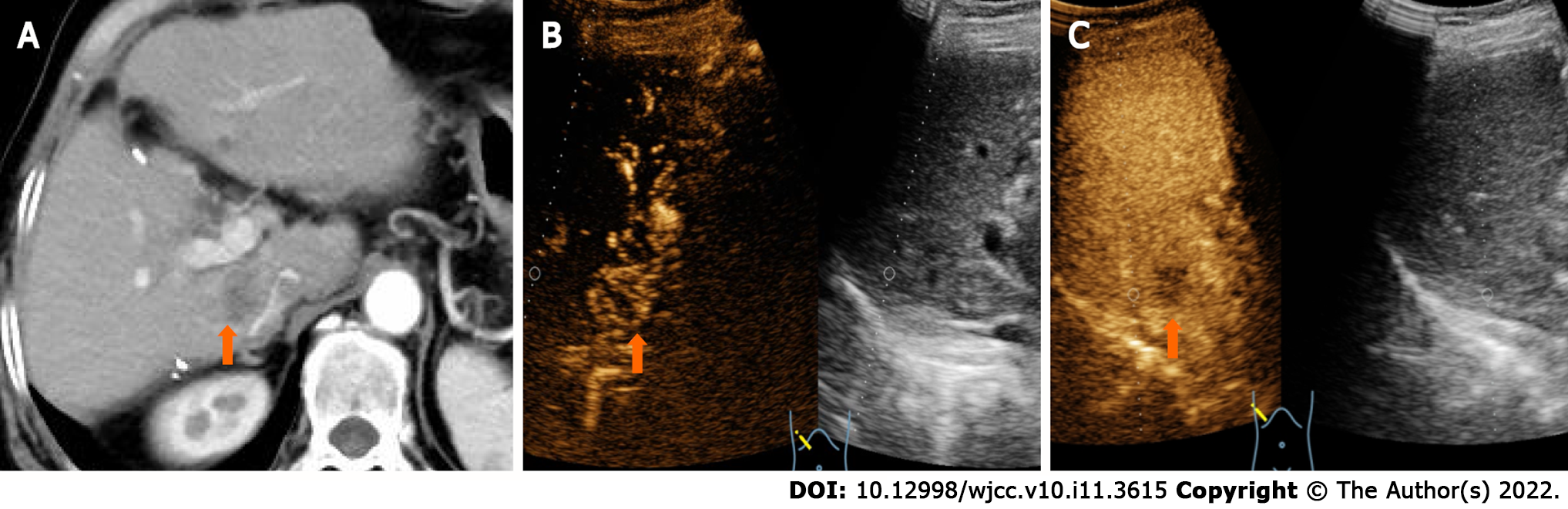
CT showed a slightly enlarged spleen. S4 showed a hypoattenuating mass (Figure 1A) (approximately 2.1 cm × 2.0 cm), which presented with marked enhancement in the arterial phase (Figure 1B) and isoenhancement in the portal venous phase (Figure 1C). S6 revealed a hypoattenuating mass (Figure 2A) (an area of approximately 3.0 cm × 2.7 cm), which presented with an annular weak enhancement in the arterial phase (Figure 2B) and mild hypoenhancement in the portal venous phase (Figure 2C). The diagnosis was considered to be cirrhosis with a malignant tumor of the liver. Ultrasound indicated that the liver parenchyma was thickened and uneven. A quasicircular hypoechoic nodule with a size of approximately 2.1 cm × 2.0 cm was found in S4 alongside the gallbladder (Figure 1D); it had a clear boundary and a regular shape and pushed and squeezed the gallbladder. The gallbladder wall was continuous and complete, and a mild blood flow signal was observed inside of the nodule (Figure 1E). S6 indicated a hypoechoic nodule with a size of approximately 3.9 cm × 3.5 cm (Figure 2D), with an irregular shape and an unclear boundary. The nodule protruded outward and pushed and squeezed the right kidney. The capsule of the right kidney was intact. Additionally, short-line blood flow signals could be observed inside of the nodule (Figure 2E). No enlarged lymph nodes were found in the abdominal cavity. On contrast-enhanced ultrasound (CEUS) (Sonazoid 0.6 mL bolus injection, Philips EPIQ7, and C5-1 convex array probe), the S4 nodule showed rapid hyperenhancement in the arterial phase (Figure 1F), mild hyperenhancement in the portal venous phase (Figure 1G) and mild hypoenhancement in the post vascular phase (Figure 1H). The S6 nodule indicated rim hyperenhancement in the arterial phase (Figure 2F); in addition, washing out began in the late arterial phase (27 s) (Figure 2G), the portal phase showed hypoenhancement, and the post vascular phase showed marked hypoenhancement (Figure 2H). The ultrasound suggested liver cirrhosis; additionally, the S4 hypoechoic nodule was considered to be HCC, and the S6 hypoechoic nodule was considered to be a malignant liver tumor (the ultrasound manifestations of the two intrahepatic nodules are shown in Table 1).
| Nodules | Location | Size (cm) | Boundary | Arterial phase | Portal phase | Post-vascular phase |
| HCC | S4 | 2.1 × 2.0 | Clear | Hyperenhancement | Hyperenhancement | Mild hypoenhancement |
| CHC | S6 | 3.0 × 2.7 | Unclear | Rim enhancement | Marked hypoenhancement | Marked hypoenhancement |
Synchronous CHC and HCC.
The patient underwent a complex liver cancer resection, cholecystectomy and partial resection of the right adrenal gland.
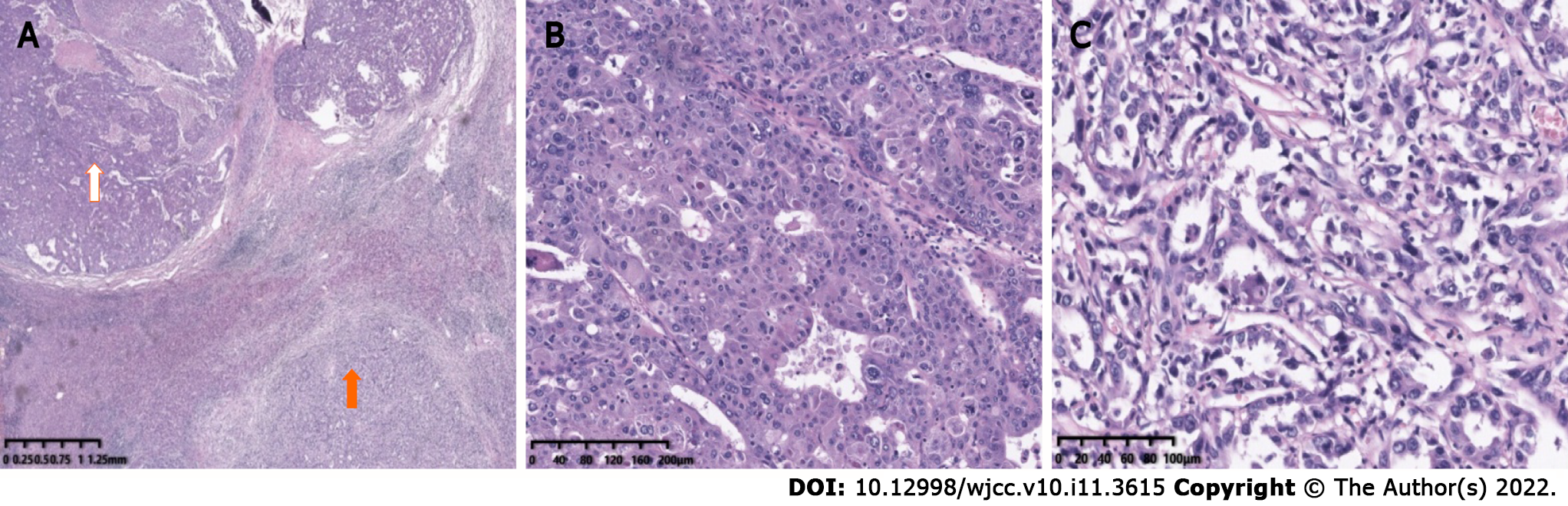
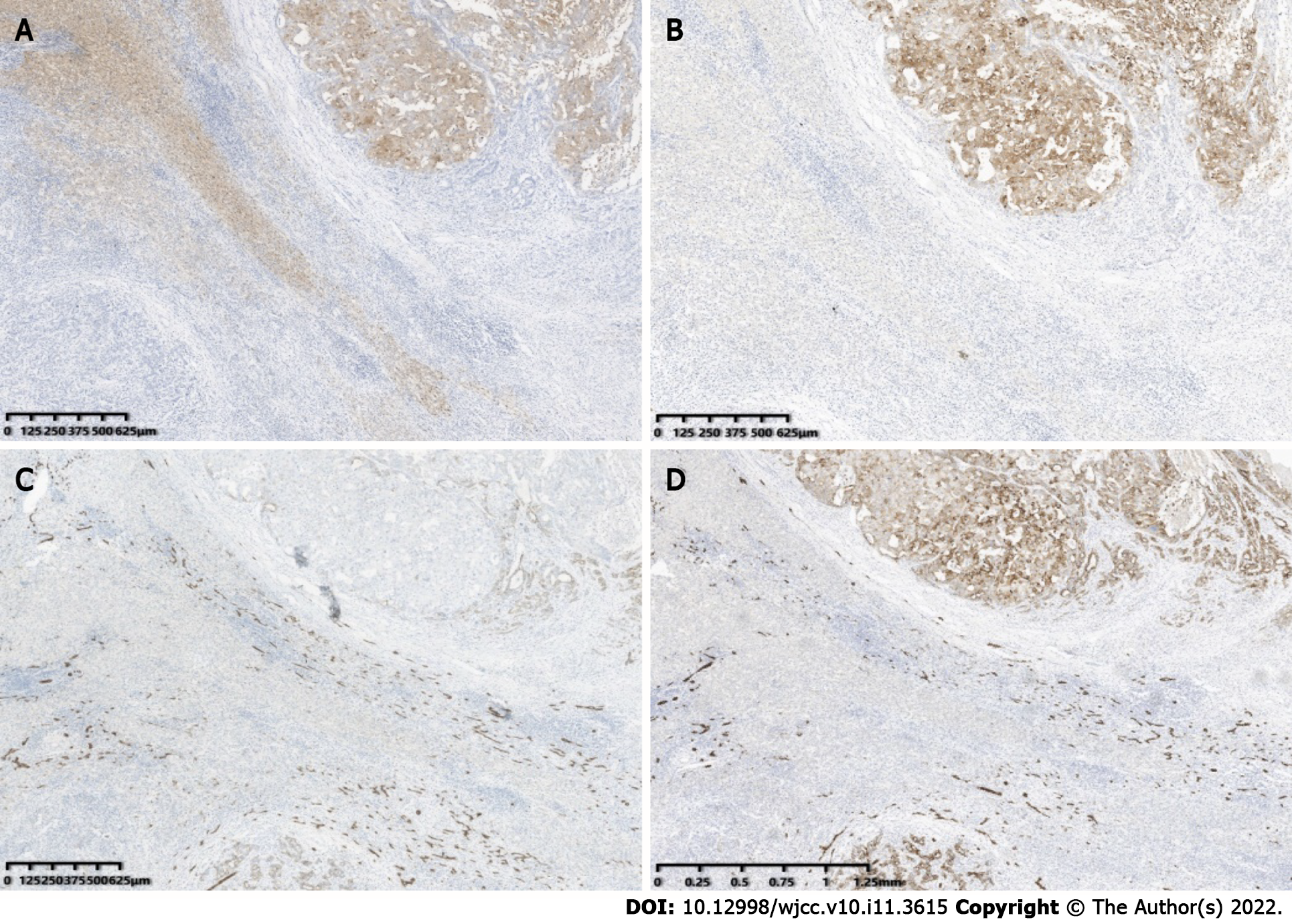
Postoperative pathology confirmed the S4 nodule a HCC (Figure 1I) and the S6 nodule a CHC (Figure 3). As to the S6 lesion, positive expression of Arginase 1 (Arg 1) (Figure 4A) and Glypican-3 (GPC-3) (Figure 4B) in the hepatocellular carcinoma component, and Cytokeratin 7 (CK7) (Figure 4C) and Cytokeratin 19 (CK19) (Figure 4D) in the cholangiolocarcinoma components were confirmed by immunohistochemical analysis. No metastasis was found in the gallbladder or in the right adrenal gland.
The patient underwent follow-up examinations at 7 mo after the operation. A newly found liver lesion located in S1 showing internal hypoenhancement and mild peripheral hyperenhancement was detected by contrast-enhanced computed tomography (CECT) (Figure 5A). The lesion manifested peripherial hyperenhancement (Figure 5B) in the arterial phase followed by early washout in the portal venous phase (Figure 5C) on CEUS. Both CECT and CEUS considered this lesion a malignancy.
Synchronous CHC and HCC is an uncommon condition that very few literatures had reported previously. Though rarely occurs, this disorder typically has a poor prognosis. Because of overlapping imaging features with HCC and atypical clinical manifestation, effective diagnosis of this disease can be challenging[6]. Patients afflicted with synchronous CHC and HCC has little or no response to therapeutic drugs due to unique fiber components of ICC in CHC. Therefore, a series of local treatments, including TACE and chemotherapy, cannot significantly benefit these patients[6]. Previous studies showed that the survival rate of patients with CHC, who underwent liver transplantation showed inferior survival in comparison to those with HCC alone, and the role and indications of liver transplantation in combined tumor have yet to be defined[7-9]. Therefore, surgery is currently the best treatment choice, but the prognosis is poor due to a high incidence of vascular invasion and lymph node metastasis; additionally, the average relapse time after radical resection is only 5.4 mo[10,11]. Thus, an accurate preoperative diagnosis is of great significance for clinical decision-making and good prognosis.
Although the CEUS features of CHC partially overlap with those of HCC and ICC, CHC still possesses its own clinical characteristics. HCC usually occurs in patients with chronic hepatitis B or cirrhosis. On CEUS images, rapid arterial phase hyperenhancement (APHE) resulted from the formation of neoangiogenesis, and washout during postarterial phases due to reduced or absent of normal structure of portal triads, are identified as characterics of HCC[12]. According to the criteria of CEUS LI-RADS V2017 and related research[13,14], typical HCC is characterized by APHE (in whole or in part, not rim or peripheral discontinuous globular enhancement) with mild and late washout (> 60 s). Rim-like hyperenhancement, early (< 60 s) washout and marked washout within 120 s are specific enhancement patterns of ICC on CEUS. Arterial rim hyperenhancement pattern of ICC is associated with a high degree of malignant cell proliferation in the periphery while necrosis or fibrosis in the center of the tumor on pathology[15,16]. The ultrasound manifestations of CHC are related to the proportion of HCC and ICC components in the mass. The enhancement pattern of HCC-dominant lesions is similar to that of HCC, whereas those of ICC-dominant lesions is similar to that of ICC. The amount of the HCC component may be the main determinant of radiologic LI-RADS categories of hepatocellular-cholangiocarcinoma; tumors of LR-4 or LR-5 categories were associated with a larger proportion of the HCC component and smaller or none proportion of the cholangiocarcinomas component[17]. According to the CEUS LI-RADS criteria, most CHCs are diagnosed as LR-M, and studies have showed that the disease-free survival rate of these patients is low[18-20]. The enhancement pattern of CHC on CEUS is also associated with nodule size. When the nodule is smaller than or equal to 3 cm, the enhancement pattern is similar to that of HCC; when the nodule is larger than 3 cm, the enhancement mode is similar to that of ICC. With an increase in lesion diameter, the manifestations of CHC in enhanced images change from HCC-like to ICC-like[21]. On CECT, CHC commonly shows central delayed enhancement in the delayed phase, whereas it shows marked washout on CEUS images. This heterogeneity rarely appears in HCC, which is helpful in distinguishing between the two entities. In our case, the S4 nodule showed rapid and hyperenhancement in the arterial phase followed by mild and late washout in the delayed phase, a typical manifestation of CEUS LR-5 category. The S6 nodule displayed peripheral rim-like hyperenhancement in the arterial phase, followed by early washout, which should be classified as a LR-M nodule. Both of the nodules were differ in enhancement pattern, and onset and degree of washout. The discrepancy between the simultaneously elevated level of AFP and CA19-9 and the CEUS patterns (i.e., the CEUS mode of ICC presents upon the increase in AFP and the CEUS mode of HCC presents upon the increase in CA19-9) have been reported to be the diagnostic criteria that can improve the accurate diagnostic rate of CHC[22]. Although some of the previously described features can help us distinguish CHC from HCC, accurate diagnosis of some cases remained tough before operations. Alternatively, ultrasound-guided puncture biopsy is necessary in such circumstances.
In summary, the CEUS manifestations of HCC and CHC are different. CEUS combined with clinical information (history of chronic hepatitis B and the synchronously elevated level of AFP and CA19-9) may indicate synchronous CHC and HCC, which help in effective diagnosis, clinical decision-making and better prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Caiati C, Sintusek P S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55840] [Article Influence: 7977.1] [Reference Citation Analysis (132)] |
| 2. | Xie DY, Fan HK, Ren ZG, Fan J, Gao Q. Identifying Clonal Origin of Multifocal Hepatocellular Carcinoma and Its Clinical Implications. Clin Transl Gastroenterol. 2019;10:e00006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Matsumoto Y, Fujii H, Matsuda M, Kono H. Multicentric occurrence of hepatocellular carcinoma: diagnosis and clinical significance. J Hepatobiliary Pancreat Surg. 2001;8:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Feo F, Pascale RM. Multifocal hepatocellular carcinoma: intrahepatic metastasis or multicentric carcinogenesis? Ann Transl Med. 2015;3:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 5. | Ide R, Oshita A, Nishisaka T, Nakahara H, Aimitsu S, Itamoto T. Primary biliary cholangitis metachronously complicated with combined hepatocellular carcinoma-cholangiocellular carcinoma and hepatocellular carcinoma. World J Hepatol. 2017;9:1378-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Connell LC, Harding JJ, Shia J, Abou-Alfa GK. Combined intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Chin Clin Oncol. 2016;5:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, Giardini V. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Song S, Moon HH, Lee S, Kim TS, Shin M, Kim JM, Park JB, Kwon CH, Kim SJ, Lee SK, Joh JW. Comparison between resection and transplantation in combined hepatocellular and cholangiocarcinoma. Transplant Proc. 2013;45:3041-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Magistri P, Tarantino G, Serra V, Guidetti C, Ballarin R, Di Benedetto F. Liver transplantation and combined hepatocellular-cholangiocarcinoma: Feasibility and outcomes. Dig Liver Dis. 2017;49:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Kim SH, Park YN, Lim JH, Choi GH, Choi JS, Kim KS. Characteristics of combined hepatocelluar-cholangiocarcinoma and comparison with intrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2014;40:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Stavraka C, Rush H, Ross P. Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. J Hepatocell Carcinoma. 2019;6:11-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Fowler KJ, Burgoyne A, Fraum TJ, Hosseini M, Ichikawa S, Kim S, Kitao A, Lee JM, Paradis V, Taouli B, Theise ND, Vilgrain V, Wang J, Sirlin CB, Chernyak V. Pathologic, Molecular, and Prognostic Radiologic Features of Hepatocellular Carcinoma. Radiographics. 2021;41:1611-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Kono Y, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, Willmann JK, Wilson SR, Santillan C, Kambadakone A, Mitchell D, Vezeridis A, Sirlin CB. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS®): the official version by the American College of Radiology (ACR). Ultraschall Med. 2017;38:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | Sugimoto K, Kakegawa T, Takahashi H, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Kasai Y, Itoi T. Usefulness of Modified CEUS LI-RADS for the Diagnosis of Hepatocellular Carcinoma Using Sonazoid. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Xu HX, Chen LD, Liu LN, Zhang YF, Guo LH, Liu C. Contrast-enhanced ultrasound of intrahepatic cholangiocarcinoma: correlation with pathological examination. Br J Radiol. 2012;85:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Li R, Yuan MX, Ma KS, Li XW, Tang CL, Zhang XH, Guo DY, Yan XC. Detailed analysis of temporal features on contrast enhanced ultrasound may help differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma in cirrhosis. PLoS One. 2014;9:e98612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Choi SH, Jeon SK, Lee SS, Lee JM, Hur BY, Kang HJ, Kim H, Park Y. Radio-pathologic correlation of biphenotypic primary liver cancer (combined hepatocellular cholangiocarcinoma): changes in the 2019 WHO classification and impact on LI-RADS classification at liver MRI. Eur Radiol. 2021;31:9479-9488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Yang J, Huang JY, Chen X, Ling WW, Luo Y, Shi YJ, Liu JB, Lu Q, Lyshchik A. Combined hepatocellular-cholangiocarcinoma: can we use contrast-enhanced ultrasound Liver Imaging Reporting and Data System (LI-RADS) to predict the patient's survival? Eur Radiol. 2021;31:6397-6405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Jeon SK, Joo I, Lee DH, Lee SM, Kang HJ, Lee KB, Lee JM. Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol. 2019;29:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Zhang HC, Zhu T, Hu RF, Wu L. Contrast-enhanced ultrasound imaging features and clinical characteristics of combined hepatocellular cholangiocarcinoma: comparison with hepatocellular carcinoma and cholangiocarcinoma. Ultrasonography. 2020;39:356-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Zhou Y, Li D, Long L, Ding JM, Wang FM, Wang YD, Zhou HY, Jing X. Analysis of enhancement patterns and combined diagnosis of combined hepatocellular-cholangiocarcinoma: CEUS, CECT/MRI and tumor markers. Chin J Ultrasonogr. 2020;29:754-760. [DOI] [Full Text] |
| 22. | Sagrini E, Iavarone M, Stefanini F, Tovoli F, Vavassori S, Maggioni M, Renzulli M, Salvatore V, Stefanescu H, Colombo M, Bolondi L, Piscaglia F. Imaging of combined hepatocellular-cholangiocarcinoma in cirrhosis and risk of false diagnosis of hepatocellular carcinoma. United European Gastroenterol J. 2019;7:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |