Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3601
Peer-review started: December 6, 2021
First decision: January 25, 2022
Revised: February 3, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 123 Days and 8.5 Hours
Del(5q) is the most common molecular event in myelodysplastic syndrome (MDS), accounting for 10%-15% of cases. Inv(3) is an adverse cytogenetic abnormality observed in less than 1% of MDS patients. Few studies have reported the coexistence of del(5q) and inv(3) in MDS. Therefore, the pathological mechanism, treatment strategy and prognosis of this subtype need to be elucidated.
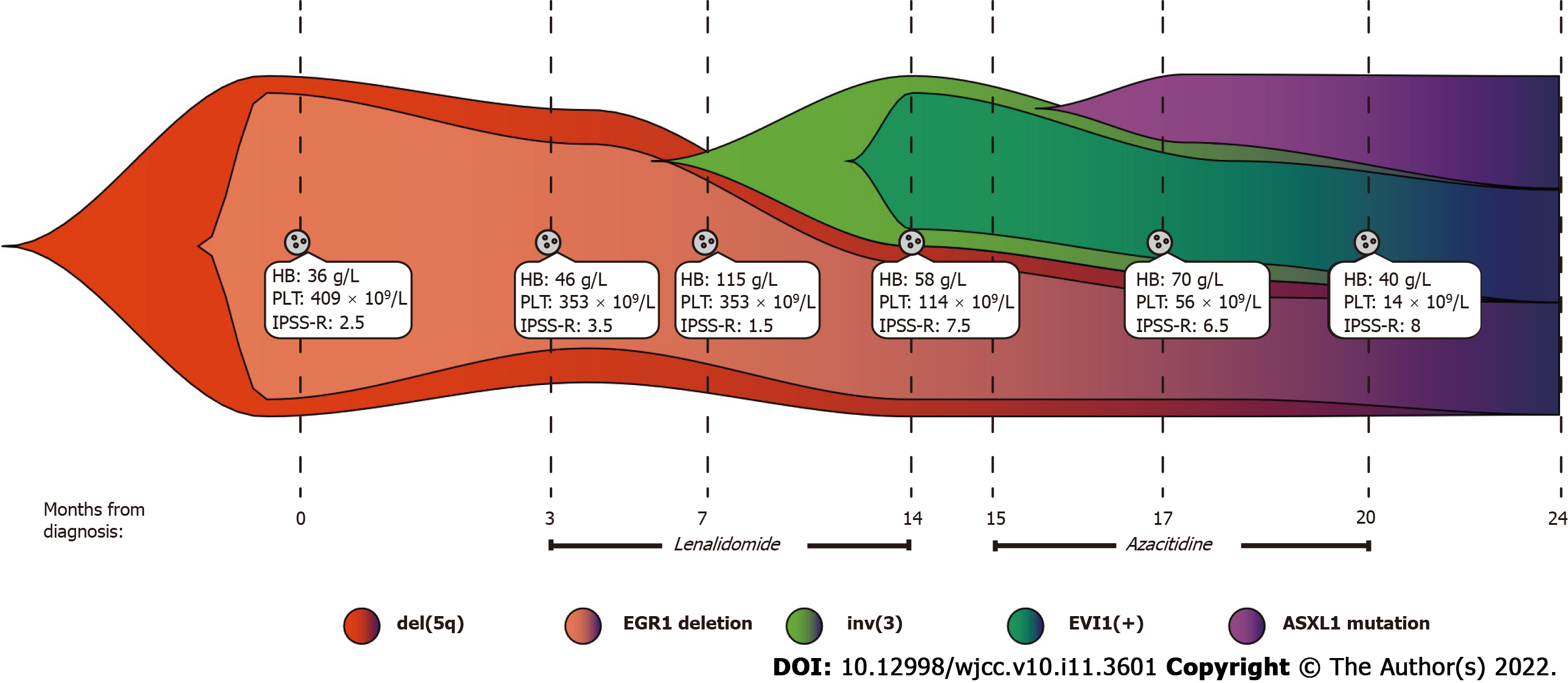
A 66-year-old woman was admitted to the hospital due to chest tightness and shortness of breath. Combining clinical assessments with laboratory examinations, the patient was diagnosed with MDS containing both del(5q) and inv(3). Considering the deletion of chromosome 5q, we first treated the patient with lenalidomide. When drug resistance arose, we tried azacitidine, and the patient had a short remission. Finally, the patient refused treatment with haematopoietic stem cell transplantation and died of severe infection four months later.
MDS patients with del(5) and inv(3) have a poor prognosis. Azacitidine may achieve short-term remission for such patients.
Core Tip: We report a rare case of myelodysplastic syndrome (MDS) with two chromosomal structural abnormalities, del(5q) and inv(3). The patient evolved from the initial del(5q) to inv(3) combined with del(5q). Considering the deletion of chromosome 5q, we first treated the patient with lenalidomide. When drug resistance arose, we tried azacitidine, and the patient had a short remission. Finally, the patient refused treatment with haematopoietic stem cell transplantation (HSCT), and her condition gradually deteriorated until she was discharged from the hospital. In this rare and contradictory situation, we found that MDS patients with coexisting del(5q) and inv(3) may have a poor prognosis. However, azacitidine may play a role to some extent in MDS with del(5q) and inv(3), and HSCT may be the only way to cure the disease.
- Citation: Liang HP, Luo XC, Zhang YL, Liu B. Del(5q) and inv(3) in myelodysplastic syndrome: A rare case report. World J Clin Cases 2022; 10(11): 3601-3608
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3601.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3601
Myelodysplastic syndrome (MDS) is defined as a typical heterogeneous group of clonal haematopoietic disorders characterized by dysplastic and ineffective haematopoiesis, and approximately 30% of patients progress to acute myeloid leukaemia (AML)[1,2]. The incidence of MDS is associated with age, especially in people 60 years older, and males are more susceptible than females[3]. MDS patients generally have poor outcomes, with a median overall survival of 5 years[2]. According to the International Prognostic Scoring System (IPSS) and revised International Prognostic Scoring System (IPSS-R), cytogenetic abnormalities, especially certain unbalanced abnormalities, have a profound impact on the prognosis of MDS patients[4]. Unbalanced chromosomal abnormalities caused by partial acquisition or deletion of chromosomes are common in MDS[5]. These abnormalities often occur during tumorigenesis and play a crucial role in MDS progression.
Here, we report a case of an MDS patient with clonal progression from del(5q) to inv(3) and del(5q), who was treated with azacitidine after lenalidomide resistance. Furthermore, we will summarize the genetic abnormalities and treatment strategies to add a corresponding contribution to the treatment and prognosis of these patients.
In September 2020, a 66-year-old woman was admitted to our hospital for progressive chest tightness and shortness of breath.
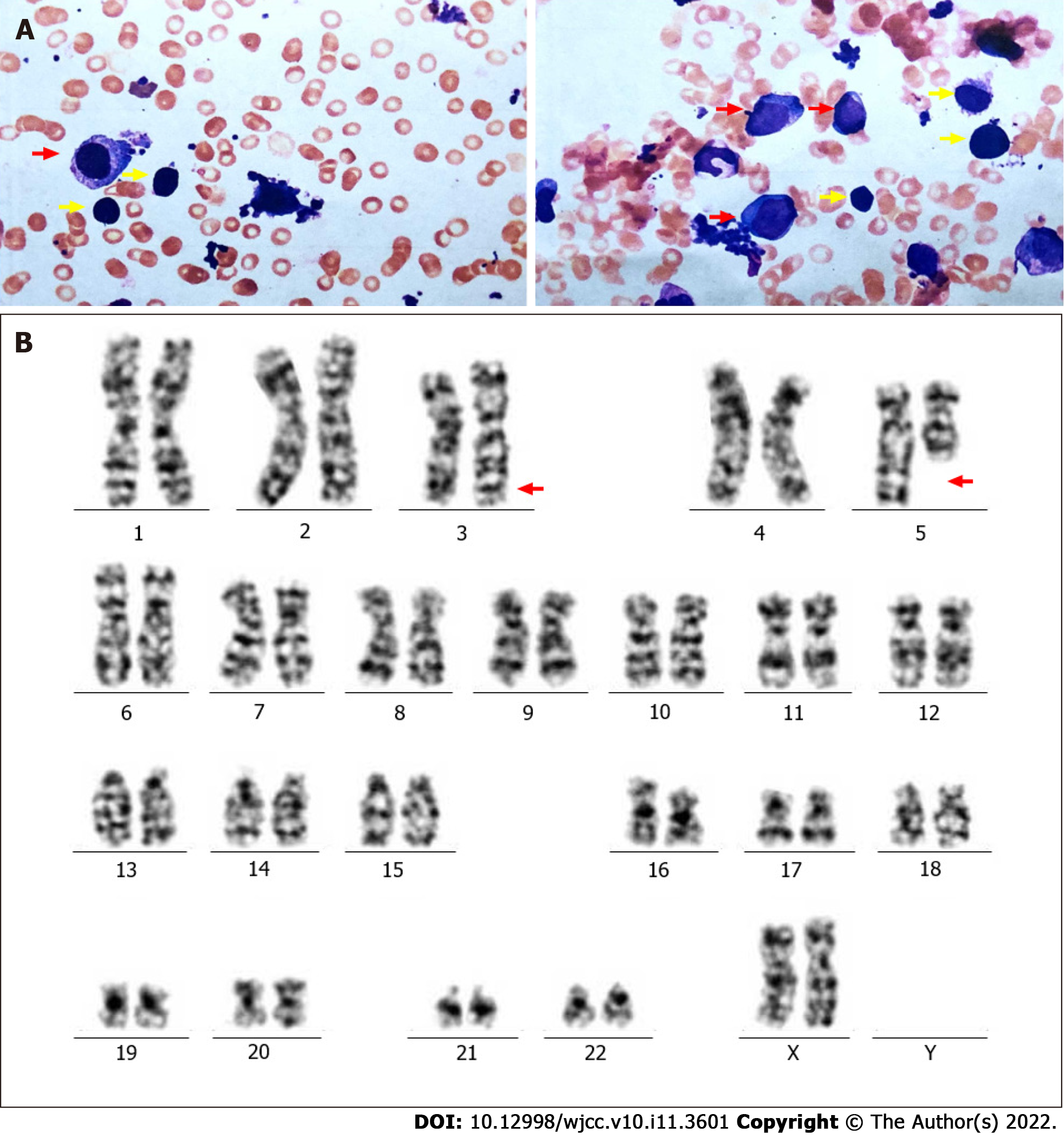
One year prior, the patient had been admitted to the hospital with severe anaemia and thrombocytosis. Physical examination showed that the patient had an anaemic appearance. The results of peripheral blood examination were as follows: red blood cell (RBC) count, 1.0 × 1012/L; platelet (PLT) count, 409 × 109/L; haemoglobin (HB), 36 g/L; creatinine, 0.55 mg/dL; and lactate dehydrogenase (LDH), 418 U/L. Bone marrow trephine biopsy revealed more than 10% abnormal megakaryocytes (single round nuclei and cytosolic lobulated micronuclei) (Figure 1A). Fluorescence in situ hybridization (FISH) indicated deletion of the EGR1 (5q31) gene (Figure 1B). Furthermore, the karyotype was described as 46, XX, del(5)(q13q31) by G band staining (Figure 1C). Based on clinical manifestations and laboratory tests, the patient was diagnosed with low-risk MDS (low risk, IPSS-R = 2.5). The endothelial activation and stress index (EASIX) was 0.56. EASIX is an independent prognostic factor for lower-risk MDS patents that was calculated by the following formula: LDH (U/L) × creatinine (mg/dL)/PLT (nL)[6]. The patient was advised to be treated with lenalidomide. The dosing schedule was 10 mg/d or 21 d in a 28-d cycle. After three cycles of treatment, peripheral blood examination showed HB 97 g/L and PLT 341 × 109/L.
The patient was previously healthy. There was no disease history in other systems.
No contributory personal history or similar family history.
Physical examination showed that the patient had a moderate anaemic appearance. Her vital signs were stable, with no other positive findings.
The results of the peripheral blood examination were as follows: RBC, 2.27 × 1012/L; WBC, 1.93 × 109/L; PLT, 114 × 109/L; and HB, 58 g/L.
Bone marrow aspirate smears showed hypercellularity with marked myeloid and erythroid hypoplasia and a blast cell count of 16% (Figure 2A). Another karyotype examination revealed 46, XX, inv(3)(q21q26), and del(5)(q13q31) (Figure 2B). qRT–PCR showed that the EVI1 expression level was 90.63%, which was classified as high expression.
According to the IPSS-R, the patient's diagnosis was revised to high-risk MDS (very high risk, IPSS-R = 7.5).
We continued to treat the patient with lenalidomide. In less than one treatment cycle, the patient rapidly developed resistance to the drug. Subsequently, we tried azacitidine as a treatment and administered 75 mg/m2/d intravenously for 7 consecutive days every 28 d. After two courses, haematology showed HB 70 g/L and PLT 56 × 109/L. After the fourth cycle, peripheral blood examination revealed HB 40 g/L and PLT 14 × 109/L. Bone morrow aspirate smears revealed that nucleated cells accounted for 6% of the cell population. A mutation of the ASXL1 gene [NM 015338:c.4232_4233delinsA(p. W1411*) exon 12] with a variant allele frequency of 33.1% was detected. Subsequently, her medical condition gradually deteriorated. In view of the present situation, we recommended HSCT.
The patient was discharged and wilfully refused HSCT. After four months, the patient died of the infection.
We report a rare case of MDS with clonal evolution from del(5q) to inv(3) (Figure 3). MDS with del(5q), also known as 5q-syndrome, is a specific type of MDS that has a better prognosis than other subtypes of MDS. The median expected survival time of this syndrome is approximately 58 mo[7]. Deletion of chromosome arm 5q results in the deletion of genes located on this chromosome, including SPARC, EGR1, CTNNA1, APC and NPM1[8]. Based on this, we used LSI EGR1 and D5S23 and a D5S721 dual colour probe to detect del(5q). MDS with inv(3)/t(3) is considered to be a rare event (< 1%); it is an invasive disease with a high risk of developing AML. Furthermore, high expression of EVI1 was observed with chromosome 3 abnormalities in our case. EVI1 is an oncogenic transcriptional regulator that may be involved in the proliferation and maintenance of haematopoietic stem cells, and its abnormally high expression often promotes disease progression[9]. In addition, ASXL1 mutations that frequently occur in MDS were detected in our case and predict an adverse outcome[10]. Therefore, the patient in our report contained "dominant" karyotypes [del(5q)], "inferior" karyotypes [inv(3)] and harmful ASXL1 mutations. However, the prognostic tendency of these patients remains elusive.
Combining bone marrow cytogenetics, the percentage of bone marrow blasts and cytopenia, our patient was classified as low risk according to the IPSS-R at primary diagnosis. In recent years, cardiovascular disease has been considered to be the second most common cause of death among patients with low-risk MDS after haematological complications[11]. Therefore, we used EASIX to assess the patient’s cardiovascular risk, which was 0.56[6]. Because the patient had no previous history of cardiovascular disease and the cardiovascular examination results were negative at admission, we alleviated the patient's cardiovascular disease concerns. Considering the IPSS-R and EASIX, we preliminarily evaluated the patient had a good prognosis.
Lenalidomide therapy is initially recommended based on age, general conditions, and cytogenetic abnormalities. Since 2005, the Food and Drug Administration of the United States has approved the use of lenalidomide for the treatment of transfusion-dependent low-risk MDS with or without del(5q), and it has been indicated that lenalidomide could reduce transfusion requirements and reverse cytogenetic abnormalities[12]. Lenalidomide is the first and only treatment for cytogenetically defined subsets of MDS disease, especially MDS with del(5q). However, not all patients achieve a long-term response. It was reported that approximately half of patients with del(5q) lost response or progression after 2-3 years of treatment[13]. Indeed, lenalidomide resistance has become a common event in the treatment of MDS.
In our case, lenalidomide treatment initially showed a good response in the patient, but the patient rapidly developed drug resistance after the first remission. Based on the available data, we found that the occurrence of primary resistance to lenalidomide in MDS is mainly related to TP53 mutations. We reviewed the role of TP53 mutations and abnormal p53 pathway activation in myeloid malignant tumours. In del (5q) patients who had a high mutation rate of TP53, the cytogenetic complete remission rate was less than 12% after treatment with lenalidomide[7]. Therefore, it is plausible that there is a high correlation between TP53 mutations and lenalidomide resistance. In addition, lenalidomide upregulates RUNX1 expression in a CRBN- and TP53 -dependent manner in del (5q) MDS, and RUNX1 induces megakaryocyte differentiation and apoptosis assisted by GATA2. As a result, lenalidomide resistance occurs when RUNX1 is mutated or downregulated[14]. The secondary or acquired resistance to lenalidomide is associated with the overexpression of PP2A. The overexpression of PP2A leads to the degradation of p53 in red blood cell precursors and the instability of β-catenin, which is more conducive to the evolution of del(5q) clones[15]. However, TP53 and RUNX1 mutations were not detected in our case. The acquired resistance of our patients to lenalidomide may be related to PP2A abnormalities, but further sufficient data is required for further exploration.
After the lenalidomide treatment failed, inv(3) with EVI1 overexpression and ASXL1 mutations occurred in the patient. Based on clinical assessments and laboratory examination, the patient's diagnosis was revised to high-risk MDS. We then tried to treat the patient with azacytidine, which is a demethylation drug. Demethylation drugs mainly include azacitidine and decitabine, both of which can inhibit DNA methylation by binding to DNA. Interestingly, azacitidine also binds to RNA to inhibit RNA synthesis and protein metabolism[16]. Sallman et al[16] reported a study about the response to azacitidine in del(5q) MDS patients after lenalidomide resistance. Among 18 del(5q) MDS patients treated with azacytidine, the overall response rate was 56%, including a complete response rate of 5.6%, a marrow complete response rate of 11.1%, and a haematological improvement rate of 38.9%. Azacitidine had the same effect in del(5q) and non-del(5q) patients[17]. In the study by Wanquet et al[18], 157 AML/MDS patients with chromosome 3q abnormalities and 27 patients with isolated EVI1 overexpression were treated with azacitidine. The overall response rate was 50%, including a complete remission rate of 29%, and the median overall survival time was 10.6 mo. AML/MDS with 3q abnormalities has a special response to azacitidine, and azacitidine is an appropriate choice before the patient receives allohaematopoietic stem cell transplantation[18]. To date, there have been a few reports on the treatment of MDS with decitabine[3]. Therefore, we believe that azacitidine is a reasonable option for the treatment of MDS after the failure of lenalidomide. Our patient experienced a short-term improvement after 2 courses of azacitidine treatment.
The condition of our patient worsened again after a short period of time, and we considered HSCT. At present, for both low-risk and high-risk MDS patients, HSCT is still the only curative treatment[19]. Patients under 65 years of age and suitable healthy elderly patients should be strongly recommended for HSCT with a suitable donor with the same human leukocyte antigen[20]. Among high-risk MDS patients undergoing HSCT, 40-50% of patients have achieved a prolonged disease-free survival and have improved over the years[21]. However, the optimal timing of HSCT and the specific chemical regimen before HSCT treatment is still a controversial issue. It is generally believed that an increase in the percentage of bone marrow blasts, especially if it is greater than 10%, is associated with a higher risk of recurrence[22]. In addition, the existence of poor prognostic mutations, especially mutations in TP53, ASXL1 and RUNX1, should be considered for the use of HSCT to reduce the risk of recurrence[23]. However, in our case, when we recommended that the patient undergo HSCT, the patient rejected the recommendation for unknown reasons. Subsequently, the patient chose to leave the hospital voluntarily, and we learned during follow-up that the patient died of serious infection after four months.
In summary, we report a rare case of MDS with clonal evolution from del(5) to inv(3). Although lenalidomide and azacitidine provided temporary remission to the patient, the patient inevitably has a poor prognosis. The complex and heterogeneous pathophysiology of MDS is still the main reason for the limited effectiveness of current treatments; thus, emerging therapeutic strategies are still urgently needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fazilat-Panah D, Iran; Papadopoulos VP, Greece S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Gorshein E, Weber UM, Gore S. Higher-risk myelodysplastic syndromes with del(5q): does the del(5q) matter? Expert Rev Hematol. 2020;13:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Menssen AJ, Walter MJ. Genetics of progression from MDS to secondary leukemia. Blood. 2020;136:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 3. | Garcia-Manero G, Chien KS, Montalban-Bravo G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am J Hematol. 2020;95:1399-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 4. | Hosono N. Genetic abnormalities and pathophysiology of MDS. Int J Clin Oncol. 2019;24:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Ogawa S. Genetics of MDS. Blood. 2019;133:1049-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 6. | Merz A, Germing U, Kobbe G, Kaivers J, Jauch A, Radujkovic A, Hummel M, Benner A, Merz M, Dreger P, Luft T. EASIX for prediction of survival in lower-risk myelodysplastic syndromes. Blood Cancer J. 2019;9:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Lee JH, List A, Sallman DA. Molecular pathogenesis of myelodysplastic syndromes with deletion 5q. Eur J Haematol. 2019;102:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 8. | Jädersten M, Karsan A. Clonal evolution in myelodysplastic syndromes with isolated del(5q): the importance of genetic monitoring. Haematologica. 2011;96:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Birdwell C, Fiskus W, Kadia TM, DiNardo CD, Mill CP, Bhalla KN. EVI1 dysregulation: impact on biology and therapy of myeloid malignancies. Blood Cancer J. 2021;11:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Thol F, Friesen I, Damm F, Yun H, Weissinger EM, Krauter J, Wagner K, Chaturvedi A, Sharma A, Wichmann M, Göhring G, Schumann C, Bug G, Ottmann O, Hofmann WK, Schlegelberger B, Heuser M, Ganser A. Prognostic significance of ASXL1 mutations in patients with myelodysplastic syndromes. J Clin Oncol. 2011;29:2499-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1338] [Cited by in RCA: 1876] [Article Influence: 234.5] [Reference Citation Analysis (0)] |
| 12. | List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, Powell B, Greenberg P, Thomas D, Stone R, Reeder C, Wride K, Patin J, Schmidt M, Zeldis J, Knight R; Myelodysplastic Syndrome-003 Study Investigators. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 968] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 13. | Talati C, Sallman D, List AF. SOHO State of the Art and Next Questions: Management of Myelodysplastic Syndromes With Deletion 5q. Clin Lymphoma Myeloma Leuk. 2018;18:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Martinez-Høyer S, Deng Y, Parker J, Jiang J, Mo A, Docking TR, Gharaee N, Li J, Umlandt P, Fuller M, Jädersten M, Kulasekararaj A, Malcovati L, List AF, Hellström-Lindberg E, Platzbecker U, Karsan A. Loss of lenalidomide-induced megakaryocytic differentiation leads to therapy resistance in del(5q) myelodysplastic syndrome. Nat Cell Biol. 2020;22:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Sallman DA, Wei S, List A. PP2A: The Achilles Heal in MDS with 5q Deletion. Front Oncol. 2014;4:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Sallman DA, Barnard J, Al Ali NH, Garcia-Manero G, Sekeres MA, DeZern A, Steensma DP, Roboz G, Jabbour E, Maciejewski JP, Pierce S, Padron E, Lancet JE, Kantarjian H, List AF, Komrokji RS. Hypomethylating Agent Therapy in Myelodysplastic Syndromes With Chromosome 3 Abnormalities. Clin Lymphoma Myeloma Leuk. 2020;20:e597-e605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Iizuka H, Yoshimi A, Yamamoto G, Masuda A, Nannya Y, Ichikawa M, Yatomi Y, Kurokawa M. Effective azacitidine treatment for myelodysplastic syndrome transformed from essential thrombocythemia. Rinsho Ketsueki. 2013;54:468-472. [PubMed] |
| 18. | Wanquet A, Prebet T, Berthon C, Sebert M, Roux C, Kulasekararaj A, Micol JB, Esterni B, Itzykson R, Thepot S, Recher C, Delaunay J, Dreyfus F, Mufti G, Fenaux P, Vey N. Azacitidine treatment for patients with myelodysplastic syndrome and acute myeloid leukemia with chromosome 3q abnormalities. Am J Hematol. 2015;90:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Garderet L, Ziagkos D, van Biezen A, Iacobelli S, Finke J, Maertens J, Volin L, Ljungman P, Chevallier P, Passweg J, Schaap N, Beelen D, Nagler A, Blaise D, Poiré X, Yakoub-Agha I, Lenhoff S, Craddock C, Schots R, Rambaldi A, Sanz J, Jindra P, Mufti GJ, Robin M, Kröger N. Allogeneic Stem Cell Transplantation for Myelodysplastic Syndrome Patients with a 5q Deletion. Biol Blood Marrow Transplant. 2018;24:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Fenaux P, Haase D, Santini V, Sanz GF, Platzbecker U, Mey U; ESMO Guidelines Committee. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†☆. Ann Oncol. 2021;32:142-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 21. | de Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I, Mufti GJ, Fenaux P, Sanz G, Martino R, Alessandrino EP, Onida F, Symeonidis A, Passweg J, Kobbe G, Ganser A, Platzbecker U, Finke J, van Gelder M, van de Loosdrecht AA, Ljungman P, Stauder R, Volin L, Deeg HJ, Cutler C, Saber W, Champlin R, Giralt S, Anasetti C, Kröger N. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129:1753-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 22. | Fenaux P, Platzbecker U, Ades L. How we manage adults with myelodysplastic syndrome. Br J Haematol. 2020;189:1016-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Della Porta MG, Gallì A, Bacigalupo A, Zibellini S, Bernardi M, Rizzo E, Allione B, van Lint MT, Pioltelli P, Marenco P, Bosi A, Voso MT, Sica S, Cuzzola M, Angelucci E, Rossi M, Ubezio M, Malovini A, Limongelli I, Ferretti VV, Spinelli O, Tresoldi C, Pozzi S, Luchetti S, Pezzetti L, Catricalà S, Milanesi C, Riva A, Bruno B, Ciceri F, Bonifazi F, Bellazzi R, Papaemmanuil E, Santoro A, Alessandrino EP, Rambaldi A, Cazzola M. Clinical Effects of Driver Somatic Mutations on the Outcomes of Patients With Myelodysplastic Syndromes Treated With Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol. 2016;34:3627-3637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 24. | Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SM, Miyazaki Y, Pfeilstöcker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454-2465. [PubMed] |