Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3579
Peer-review started: December 7, 2021
First decision: January 25, 2022
Revised: February 7, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 122 Days and 9.1 Hours
Juvenile dermatomyositis (JDM) is an idiopathic inflammatory myopathy that occurs in childhood. It is characterized by muscle weakness and a characteristic rash. Previous literature reports have rarely described JDM with severe skin ulcers and infections.
Herein, we describe a case of a 2-year-old female patient who suffered from JDM, whose myositis-specific autoantibodies were positive for anti-nuclear matrix protein 2 antibody, with progressively worsening skin ulcers and severe infections. The patient was treated with glucocorticoids and various immunosuppressants. Nevertheless, further progression of the disease and the combination of primary disease and severe infection in the later period were fatal.
In children, anti-nuclear matrix protein 2+ JDM combined with skin ulcers often indicates severe disease. In such cases, personalized treatment for the primary disease and infection prevention and control are essential.
Core Tip: Juvenile dermatomyositis (JDM) is a rare systemic autoimmune disease characterized by specific skin lesions, chronic muscle inflammation, and systemic vasculitis. We report a very rare case of JDM with severe skin ulcers and infections. By reporting the disease development and treatment of this case of a patient positive for anti-nuclear matrix protein 2 (NXP2) antibody combined with skin ulcers and performing a comprehensive literature review, we summarize JDM with skin ulcers, the clinical characteristics of JDM combined with positivity for anti-NXP2 antibody, and treatment measures for severe JDM.
- Citation: Wang YT, Zhang Y, Tang T, Luo C, Liu MY, Xu L, Wang L, Tang XM. Anti-nuclear matrix protein 2+ juvenile dermatomyositis with severe skin ulcer and infection: A case report and literature review. World J Clin Cases 2022; 10(11): 3579-3586
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3579.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3579
Juvenile dermatomyositis (JDM) is a rare systemic autoimmune disease characterized by vascular disease that mainly affects muscles and skin, as well as the lungs, intestines, heart, and other organs[1-3]. JDM can also lead to macrophage activation syndrome, which is a potentially fatal complication of a number of rheumatological conditions[4]. JDM is the most common inflammatory myopathy in children and has been reported to affect 1.9 individuals per million children in the United Kingdom and 2.4-4.1 individuals per million children in the United States[5,6]. Skin ulcers are one of the severe manifestations of childhood dermatomyositis; however, cases of severe skin ulcers with infections are rarely reported. Here, we report a single case of a 2-year-old female patient who suffered from JDM and whose myositis-specific autoantibodies (MSAs) were positive for anti-nuclear matrix protein 2 antibody, with progressively worsening skin ulcers and severe infections.
A 2-year-old Chinese girl came to the Department of Rheumatology and Immunology with a heliotrope rash for 1 mo and muscle weakness for 10 d.
This patient developed a heliotrope rash, periorbital edema, and nailfold capillary changes within 1 mo, with symmetric proximal muscle weakness. There was no fever, cough, hoarseness, or sensory disturbance.
The patient had no significant medical or surgical history.
The child was born at full term. Her parents and other family members had no family history of autoimmune or other diseases.
After admission, the patient’s weight was 14 kg. There were heliotrope rashes on her face, periorbital edema, changes in nailfold capillaries, Gottron papules on the dorsal surface of the proximal interdigital (PIP), symmetric proximal muscle weakness of arms and legs, no erythema butterfly, and arthritis.
Investigations revealed elevated creatine kinase of 12647 U/L (reference range, 50-220 U/L), lactate dehydrogenase (LDH) of 1358 U/L (reference range, 80-300 U/L), erythrocyte sedimentation rate (ESR) of 79 mm/h (reference range, 0-20 mm/h), and ferritin of 726 ng/mL (reference range, 10-120 ng/mL). The autoantibody, immunoglobulin, and complement profiles were normal. Anti-nuclear matrix protein 2 (NXP-2) antibody was positive in the myositis spectrum, as determined by the dot-ELISA method, and no other myositis-associated autoantibodies were present (Table 1).
| Laboratory examinations | |
| CBC | WBC: 6.71 × 109/L, PLT: 165 × 109/L, L: 0.37, N: 0.54, Hb: 120 g/L, CRP < 8 mg/L |
| Biochemical examination | CK: 12647 U/L, LDH: 1358 U/L, ALT: 116.6 U/L, AST: 359.5 U/L |
| ESR | 79 mm/h |
| Ferritin | 726 ng/mL |
| Autoantibody profile | Negative |
| Immunoglobulins | Normal |
| Complements | Normal |
| MSA | Anti-nuclear matrix protein 2 antibody positive |
Magnetic resonance imaging (MRI) of the bilateral thighs revealed inflammatory changes in the musculature and subcutaneous fat layer of the thigh muscles on both sides, which were also characteristic of dermatomyositis. Electromyography (EMG) showed that the motor unit potential amplitude of the tibialis anterior and rectus femoris muscle was reduced, and the duration was shortened, thus suggesting myogenic abnormalities.
Characteristic skin lesions, proximal muscle weakness, elevated serum muscle enzyme levels, EMG myopathic abnormalities, and changes in muscle MRI findings confirmed the diagnosis of juvenile dermatomyositis.
When the patient was diagnosed with anti-nuclear matrix protein 2 (NXP2)+ juvenile dermatomyositis, she initially received intravenous immunoglobulin (IVIG; 1 g/kg) for 2 d and high-dose glucocorticoids (GC; 15 mg/kg) for 3 d, after which she was treated with oral methylprednisolone (1.15 mg/kg.d), intravenous cyclophosphamide (IV CYC; 0.1 g/kg) for 2 d, methotrexate (MTX; 7.5 mg qw), hydroxychloroquine (0.05 g qd), and other symptomatic and supportive treatment. Following these treatments, the child's rash and edema basically disappeared, and her muscle strength improved.
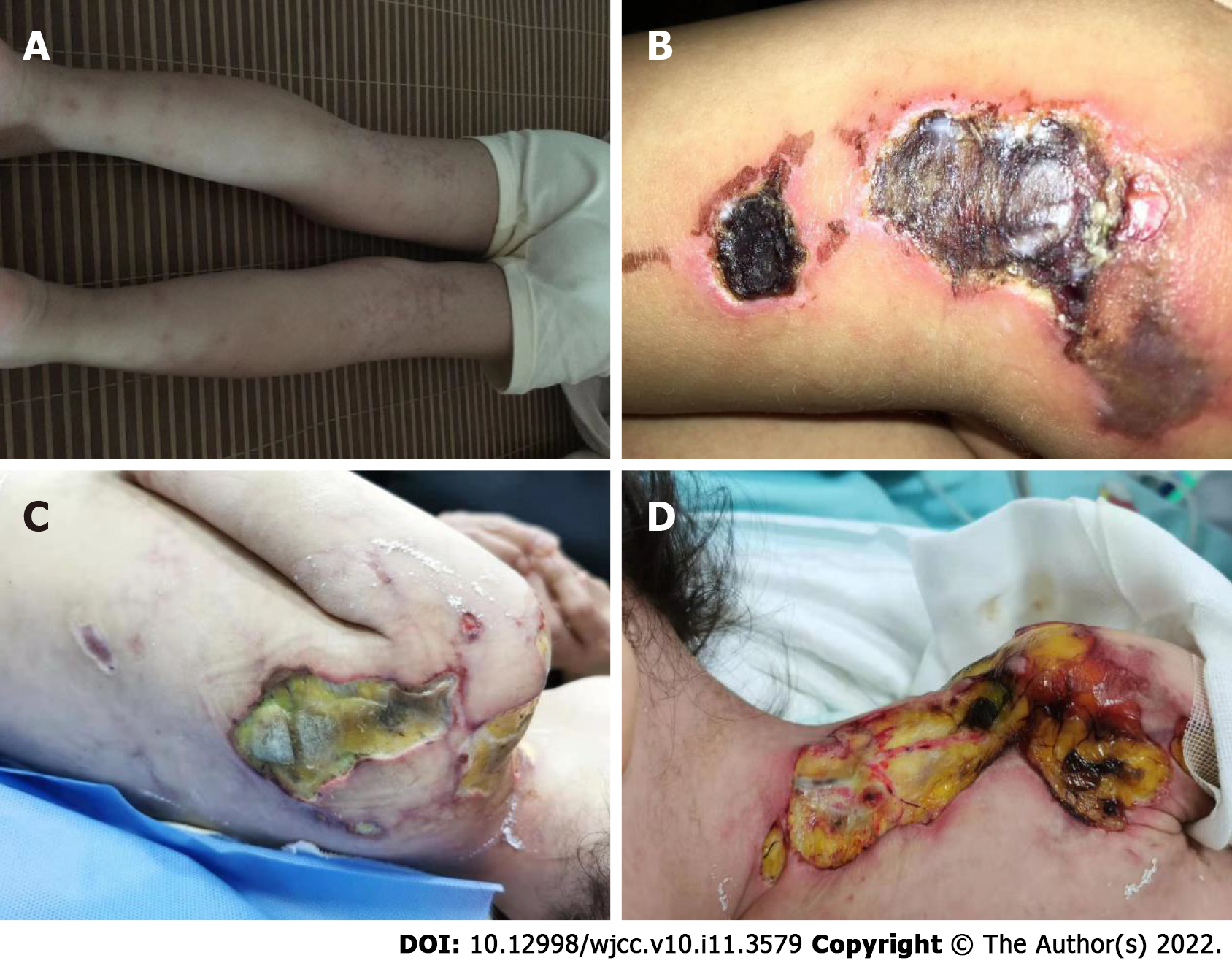
In the following 8 mo, the girl was hospitalized 9 times, and she received IVIG 9 times (1 g/kg for 2 d each time) and IV CYC 6 times, replacing MTX and hydroxychloroquine with mycophenolate mofetil (MMF) and thalidomide. From the 4th month of the illness, she was treated with tofacitinib (2.5 mg bid). In the first three months after diagnosis, the child developed livedo reticularis on the skin of the extremities (Figure 1A) and ulcers on the buttock and left upper arm, while the ulcerated surfaces gradually increased. In the 6th month, yellow necrotic fascia was visible on the left buttock (Figure 1B). After she received proper wound care, anti-infection, and adjustment of immunosuppressants, the ulcers improved (in the 8th month), showing gradual scabbing. Nonetheless, her mother discontinued the patient’s hospitalization. One month later, the patient suffered fatigue, anorexia, and multiple ulcers on the whole body again, which were worse than before. She never showed calcinosis during the whole course of the disease. After rehospitalization, she was given tocilizumab (12 mg/kg). However, when she received 20% of the infusion, the patient developed irritability, and her heart rate increased; thus, the administration of tocilizumab was stopped.
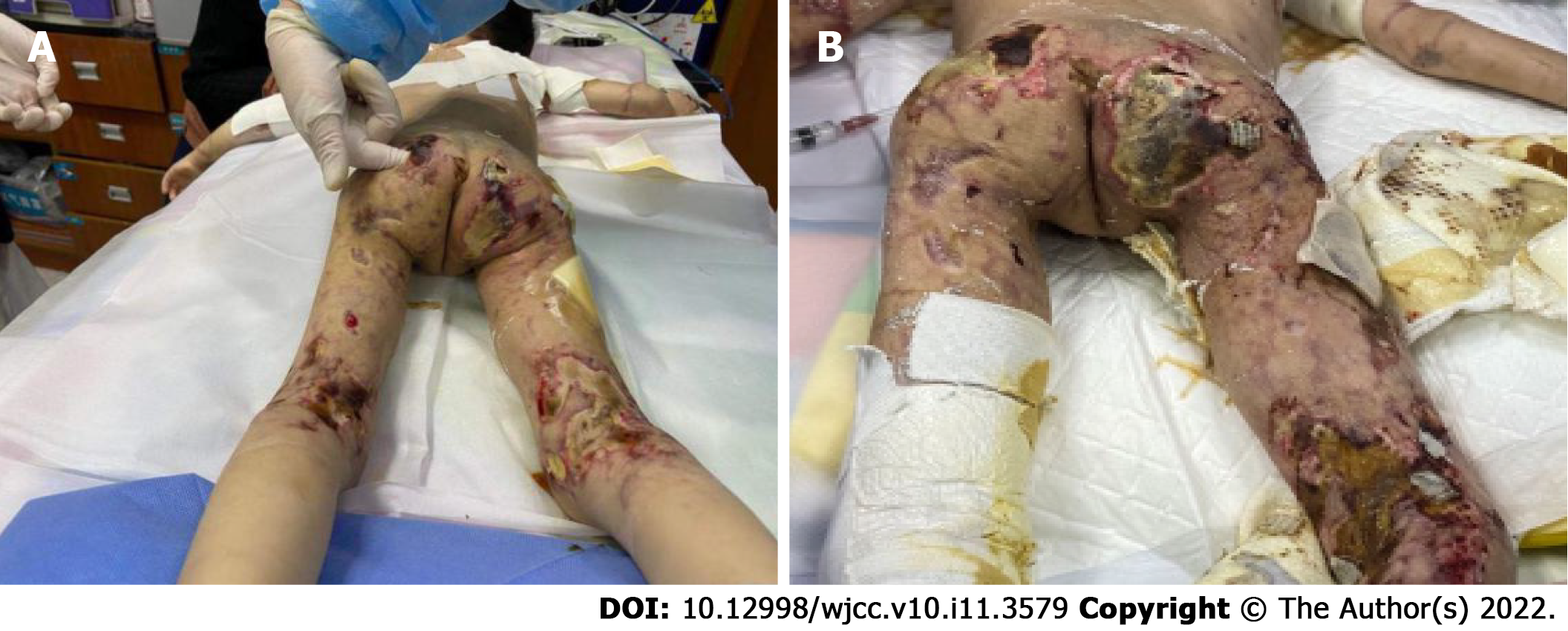
In the 10th month after diagnosis, the child was hospitalized for the last time because of bloody stools, fever, anorexia, listlessness, and multiple painful skin ulcers throughout the whole body (Figure 1C and D). The patient could not move on the bed by herself because of low muscle strength. Laboratory examinations revealed CK of 289 U/L, LDH of 689 U/L, and ESR of 92 mm/hr. Skin tissue biopsy of the left upper arm and left neck suggested epidermal necrosis, hyperplasia of subepidermal fibers and fatty tissues, visible vitreous and mucous changes, multifocal necrosis of the subepidermis and dermis, and focal chronic inflammatory cell infiltration. We used ceftazidime to fight infections and added vancomycin after 2 d. Pathogen detection in the pus from the buttock suggested Escherichia coli. Accordingly, the antibiotics were adjusted to vancomycin and meropenem; however, the ulcers further deepened (Figure 2A and B). After 9 d, re-examination of the culture revealed Pseudomonas aeruginosa and Staphylococcus epidermidis. Based on susceptibility testing, the antibiotics were changed to amikacin and ciprofloxacin. Other treatments included adjusting oral methylprednisolone at 6 mg qd (0.5 mg/kg·d) to intravenous methylprednisolone at 10 mg bid (2 mg/kg·d). During this period, the systemic dressing was changed at least 3 times per week, while IVIG, human albumin, component blood transfusion, and other symptomatic and supportive treatments were intermittently given.
On the 48th day after admission, gastrointestinal bleeding and shock occurred, after which the girl was transferred to the ICU. She was discharged 6 d later and died a few days after leaving the hospital.
JDM is a rheumatic disease that occurs in childhood, with a mortality rate reaching approximately < 4%, which is second only to systemic lupus erythematosus[7,8]. With early treatment, 30%-50% of patients are likely to achieve remission within 2-3 years from the onset of the disease. In addition to the characteristic skin lesions, other criteria include symmetric proximal muscle weakness, elevated serum muscle enzyme levels, myopathic changes on electromyogram, and typical muscle biopsy results[9].
An ulcer is one of the most serious skin manifestations of JDM and is widely regarded as an indication for more intensive treatment[10]. In different cohorts, the incidence of JDM skin ulcers has been reported to range from 2.6%-23%[7,11,12]. Rare cases have shown such severe skin ulceration with multiple pathogenic infections. Cutaneous ulceration may occur on any soft tissue in JDM, especially the armpits, elbows, or pressure points. Although rare, gluteal ulcers are more likely to worsen due to irritation caused by stool, urine, friction, and maceration, especially in infants and young children[13]. In our case, the child’s gluteal ulcer had a protracted condition that gradually expanded and deepened and was accompanied by refractory bacterial infection. Infection and JDM promote each other, which increases the difficulty of treatment and leads to prolonged and unhealed ulcers. A case of JDM in an infant with gluteal ulcer has been reported in Japan. After treatment with high-dose glucocorticoids with cyclophosphamide and MTX, the ulcer gradually healed[13]; however, this case was not accompanied by serious infection. To prevent infection and promote ulcer healing, topical treatments and routine care for skin ulcers are necessary. In 2020, guidelines for skin ulcers related to connective tissue diseases were published in Japan, which in detail described systemic and local medication, nursing, and other treatment methods for skin ulcers and for different connective tissue diseases[14].
Persistent progression of skin ulcers has also been associated with neglected assessment of skin manifestations and severity of JDM. An expert group recommends that the follow-up of patients with JDM should focus more on the evaluation of the skin, including the use of multiple scales[8].The Cutaneous Dermatomyositis Disease Activity and Severity Index(CDASI) and the Cutaneous Assessment Tool (CAT)have good interrater reliability and correlation with other measures of activity and damage in children with JDM[15]. The child in our case had prominent skin damage in the later stage. Early assessment of skin may help with follow-up treatment. Additional assessment tools can be used in clinical practice to more comprehensively assess disease activity[16].
In recent years, JDM combined with different MSAs has received extensive clinical attention. Different MSAs have been associated with different clinical phenotypes, prognoses, and risks of associated malignancy. In United States and European cohorts, MSAs were reported to be present in approximately 70% of JDM cases[17,18].
NXP2 is a protein involved in transcription and RNA metabolism regulation[19]. The autoantibody was first identified in 1997 in childhood myositis and was considered a key biomarker for the diagnosis of idiopathic inflammatory myopathy[20]. Clinically, anti-NXP2+ JDM often manifests as obvious skin rash, muscle weakness, dysphagia, calcinosis, limb edema, younger age of onset and less remission at 2 years[16]. The incidence of anti-NXP2+ JDM is 20%-25% among JDM cases, making anti-NXP2 a common type of antibody[19]. Two different studies in China have revealed detection rates of anti-NXP2 antibodies in JDM of 30.6% and 20%[21,22]. Albayda et al[23] found that NXP2+ dermatomyositis with limb weakness and neck muscle weakness were more serious than NXP2- dermatomyositis; accordingly, as our patient showed severe weakness. Another feature of NXP2+ JDM is calcinosis, although this was not present in our case. Early diagnosis and treatment of JDM can prevent the occurrence of calcinosis[24]. As there is no definitive treatment for severe calcinosis, surgical treatment is often necessary; however, there is still the possibility of recurrence[25]. Although most NXP2+ JDM patients are sensitive to GC, some patients are prone to severe and refractory JDM manifestations.
Immediately after the diagnosis of JDM was made, we treated this patient with corticosteroids and immunosuppressants. Systemic corticosteroids are the gold-standard initial treatment for JDM. However, they should not be used as monotherapy because this approach is frequently ineffective and associated with the development of unacceptable long-term adverse effects[26]. Immunosuppressants have steroid protection and are recommended even for mild cases to minimize the adverse effects of long-term GC treatment. Treatments used for refractory disease include IVIG, cyclophosphamide, cyclosporine, azathioprine, MMF, hydroxychloroquine, tacrolimus, rituximab, infliximab, and autologous stem cell transplantation[8]. Some studies suggest that treatment with GC and cyclophosphamide combined with calcineurin inhibitors is very important for dermatomyositis patients with skin ulcers or other severe manifestations[27]. A JAK-inhibitor (JAKi) can be used to treat JDM and has been reported to be partially effective for interstitial lung disease and cutaneous dermatomyositis[28,29]. However, in the present case, a JAKi did not stop the progression of the disease. For NXP2+ JDM and severe JDM, many studies have reported a favorable therapeutic effect of rituximab[19,30], which can reduce disease activity and reduce GC use. Additionally, rituximab can ameliorate the skin symptoms of refractory JDM and is effective for skin ulcers of JDM[19]. However, because the skin ulcers in this case were accompanied by severe infection, we were worried that the use of rituximab would further aggravate the infection, so it was not used. We tried tocilizumab, but the patient could not tolerate it. Plasmapheresis can be considered for the treatment of cases refractory to immunosuppressants[8].
For JDM-related skin ulcers, steroids and immunosuppressants should be first used to control the primary disease. However, if skin ulcers are complicated by infections, this type of treatment may exacerbate the ulcers due to the increased susceptibility of the patient to infection[14]. Therefore, in such cases, it is necessary to treat the primary disease and the infection simultaneously. According to the pathogenic examination and drug susceptibility results of the site of infection, appropriate antibacterial drugs were chosen, and the use of IVIG was considered. Due to the refractory nature of cutaneous vasculitis in JDM, case studies suggest that nifedipine, sildenafil, intravenous prostaglandins, and bosentan should be added as early adjuncts[26,31].
In the present case, after high-dose GC treatment, IVIG, MTX, MMF, hydroxychloroquine, a JAKi, thalidomide, and tocilizumab were given to the patient, and improvement was observed; however, the disease was not completely prevented. Further progression of the disease and the combination of primary disease and severe infection in the later period were fatal. It remains unknown whether the early use of vasodilatory agents and rituximab or plasmapheresis at the proper time could save patients’ lives, which future studies should address.
Only scarce reports of JDM with severe ulcers accompanied by infection have been reported. Skin ulcer-complicated and anti-NXP2+ JDM usually represent severe cases, and it is important to actively prevent the occurrence of infection while GC and appropriate immunosuppressive therapy are used. According to the different clinical manifestations and immunological indicators of JDM patients, appropriate assessment tools should be used to comprehensively assess the condition of JDM patients at an early stage, and individualized treatment plans should be customized.
Provenance and peer review: Unsolicited manuscript; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poddighe D, Kazakhstan; Wang CR, Taiwan S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Rider LG, Lidsley CB, Miller FW. Juvenile dermatomyositis. In: Petty RE, Laxer RM, Lindsey CB, Wedderburn LR, editors. Textbook of pediatric rheumatology, 7th ed. Philadelphia: Elsevier Saunder; 2016: 351-384. |
| 2. | Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 3. | Varnier GC, Pilkington CA, Wedderburn LR. Juvenile dermatomyositis: novel treatment approaches and outcomes. Curr Opin Rheumatol. 2018;30:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Poddighe D, Dauyey K. Macrophage activation syndrome in juvenile dermatomyositis: a systematic review. Rheumatol Int. 2020;40:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Mendez EP, Lipton R, Ramsey-Goldman R, Roettcher P, Bowyer S, Dyer A, Pachman LM; NIAMS Juvenile DM Registry Physician Referral Group. US incidence of juvenile dermatomyositis, 1995-1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Gowdie PJ, Allen RC, Kornberg AJ, Akikusa JD. Clinical features and disease course of patients with juvenile dermatomyositis. Int J Rheum Dis. 2013;16:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Mathiesen PR, Zak M, Herlin T, Nielsen SM. Clinical features and outcome in a Danish cohort of juvenile dermatomyositis patients. Clin Exp Rheumatol. 2010;28:782-789. [PubMed] |
| 8. | Bellutti Enders F, Bader-Meunier B, Baildam E, Constantin T, Dolezalova P, Feldman BM, Lahdenne P, Magnusson B, Nistala K, Ozen S, Pilkington C, Ravelli A, Russo R, Uziel Y, van Brussel M, van der Net J, Vastert S, Wedderburn LR, Wulffraat N, McCann LJ, van Royen-Kerkhof A. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann Rheum Dis. 2017;76:329-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 9. | Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3355] [Cited by in RCA: 3490] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 10. | Martin N, Krol P, Smith S, Beard L, Pilkington CA, Davidson J, Wedderburn LR; Juvenile Dermatomyositis Research Group (JDRG). Comparison of children with onset of juvenile dermatomyositis symptoms before or after their fifth birthday in a UK and Ireland juvenile dermatomyositis cohort study. Arthritis Care Res (Hoboken). 2012;64:1665-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Sun C, Lee JH, Yang YH, Yu HH, Wang LC, Lin YT, Chiang BL. Juvenile dermatomyositis: a 20-year retrospective analysis of treatment and clinical outcomes. Pediatr Neonatol. 2015;56:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Sag E, Demir S, Bilginer Y, Talim B, Haliloglu G, Topaloglu H, Ozen S. Clinical features, muscle biopsy scores, myositis specific antibody profiles and outcome in juvenile dermatomyositis. Semin Arthritis Rheum. 2021;51:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Wakiguchi H, Takei S, Imanaka H, Hiraki T, Higashi M, Yamatou T, Yamasaki Y, Kubota T, Kawano Y. Severe gluteal skin ulcers in an infant with juvenile dermatomyositis. Eur J Dermatol. 2016;26:192-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Fujimoto M, Asai J, Asano Y, Ishii T, Iwata Y, Kawakami T, Kodera M, Abe M, Amano M, Ikegami R, Isei T, Isogai Z, Ito T, Inoue Y, Irisawa R, Ohtsuka M, Omoto Y, Kato H, Kadono T, Kaneko S, Kanoh H, Kawaguchi M, Kukino R, Kono T, Koga M, Sakai K, Sakurai E, Sarayama Y, Shintani Y, Tanioka M, Tanizaki H, Tsujita J, Doi N, Nakanishi T, Hashimoto A, Hasegawa M, Hayashi M, Hirosaki K, Fujita H, Fujiwara H, Maekawa T, Matsuo K, Madokoro N, Motegi SI, Yatsushiro H, Yamasaki O, Yoshino Y, Pavoux AJL, Tachibana T, Ihn H; Japanese Dermatological Association Guidelines. Wound, pressure ulcer and burn guidelines - 4: Guidelines for the management of connective tissue disease/vasculitis-associated skin ulcers. J Dermatol. 2020;47:1071-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Tiao J, Feng R, Berger EM, Brandsema JF, Coughlin CC, Khan N, Kichula EA, Lerman MA, Lvovich S, McMahon PJ, Rider LG, Rubin AI, Scalzi LV, Smith DM, Taxter AJ, Treat JR, Williams RP, Yum SW, Okawa J, Werth VP. Evaluation of the reliability of the Cutaneous Dermatomyositis Disease Area and Severity Index and the Cutaneous Assessment Tool-Binary Method in juvenile dermatomyositis among paediatric dermatologists, rheumatologists and neurologists. Br J Dermatol. 2017;177:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Kim H, Huber AM, Kim S. Updates on Juvenile Dermatomyositis from the Last Decade: Classification to Outcomes. Rheum Dis Clin North Am. 2021;47:669-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Tansley SL, Betteridge ZE, Gunawardena H, Jacques TS, Owens CM, Pilkington C, Arnold K, Yasin S, Moraitis E, Wedderburn LR, McHugh NJ; UK Juvenile Dermatomyositis Research Group. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther. 2014;16:R138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Rider LG, Nistala K. The juvenile idiopathic inflammatory myopathies: pathogenesis, clinical and autoantibody phenotypes, and outcomes. J Intern Med. 2016;280:24-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Aggarwal R, Loganathan P, Koontz D, Qi Z, Reed AM, Oddis CV. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology (Oxford). 2017;56:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Oddis CV, Fertig N, Goel A, Espada G, Confalone Gregorian M, Cocco JAM, Londino AV. Clinical and serological characterization of the anti-MJ antibody in childhood myositis. Arthritis Rheum. 1997;40:S139. |
| 21. | Wang X, Ding Y, Zhou Z, Hou J, Xu Y, Li J. Clinical characteristics and poor predictors of anti-NXP2 antibody-associated Chinese JDM children. Pediatr Rheumatol Online J. 2021;19:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Li DM, Wang L, Liu MY, Xu L, Tang XM. [The analysis of clinical phenotypes and autoantibodies in juvenile dermatomyositis]. Zhonghua Er Ke Za Zhi. 2020;58:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Albayda J, Pinal-Fernandez I, Huang W, Parks C, Paik J, Casciola-Rosen L, Danoff SK, Johnson C, Christopher-Stine L, Mammen AL. Antinuclear Matrix Protein 2 Autoantibodies and Edema, Muscle Disease, and Malignancy Risk in Dermatomyositis Patients. Arthritis Care Res (Hoboken). 2017;69:1771-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | Hoeltzel MF, Oberle EJ, Robinson AB, Agarwal A, Rider LG. The presentation, assessment, pathogenesis, and treatment of calcinosis in juvenile dermatomyositis. Curr Rheumatol Rep. 2014;16:467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Kobayashi I, Akioka S, Kobayashi N, Iwata N, Takezaki S, Nakaseko H, Sato S, Nishida Y, Nozawa T, Yamasaki Y, Yamazaki K, Arai S, Nishino I, Mori M. Clinical practice guidance for juvenile dermatomyositis (JDM) 2018-Update. Mod Rheumatol. 2020;30:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Waldman R, DeWane ME, Lu J. Dermatomyositis: Diagnosis and treatment. J Am Acad Dermatol. 2020;82:283-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 27. | Ishigaki K, Maruyama J, Hagino N, Murota A, Takizawa Y, Nakashima R, Mimori T, Setoguchi K. Skin ulcer is a predictive and prognostic factor of acute or subacute interstitial lung disease in dermatomyositis. Rheumatol Int. 2013;33:2381-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Kurasawa K, Arai S, Namiki Y, Tanaka A, Takamura Y, Owada T, Arima M, Maezawa R. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology (Oxford). 2018;57:2114-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 29. | Moghadam-Kia S, Charlton D, Aggarwal R, Oddis CV. Management of refractory cutaneous dermatomyositis: potential role of Janus kinase inhibition with tofacitinib. Rheumatology (Oxford). 2019;58:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, Barohn RJ, Feldman BM, Harris-Love MO, Koontz DC, Fertig N, Kelley SS, Pryber SL, Miller FW, Rockette HE; RIM Study Group. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 31. | Combalia A, Giavedoni P, Tamez L, Grau-Junyent JM, Mascaró JM Jr. Bosentan for Cutaneous Ulcers in Anti-MDA5 Dermatomyositis. JAMA Dermatol. 2018;154:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |