Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3496
Peer-review started: August 9, 2021
First decision: November 17, 2021
Revised: December 5, 2021
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 241 Days and 19.8 Hours
Ductal carcinoma in situ (DCIS) arising within fibroadenoma is a type of tumor that is rarely encountered in clinic, with only about 100 cases of carcinoma arising within a fibroadenoma reported in the literature. Here, we present two cases of breast DCIS arising within a fibroadenoma and discuss their clinical and imaging findings as well as treatment.
The patients did not have cancer-related personal and family histories. Case 1 (a 49-year-old woman) was diagnosed with a bilateral breast nodule in May 2018 and was followed (preoperative imaging data including ultrasound and mammography) for 3 years; she underwent an excisional biopsy to address an enlargement in nodule size. Case 2 (a 37-year-old woman) was diagnosed with a left breast nodule in June 2021 and consequently received vacuum-assisted biopsy of the tumor which appeared as “irregularly shaped” and “unevenly textured” tissue on ultrasound. The pathological diagnosis was clear in both cases. Both patients underwent breast-conserving surgery and sentinel lymph node biopsy. The two cases received or planned to receive radiotherapy as well as endocrine therapy (tamoxifen).
Breast DCIS arising within a fibroadenoma is rare, but patients treated with radiotherapy and endocrine therapy can have good prognosis.
Core Tip: Breast ductal carcinoma in situ arising within a fibroadenoma is a rare event. We present 2 such cases and discuss their clinical and imaging findings as well as treatment. Both patients underwent breast-conserving surgery and sentinel lymph node biopsy. The 2 cases received or planned to receive radiotherapy as well as endocrine therapy (tamoxifen). More sections are needed to reduce the missed rate. Complete follow-up data with preoperative imaging can help with decision-making during patient follow-up.
- Citation: Wu J, Sun KW, Mo QP, Yang ZR, Chen Y, Zhong MC. Preoperational diagnosis and management of breast ductal carcinoma in situ arising within fibroadenoma: Two case reports. World J Clin Cases 2022; 10(11): 3496-3504
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3496.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3496
Fibroadenoma is the most common breast tumor found in young women[1,2]. Ductal carcinoma in situ (DCIS) arising within a fibroadenoma is a specific pathological type of tumor that is rarely encountered in the clinic[3]. Its incidence ranges from 0.02% to 0.125%[4-6] and it is usually discovered by chance during pathological examination of fibroadenoma resected tissue. So far, approximately 100 cases for carcinoma arising within a fibroadenoma have been reported worldwide, including intraductal carcinoma, lobular carcinoma in situ and invasive carcinoma, most of which lack preoperative follow-up data. Herein, we present 2 cases of breast DCIS arising within a fibroadenoma and discuss their clinical and imaging findings as well as treatment.
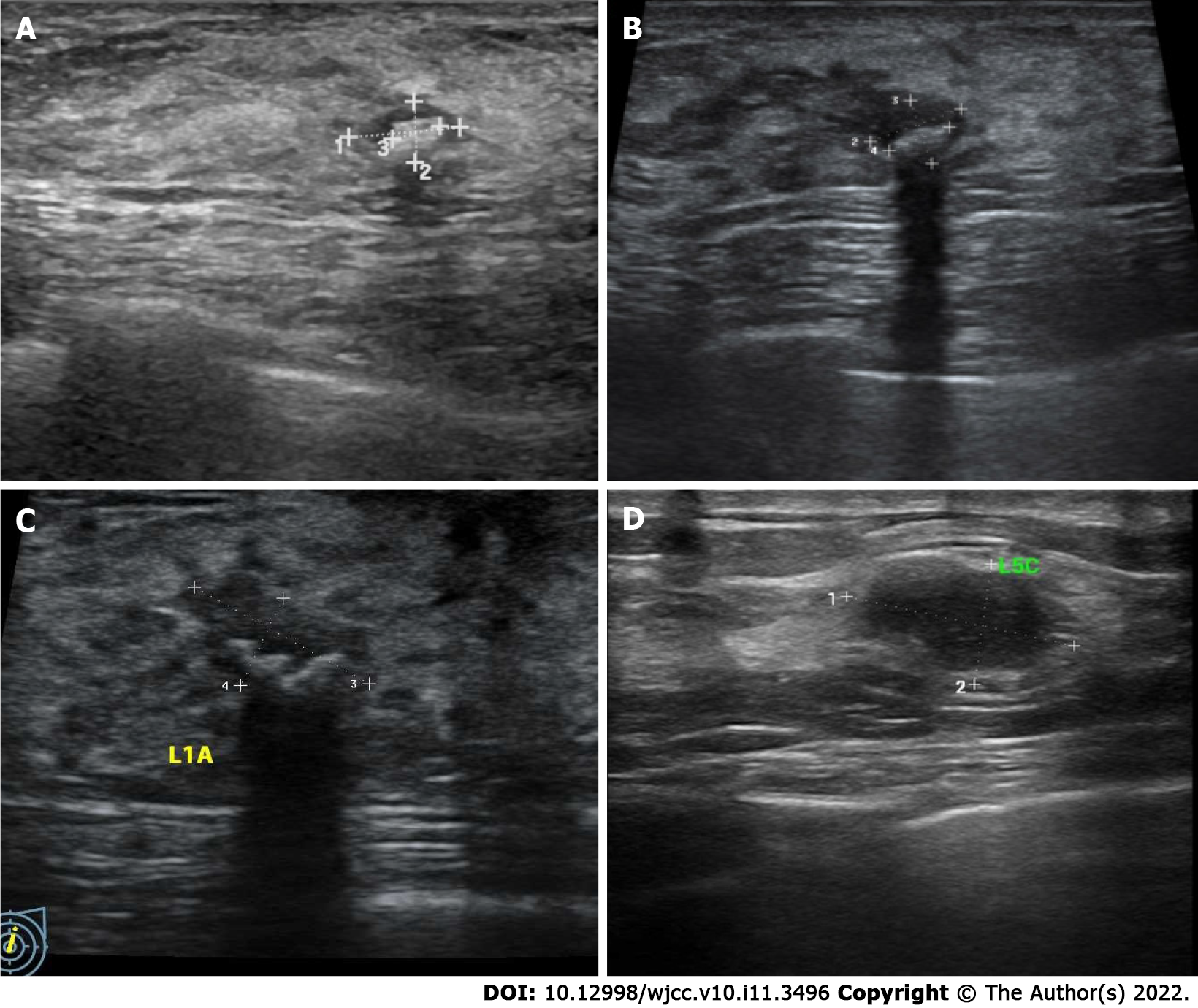
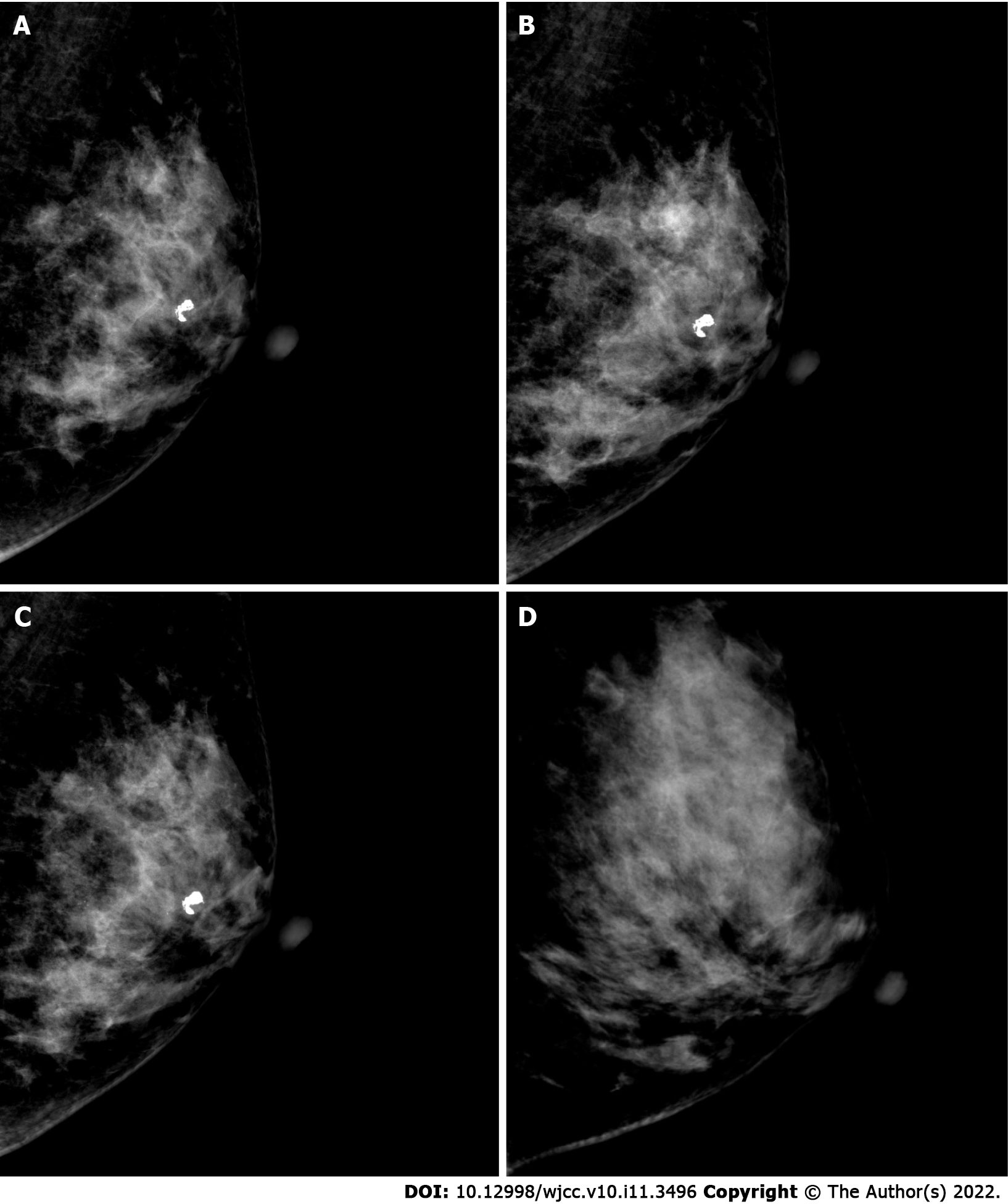
Case 1: A 49-year-old woman was diagnosed with a bilateral breast nodule in May 2018 (Figure 1A). The first ultrasound diagnosis showed a breast nodule of 8 mm × 5 mm × 7 mm in size, located in the left breast at the one o’clock direction. The tissue was hypoechoic, with an internal visible bright spot that was about 4 mm in length. Mammography suggested coarse granular calcified nodules behind the left areola and small nodules in the upper quadrant of the right breast (Figure 2A). No palpable masses were noted in either breast. It was recommended that she undergo regular ultrasound review (which was performed again in March 2019 and June 2021) and received no interventional treatment. In March 2019, ultrasound (Figure 1B) and mammography (Figure 2B) of these breast nodules showed no differences compared with the previous diagnosis. Yet, in June 2021 the ultrasound suggested an increase in the size (11 mm × 6 mm × 11 mm) of the breast nodule behind the left areola at the one o’clock direction. Moreover, coarse calcification plaques about 4 mm in length with Breast Imaging Reporting and Data System (BIRADS) grade IVA were found in the interior of the nodule. Mammography findings were similar to those observed previously. The patient had no remarkable physical complaints.
Case 2: A 37-year-old woman was diagnosed with a left breast nodule in June 2021; yet, a specific size was not recorded. She was instructed to have regular ultrasound review, and no interventional treatment was given. The patient visited the outpatient clinic in July 2021 and underwent further ultrasound examination, which showed a nodule in the left breast at the five o’clock position. The nodule was approximately 15 mm × 9 mm × 11 mm in size with irregular morphology and uneven internal echogenicity but without calcification inside, and of BIRADS grade III. Mammography suggested no other abnormalities. During the history-taking, she revealed no remarkable physical complaints.
Case 1 and case 2 have none present illness.
Case 1: The patient’s previous medical history was unremarkable.
Case 2: The patient was diagnosed with depression for over 9 years. Oral paroxetine was used to control symptoms.
Case 1 and case 2 had no personal or family histories of breast malignancy.
Case 1: Physical examination revealed the blood pressure to be 135/89 mmHg. No abnormalities were found in the cardiopulmonary and abdominal regions. The nipples were symmetrical, and the skin of both breasts was unremarkable, with no inversion of the nipple on either side and no significant nipple bleeding. No palpable masses were noted in either of the breasts, and no enlarged lymph nodes were palpable in the axilla and supraclavicular regions.
Case 2: Blood pressure was 122/79 mmHg. Physical examination of the cardiopulmonary and abdominal regions revealed no abnormalities. The nipples were symmetrical, and the skin of both breasts was unremarkable, with no inversion of the nipple on either side and no significant nipple bleeding. A nodule of about 15 mm × 10 mm in size with moderate texture, poorly defined borders, and smooth surface was palpable in the outer lower quadrant of the left breast. No palpable mass was detected in the right breast. No enlarged lymph nodes were palpable in the axilla and supraclavicular regions.
Case 1: Sex hormone tests suggested estradiol < 10 pg/mL, follicle-stimulating hormone 24.86 IU/L and luteinizing hormone 15.19 IU/L. The levels of carcinoembryonic antigen, cancer antigen (CA) 125, CA153 and CA199 were within normal limits.
Case 2: Carcinoembryonic antigen and CA199 levels were within normal limits.
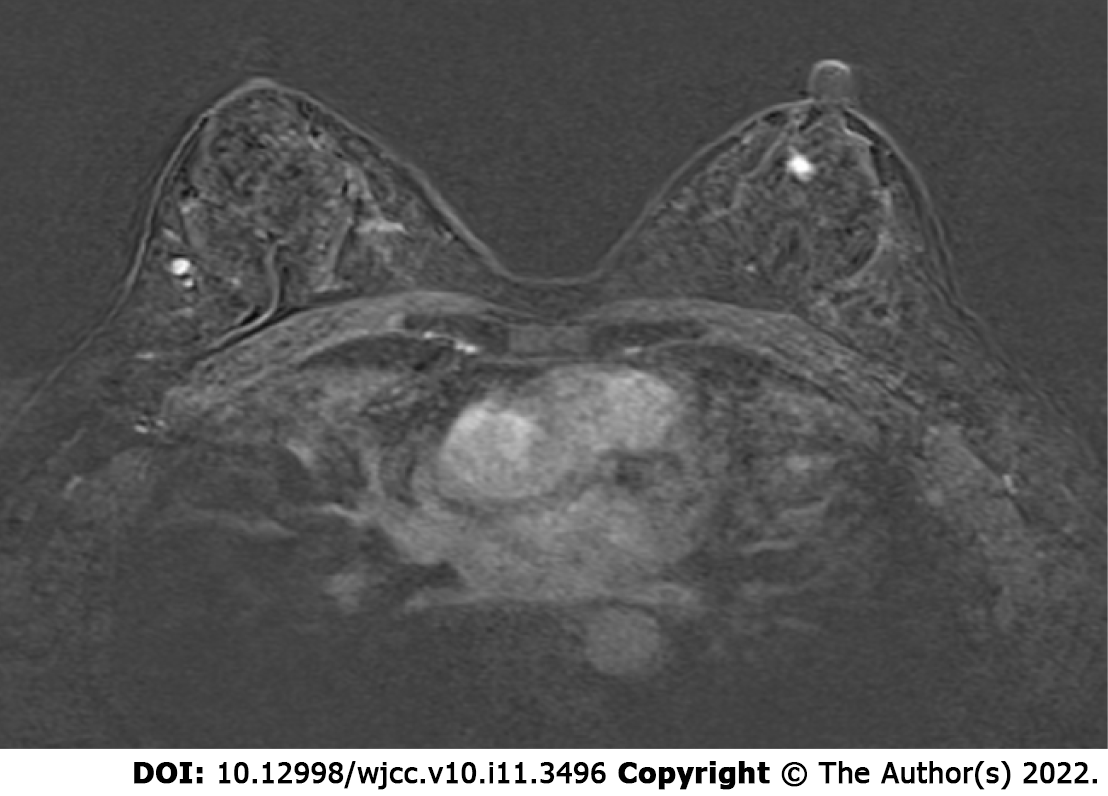
Case 1: Breast and axillary lymph node ultrasound showed the nodule behind the left areola at the one o’clock position of the left breast was 11 mm × 6 mm × 11 mm in size with coarse calcified plaques about 4 mm in length diameter and BIRADS grade IVA (Figure 1C). The remaining bilateral breast had multiple nodules and BIRADS grade II-III. Mammography suggested a coarse granular calcified nodule posterior to the left areola, BIRADS category 2 (Figure 2C). Dynamic contrast-enhanced magnetic resonance imaging of the breast showed a left posterior papillary nodule, approximately 7 mm × 6 mm in size with a clear border and slightly lobulated appearance, a type II pattern on enhancement curve and lipohyalinosis hypointense foci on T1-weight imaging and T2-weighted imaging with possible posterior calcification and BIRADS 4a. There were multiple small nodules (BIRADS 3) throughout both breasts (Figure 3).
Case 2: Breast and axillary lymph node ultrasound showed the nodule located at the five o’clock direction in the left breast. The nodule was approximately 15 mm × 9 mm × 11 mm in size with irregular morphology, uneven internal echogenicity, without calcification inside, and BIRADS grade III (Figure 1D). Mammography suggested mammary hyperplasia and BIRADS category 3 (Figure 2D). Dynamic contrast-enhanced magnetic resonance examination of the mammary gland was not performed.
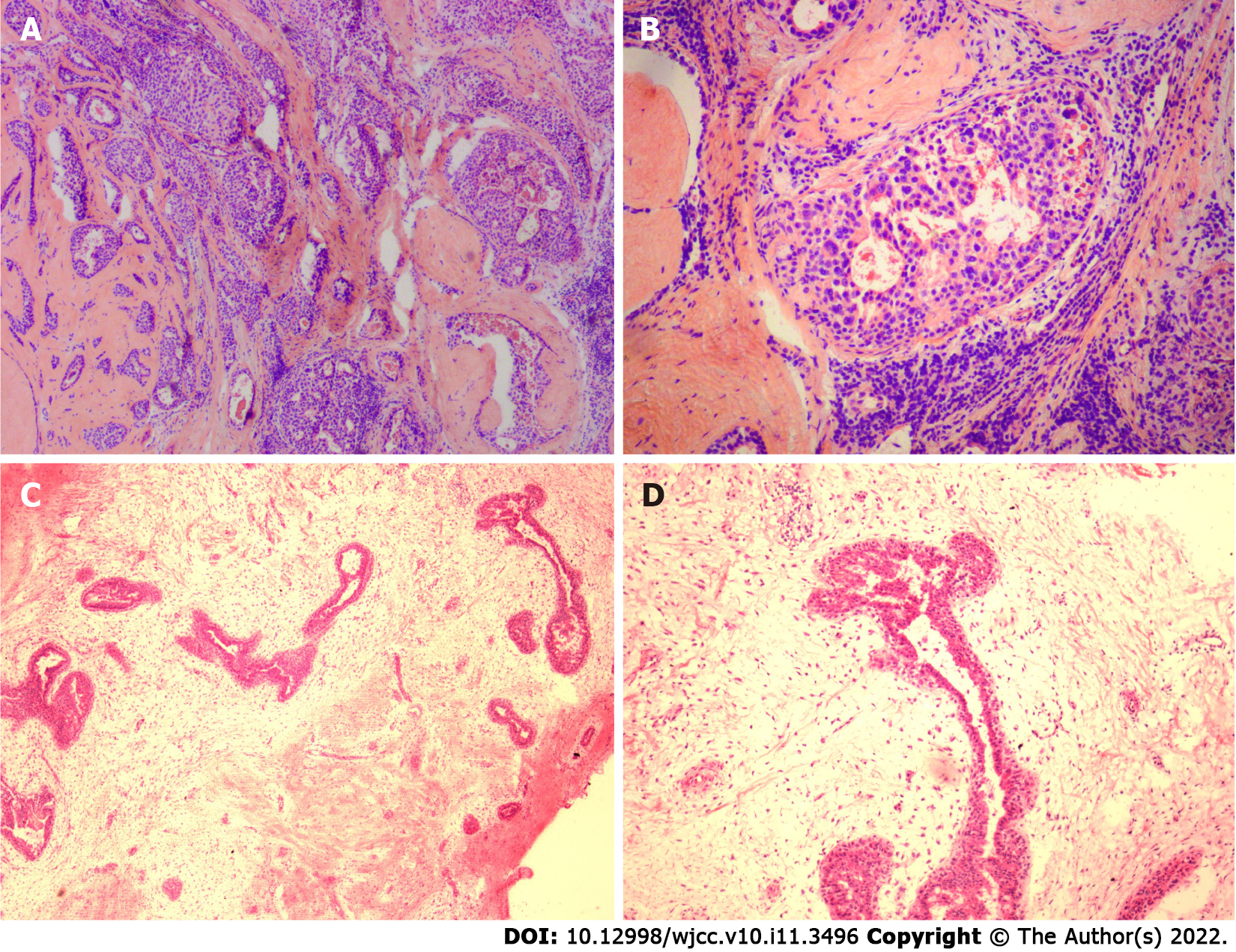
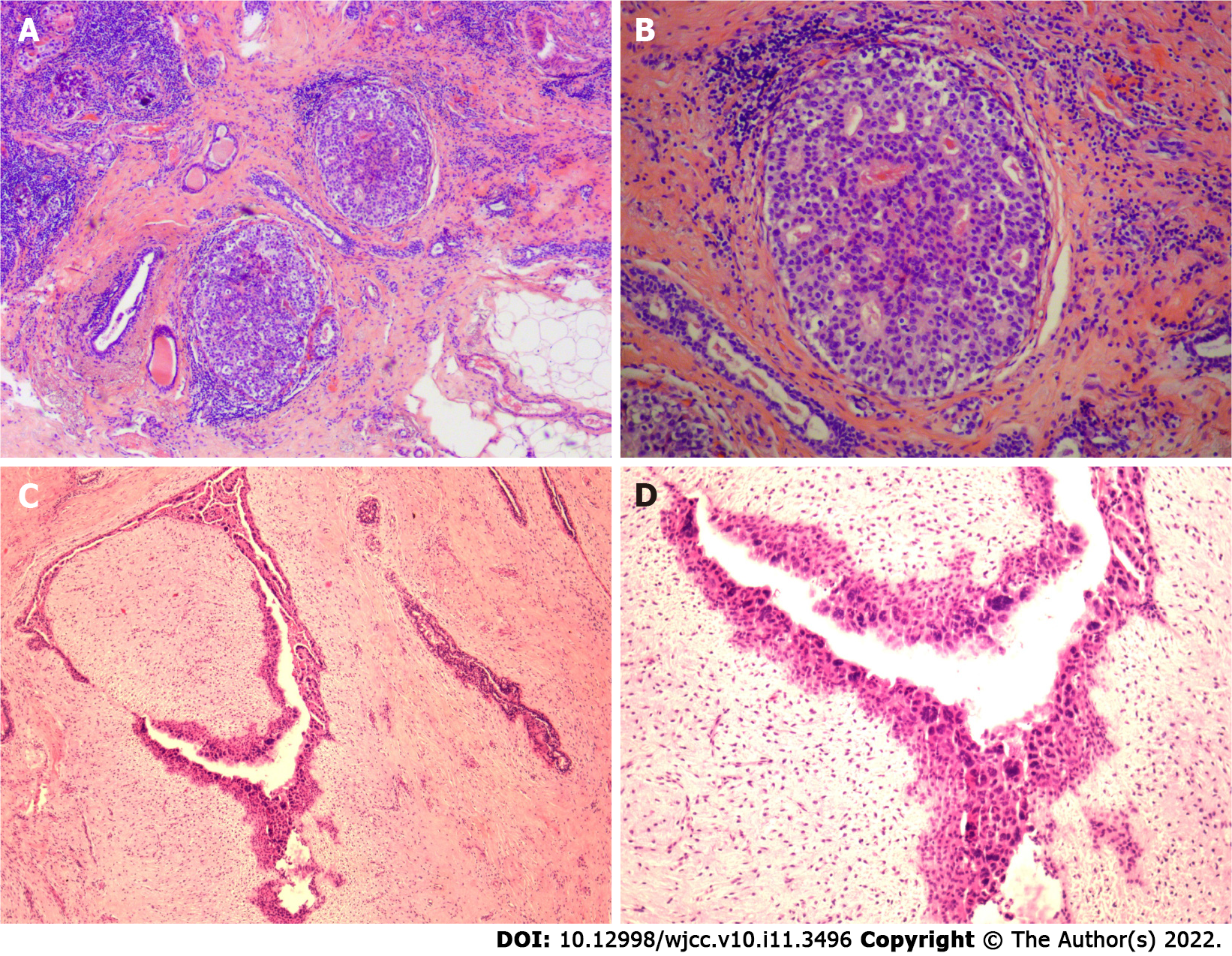
Case 1: The left breast nodule of the patient was completely resected and sent for pathological examination. Intraoperative hematoxylin and eosin-stained frozen sections results are shown in Figures 4 and 4B; paraffin sections after operation are shown in Figures 5A and 5B. DCIS on a background of fibroadenomas (all surrounded by a fibroadenomatous component) in a nested pattern was well demonstrated on both frozen and paraffin sections.
Case 2: The patient’s left breast nodule was excised by a vacuum-assisted biopsy system and then sent for pathological examination. Intraoperative hematoxylin and eosin-stained frozen section results are shown in Figure 4C and 4D and postoperative paraffin section findings in Figures 5C and 5D. The frozen section showed no dysplasia of the ductal epithelium within the fibroadenoma, either at low or medium magnification. In postoperative paraffin section analysis, a focal intraductal carcinoma component within a fibroadenoma was found.
The 2 patients were diagnosed with breast DCIS arising within fibroadenoma. Immunohistochemical examination suggested estrogen receptor positivity.
Both patients underwent breast-conserving surgery and sentinel lymph node biopsy. During the operations, the negative margin of the resected specimen and negative sentinel lymph nodes were confirmed with frozen sections.
Both patients recovered well after the operation. One patient received radiotherapy, while the other prepared to start radiotherapy (she consulted a radiotherapy physician and received an appointment for radiotherapy). The recommendation to begin endocrine therapy with tamoxifen was given. The follow-up is ongoing.
To the best of our knowledge, this is the first study that reported imaging results of DCIS arising within fibroadenoma, which might gradually develop into a prediagnostic follow-up tool that could facilitate decisions on when to perform surgical biopsies for breast nodules with an apparently benign tumor observed at follow-up. Moreover, these data demonstrate the importance of adequate sampling, which can increase the accuracy of early diagnosis. In addition, our results verified the value of frozen and paraffin sections for diagnosing this particular cancer type, thus allowing for a definite diagnosis to be made.
Breast DCIS arising within a fibroadenoma is a rare occurrence. To our knowledge, there are no previous reports on imaging data (including ultrasound and mammography) during the longer preoperative follow-up for DCIS developing in fibroadenoma. Herein, we reported 2 cases of breast DCIS arising within fibroadenoma: 1 with a short medical history and 1 with more than 3 years of follow-up by ultrasound and mammography, subsequently confirmed by pathological examination.
Preoperative color Doppler sonography has a suggestive role in helping physicians make a correct diagnosis. In addition, longer preoperative follow-up imaging data allow us to review the developmental changes in this rare disease on imaging. Our data suggested that enlarged lesions with irregular margins, uneven texture, and concomitant calcifications could be important features for detecting this type of malignancy, which is consistent with previous studies[7,8].
Without adequate sampling and sectioning, a possibility of a missed diagnosis of intraductal carcinoma or even invasive carcinoma of such a focal location within a fibroadenoma increase. In addition, it is possible that the dysplastic ductal epithelium, or even carcinoma, could not be identified because frozen sections were only selected[9], although the ability of frozen sections for the diagnosis of this particular cancer type is adequate. In addition, some experts argue that invasive carcinomas arising in fibroadenomas could have similar prognostic features as intraductal carcinomas.
These 2 cases of breast DCIS arising within a fibroadenoma also clearly suggest that fibroadenomas should not be ignored and warrant close follow-up. Based on the course of the follow-up and diagnosis in the above cases and referring to the previous literature, a significantly enlarged mammary nodule with irregular morphology or uneven internal echogenicity under ultrasound may indicate the need for biopsy or surgical intervention[10].
In general, the clinical and macroscopic features of DCIS arising within a fibroadenoma rarely differed from those of common DCIS[11]. Therefore, the treatment of this particular entity may be similar to the treatment of common DCIS. Based on the prognosis of carcinoma in situ within fibroadenomas, some scholars recommend breast-conserving surgery as the preferred treatment modality[11]. Only a small amount of adjacent breast tissue is usually included around the biopsy specimen. Therefore, biopsy is inadequate to assess adjacent mammary ducts. Re-excision is recommended. Mastectomy may also be performed if the patient wishes to approach “near-certain cure”[11]. In current National Comprehensive Cancer Network guidelines, patients suffering from DCIS treated with lumpectomy would be recommended to receive radiotherapy first, with the exception of those with lower recurrence risk factors. Whole breast radiation therapy with or without boost to tumor bed is the preferred method of radiotherapy (category I). The patients with DCIS without the high recurrence risk factors, such as age < 50 years, larger size, higher grade, palpable mass and close or involved margins, may be treated by excision alone. This propensity to excision alone would be stronger if they were estrogen receptor-positive because there are also endocrine therapies that can be used for treatment.
Despite the favorable prognosis, it remains unknown whether radiotherapy can be exempted after breast-conserving surgery in DCIS arising within a fibroadenoma. An analysis assessing 20-year mortality outcomes in patients with DCIS demonstrated no survival benefit to radiation, although it did reduce local recurrence risks significantly[12,13]. Relevant clinical studies could be designed but there are no recommendations in the current guidelines. In clinical practice, we may be more cautious in decisions about the treatment adopted for DCIS arising within a fibroadenoma. Referring to the recent guideline recommendations for common DCIS, age < 50 years was one of the recurrence risk factors. Therefore, the 2 patients received or will receive radiotherapy for treatment.
As recommended by National Comprehensive Cancer Network guidelines, DCIS patients are recommended routine genetics consultation. In addition to tumor type, age of cancer onset was also found to be a statistically significant indicator for germline referral[14]. The 2 cases involved in this report were both young patients, making genetic testing more valuable (i.e., it may find BRCA1/2 mutations, etc.). Regrettably, neither patient accepted this recommendation.
Both of the patients showed estrogen receptor positivity. They were administered endocrine therapy with tamoxifen accordingly. As recommended by the recent National Comprehensive Cancer Network guidelines, endocrine therapy is considered the risk-reduction treatment of the ipsilateral breast after surgery in patients with DCIS undergoing breast-conserving surgery, especially in patients with estrogen receptor-positive DCIS. Patients are treated with tamoxifen during the premenopausal period and with tamoxifen or an aromatase inhibitor during the postmenopausal period.
Breast DCIS arising within a fibroadenoma is rare. More sections are needed to reduce the missed rate. Complete follow-up data with preoperative imaging can help us make decisions during patient follow-up. Taken together, it remains uncertain whether a more conservative approach can be taken in the adjuvant setting for breast DCIS arising within a fibroadenoma.
The authors express special thanks to Dr Ke-Wang Sun who guided this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Menendez-Menendez J, Spain; Serrano Uson Junior PL, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Carty NJ, Carter C, Rubin C, Ravichandran D, Royle GT, Taylor I. Management of fibroadenoma of the breast. Ann R Coll Surg Engl. 1995;77:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 2. | Foster ME, Garrahan N, Williams S. Fibroadenoma of the breast: a clinical and pathological study. J R Coll Surg Edinb. 1988;33:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 3. | Fukuda M, Nagao K, Nishimura R, Matsuda M, Baba K, Ueno Y, Morinaga H, Omachi H, Hamada T. Carcinoma arising in fibroadenoma of the breast--a case report and review of the literature. Jpn J Surg. 1989;19:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Deschênes L, Jacob S, Fabia J, Christen A. Beware of breast fibroadenomas in middle-aged women. Can J Surg. 1985;28:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 5. | Dupont WD, Page DL, Parl FF, Vnencak-Jones CL, Plummer WD Jr, Rados MS, Schuyler PA. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 254] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Buzanowski-Konakry K, Harrison EG Jr, Payne WS. Lobular carcinoma arising in fibroadenoma of the breast. Cancer. 1975;35:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | El-Essawy M, Haidary A A, Khan A L. Ductal carcinoma in situ (DCIS) in breast fibroadenoma. Egypt J Radiol Nuc M. 2020;51:73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Ren Y, Li P, Yang Y, Huang Y, Zhan D, Wen B. Imaging findings of ductal carcinoma in situ arising within fibroadenoma. Breast J. 2020;26:1037-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Hirano M, Ueno E, Tanaka H, Aiyoshi Y, Soeda S. A case of ductal carcinoma arising in a fibroadenoma of the breast. J STAGE. 1991;52:1255-11258. [DOI] [Full Text] |
| 10. | Feliciano YZ, Freire R, Net J, Yepes M. Ductal and lobular carcinoma in situ arising within an enlarging biopsy proven fibroadenoma. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Diaz NM, Palmer JO, McDivitt RW. Carcinoma arising within fibroadenomas of the breast. A clinicopathologic study of 105 patients. Am J Clin Pathol. 1991;95:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Lui SA, Oh HB, Wang S, Chan CW. Ductal carcinoma in-situ arising within benign phyllodes tumours. Ann R Coll Surg Engl. 2018;100:e97-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol. 2015;1:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 373] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 14. | Vlessis K, Purington N, Chun N, Haraldsdottir S, Ford JM. Germline Testing for Patients With BRCA1/2 Mutations on Somatic Tumor Testing. JNCI Cancer Spectr. 2020;4:pkz095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |