Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3113
Peer-review started: December 20, 2021
First decision: January 18, 2022
Revised: February 6, 2022
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: April 6, 2022
Processing time: 99 Days and 1.2 Hours
Breast cancer is a malignant tumor with an unclear etiology and is the most common malignant tumor in women. Surgery is the main clinical treatment for breast cancer. Although traditional total mastectomy combined with axillary lymph node dissection is effective, it can result in shoulder dysfunction, especially in middle-aged and elderly patients with breast cancer with weak constitution and other underlying diseases. Furthermore, the postoperative quality of life is poor.
To assess breast-conserving surgery and sentinel lymph node biopsy for breast cancer treatment and their correlation with polyligand proteoglycan-1.
Overall, 80 patients with breast cancer treated in our hospital from January 2021 to July 2021 were retrospectively selected and divided into an observation group (n = 44) and control group (n = 36) according to the treatment plan. The observation group was treated with breast-conserving surgery and sentinel lymph node biopsy, and the control group was treated with total breast resection. Simultaneously, immunohistochemical staining was used to detect the expression of syndecan-1 (SDC-1) in the lesions, and its relationship with clinicopathological findings was analyzed.
Intraoperative blood loss, operation time, and hospital stay in the observation group were 65.51 ± 9.94 mL, 65.59 ± 9.40 min, and 14.80 ± 3.03 d, respectively, which were significantly lower than those in the control group (P < 0.05). The incidence of postoperative complications in the observation group was 11.36%, which was significantly lower than that in the control group (P < 0.05). The positive expression rate of SDC-1 in the observation group was 25.00%, and there was no significant difference between the groups (P > 0.05). The positive expression rate of SDC-1 in patients with American Joint Committee on Cancer (AJCC) stage II was 14.29%, which was significantly lower than that in patients with AJCC stage I (P < 0.05). The positive expression of SDC-1 had no significant relationship with age, course of disease, site, tissue type, and treatment plan (P > 0.05).
Breast preservation surgery and sentinel lymph node biopsy for breast cancer treatment have fewer complications and quicker recovery than those treated with total breast resection. Low SDC-1 expression in breast cancer lesions is related to AJCC staging.
Core Tip: Breast preservation and sentinel lymph node biopsy has shown to have a good treatment effect in patients with breast cancer, especially in older patients, as well as the significant advantage of guaranteed postoperative quality of life for patients. Polyligand proteoglycan-1 is used as a cell surface mucin polysaccharide, which is also a kind of biological macromolecular protein closely associated with tumorigenesis. In this study, we explored the relationship between breast cancer treatment and polyligand proteoglycan-1 through this study.
- Citation: Li FM, Xu DY, Xu Q, Yuan Y. Breast-conserving surgery and sentinel lymph node biopsy for breast cancer and their correlation with the expression of polyligand proteoglycan-1. World J Clin Cases 2022; 10(10): 3113-3120
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3113.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3113
In recent years, breast preservation and sentinel lymph node biopsy has shown to have a good treatment effect in patients with breast cancer, especially in older patients, as well as the significant advantage of guaranteed postoperative quality of life for patients[1-4]. The procedure is more popular in developed countries, but it has not been widely performed in China. Further exploration of the occurrence and developmental mechanism of breast cancer at the molecular level and biomarkers with high specificity and sensitivity can provide new therapeutic targets for the treatment of breast cancer[5]. Polyligand proteoglycan-1 [syndecan-1 (SDC-1)] is used as a cell surface mucin polysaccharide, which is also a kind of biological macromolecular protein closely associated with tumorigenesis; it participates in the regulation of various functions, including lymphocyte accumulation, immunomodulation, and cell matrix regulation, and is closely related to the progress of various malignant tumors[6].
The purpose of this study was to further confirm the effectiveness of breast preservation and sentinel lymph node biopsy and explore the efficacy of different treatment methods and their correlation with SDC-1 expression.
A total of 80 patients with breast cancer treated in our hospital from January 2021 to July 2021 were retrospectively selected. The inclusion criteria for patients were as follows: (1) confirmation of breast cancer by pathology; (2) classified according to American Joint Committee on Cancer (AJCC) stage I-II; (3) single lesion with a diameter < 3 cm and the distance from tumor edge to areola edge ≥ 2 cm; and (4) complete clinical follow-up data. The exclusion criteria were as follows: (1) preoperative anti-tumor therapy such as radiotherapy and chemotherapy; and (2) breast cancer combined with autoimmune diseases, blood system diseases, and other serious diseases. Patients were divided into an observation group (n = 44) and control group (n = 36) according to the treatment plan. A comparison of general clinical data between the two groups is shown in Table 1. This study was approved by the hospital ethics committee.
| Group | Cases | Age (yr) | Course of the disease (mo) | AJCC stage | Site | Tissue types | |||
| I | II | Outer quadrant | Non outer quadrant | Invasive ductal carcinoma | Others | ||||
| Observation group | 44 | 51.03 ± 12.11 | 11.06 ± 3.35 | 26 (59.09) | 18 (40.91) | 26 (59.09) | 18 (40.91) | 33 (75.00) | 11 (25.00) |
| Control group | 36 | 49.10 ± 11.45 | 10.42 ± 4.03 | 19 (52.78) | 17 (47.22) | 21 (58.33) | 15 (41.67) | 24 (66.67) | 12 (33.33) |
| t/χ2 | 0.727 | 0.776 | 0.321 | 0.878 | 0.671 | ||||
| P value | 0.470 | 0.440 | 0.571 | 0.349 | 0.413 | ||||
Total breast resection was performed in the control group. The patients were placed in supine position, under general anesthesia, with both breasts exposed and tumor boundaries marked. The nipple, areola, and skin and breast tissue 3 cm from the tumor edge were routinely removed. Axillary lymph nodes were dissected, and all lymph nodes from the leading edge of the latissimus dorsi muscle to the medial side of the pectoralis minor muscle were removed. Drainage tubes were placed after surgery and surgical incision was closed.
Breast preservation and sentinel lymph node biopsy was performed in the observation group. For breast preservation surgery, patients were administered general anesthesia, and 3 mL 1% methylene blue was injected subcutaneously into the areola. Normal tissues approximately 2 cm from the tumor were removed, and quick freezing was performed for pathological examination. If the margin was positive, total mastectomy was performed. If the results of margin examination were negative, breast preservation surgery was performed. For sentinel lymph node biopsy, an axillary and mammary fold were selected as surgical approaches, a longitudinal incision was made, subcutaneous tissue was cut, and the first blue staining lymph node was searched for in the lymphatic vessels. After the sentinel lymph node was removed, it was quickly frozen for pathological examination. If the test result was positive, axillary lymph node dissection was performed; if the test results were negative, the incision was closed, and the operation was completed.
The surgically resected tissue was fixed with 10% formaldehyde for 24 to 48 h and then embedded in paraffin. The paraffin sample was cut into 4 consecutive tissue sections with a thickness of approximately 4 mm and baked at 60℃ for 3 h. Conventional dewaxing was performed, 3% H2O2 was added for 10 min, and then incubated at room temperature. The samples were washed with phosphate-buffered saline (PBS) 3 times for 3 min each, repaired with citric acid solution, washed with PBS 3 times for 3 min each, and incubated overnight with the primary antibody (anti-SDC-1). Samples were washed with PBS 3 times for 3 min each, polymer enhancer was added, incubated at room temperature for 20 min, washed with PBS 3 times for 3 min each, enzyme-labeled anti-mouse polymer was added, and incubated at room temperature for 30 min. Samples were washed with diaminobenzidine chromogenic water, dyed with hematoxylin, and sealed by conventional dehydration. The immunohistochemistry kit was purchased from Beijing Zhongshan Biological Products Co., LTD (Beijing, China).
SPSS 22.0 software (IBM, Armonk, NY) was used for data analyses. Normally distributed data are expressed as means with standard deviations. The t-test was used for comparison between groups. Count data are expressed as n (%), and comparisons between groups was performed by χ2 test. The inspection level was set at α = 0.05.
Intraoperative blood loss, operation time, and hospital stay in the observation group were significantly lower than those of the control group (P < 0.05) (Table 2).
| Group | Cases | Intraoperative blood loss (mL) | Operation time (min) | Hospital stay (d) |
| Observation group | 44 | 65.51 ± 9.94 | 65.59 ± 9.40 | 14.80 ± 3.03 |
| Control group | 36 | 120.17 ± 13.35 | 105.51 ± 8.81 | 24.41 ± 3.80 |
| t | -20.977 | -19.435 | -12.587 | |
| P value | 0.000 | 0.000 | 0.000 |
The incidence of postoperative complications in the observation group was significantly lower than that of the control group (P < 0.05) (Table 3).
| Groups | Cases | Complication | ||||
| Hemorrhage | Effusion | Upper extremity edema | Other | Total | ||
| Observation group | 44 | 1 (2.27) | 2 (4.55) | 1 (2.27) | 1 (2.27) | 5 (11.36) |
| Control group | 36 | 3 (8.33) | 3 (8.33) | 6 (16.67) | 4 (11.11) | 16 (44.44) |
| χ2 | 11.192 | |||||
| P value | 0.000 | |||||
There was no significant difference in SDC-1 expression between groups (P > 0.05) (Table 4).
| Group | Cases | Positive expression rate of SDC-1 | χ2 | P value |
| Observation group | 44 | 11 (25.00) | 0.084 | 0.771 |
| Control group | 36 | 8 (22.22) |
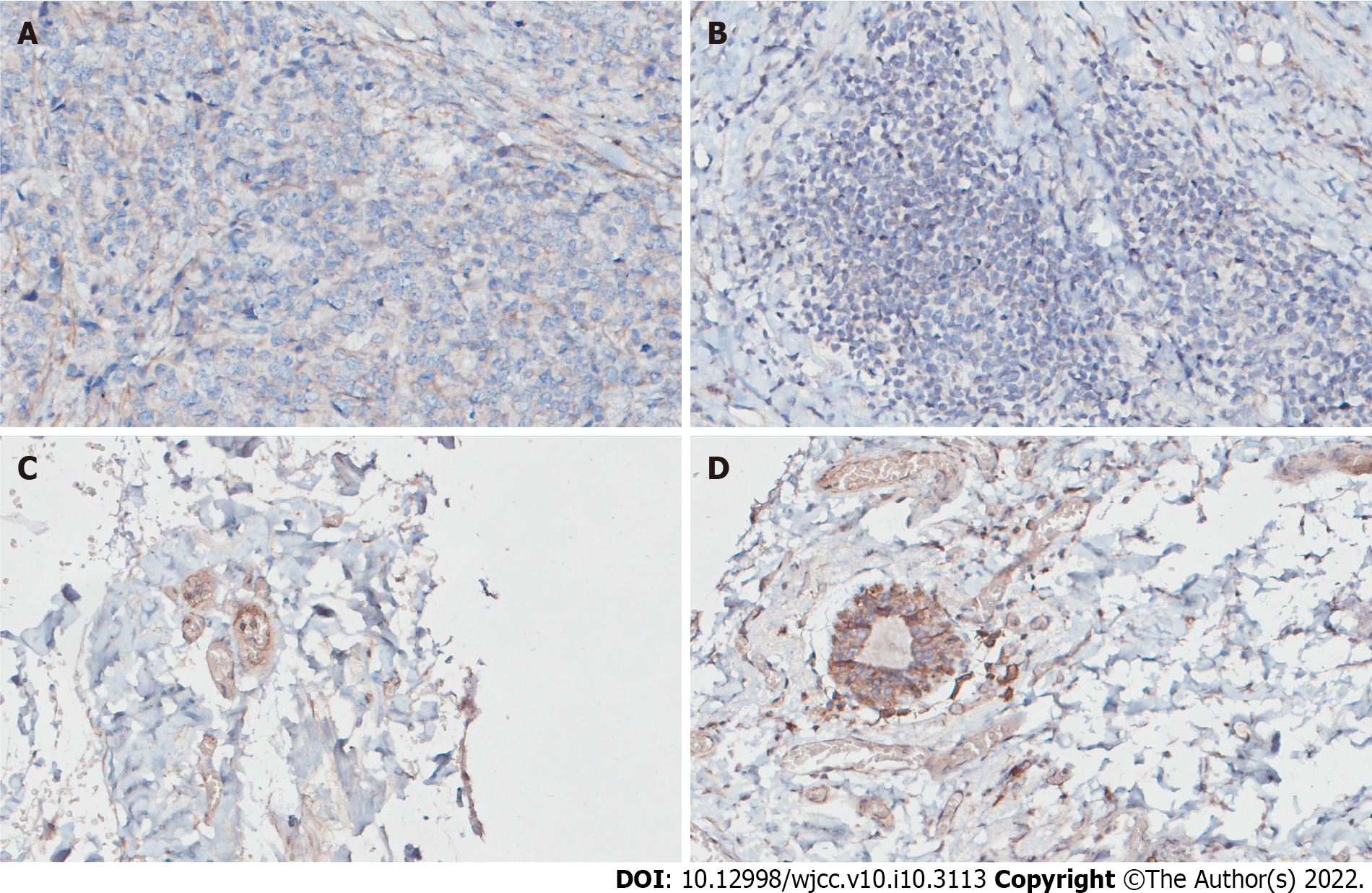
The positive expression rate of SDC-1 in patients with AJCC stage Ⅱ was 11.29%, which was significantly lower than that for AJCC stage I (P < 0.05). There was no relationship between SDC-1 positivity and age, disease course, site, tissue type, or treatment plan (P > 0.05) (Table 5, Figure 1).
| Clinicopathological features | Cases | Positive expression rate of SDC-1 | χ2 | P value |
| Age (yr) | 0.225 | 0.635 | ||
| < 50 | 30 | 8 (26.67) | ||
| ≥ 50 | 50 | 11 (22.00) | ||
| Course of the disease | 0.133 | 0.716 | ||
| < 12 mo | 35 | 9 (25.71) | ||
| ≥ 12 mo | 45 | 2 (5.71) | ||
| AJCC stage | 5.824 | 0.016 | ||
| I | 45 | 17 (37.78) | ||
| II | 35 | 5 (14.29) | ||
| Site | 0.008 | 0.931 | ||
| Outer quadrant | 47 | 11 (23.40) | ||
| Non outer quadrant | 33 | 8 (24.24) | ||
| Tissue type | 0.097 | 0.755 | ||
| Invasive ductal carcinoma | 57 | 13 (22.81) | ||
| Others | 23 | 6 (26.09) | ||
| Treatment plan | 0.084 | 0.771 | ||
| Breast conserving surgery plus sentinel lymph node biopsy | 44 | 11 (25.00) | ||
| Total mastectomy | 36 | 8 (22.22) |
Total mastectomy combined with axillary lymph node dissection is the most common treatment for patients with breast cancer, but it is poorly tolerated in some patients as there are many complications after surgery, which increase the risk of surgical site infection. Recent advances in medical and health technology have led to the development of breast-conserving surgery. After surgical resection of pathological tissues, sentinel lymph node biopsy should be performed on patients, and qualitative pathological examinations should be performed after lymphatic tissue resection. If the results are positive, mastectomy is still needed to prevent recurrence, but most patients with a negative pathological examination can achieve breast conservation[7-9].
The amount of intraoperative blood loss, operation time, and hospital stay in the observation group were significantly lower than those in the control group (P < 0.05). This may be because sentinel lymph node biopsy can predict lymph node metastasis and reduce axillary lymph node dissection treatment, thus slowing down the spread of cancer cells and improving the therapeutic effect. The focus of breast preservation surgery is to preserve the appearance and function of the patient’s breast, minimize the scope of surgical resection, and reduce intraoperative blood loss. As of 2021, the incidence of postoperative complications in the observation group was significantly lower than that in the control group (P < 0.05), and there was no significant difference in postoperative recurrence rate between the two groups, which is consistent with the results of previous studies[10]. Other studies[11-13] have confirmed that for patients with stage I-II breast cancer, combined breast preservation surgery and sentinel lymph node biopsy can reduce the occurrence of postoperative complications, accelerate recovery, contribute to anti-tumor immune function, and play a role in preventing the recurrence of advanced tumors. However, there was no significant difference in the postoperative recurrence rate between the two treatments in this study, which may be due to the small sample size.
As can be seen from the results of this study, the advantages of breast-conserving surgery combined with sentinel lymph node biopsy in the treatment of early breast cancer are evident in two aspects. First, preoperative sentinel lymph node biopsy can accurately determine whether axillary lymph node metastasis occurs. It is helpful to detect metastatic cancer cells early and take effective intervention measures in a timely manner to prevent the spread of cancer cells. Second, compared with radical surgery, breast-conserving surgery has a smaller resection area and causes less trauma to the body. Tumor metastasis can be controlled while preserving the shape of the patient’s breast as much as possible, resulting in high patient satisfaction.
SDC-1 is closely related to malignant tumors, can promote the occurrence and development of some tumors, and may promote the division and proliferation of tumor cells as well as tumor angiogenesis[14]. SDC-1 can be expressed on the surface of tumor cells from a variety of sources. After malignant transformation of a variety of tumors, SDC-1 expression on tumor cell membranes will also change[15]. SDC-1, a member of the transmembrane mucin family of adhesive molecule integrin, is mainly expressed in mammalian epithelial cells, activates corresponding cell signal transduction pathways, and exerts biological effects through a series of ligands including its own heparin sulfate side-chain and cell adhesion molecules, matrix components, growth factors, enzymes, and enzyme inhibitors[16,17]. The results of this study showed that there was no significant difference in the positive expression rate of SDC-1 between groups (P > 0.05), and the positive expression rate of SDC-1 in patients with AJCC stage II and recurrence was significantly lower than that in patients with AJCC stage I and without recurrence (P < 0.05). These results suggest that SDC-1 may be involved in the occurrence and development of breast cancer. SDC-1 may be closely related to the occurrence and development of breast cancer and the severity of disease development. This may be because it can promote angiogenesis around lesions and promote the further development of breast cancer through invasion and spreading, causing damage to adjacent tissues and organs. High expression of SDC-1 and angiogenesis is the key to the success of lesion transplant factors, which play an important role in the pathogenesis of breast cancer. Therefore, SDC-1 can be considered as a new diagnostic and treatment indicator of mammary cancer, which needs to be further studied.
In recent years, the incidence of breast cancer has increased significantly, and its clinical treatment is more complicated. Although individualized and standardized treatment strategies have been widely advocated, problems such as a high recurrence rate, many complications, and unsatisfactory long-term efficacy still exist[18-20]. Therefore, new methods of diagnosis and treatment are urgently needed. Currently, the relationship between SDC-1 expression and patients with breast cancer before and after surgery has not been indicated in clinical studies. In this study, breast preservation surgery and sentinel lymph node biopsy were performed on patients, immunohistochemistry was used to detect the positive expression of SDC-1, and the relationship between SDC-1 and clinicopathological factors of breast cancer was analyzed. These findings can provide a new basis for early diagnosis, treatment, effective control of the recurrence of breast cancer, and relevant reference values.
Breast preservation and sentinel lymph node biopsy for the treatment of breast cancer has a good clinical effect, including fewer complications and quicker recovery than total breast resection. Low SDC-1 expression in breast cancer lesions is related to AJCC staging.
Breast cancer is a malignant tumor with an unclear etiology and is the most common malignant tumor in women.
Polyligand proteoglycan-1 [syndecan-1 (SDC-1)] participates in the regulation of various functions, including lymphocyte accumulation, immunomodulation, and cell matrix regulation, and is closely related to the progress of various malignant tumors.
We explored the efficacy of different treatment methods and their correlation with SDC-1 expression.
We selected 80 patients with breast cancer and divided into an observation group (n = 44) and control group (n = 36) according to the treatment plan.
The positive expression rate of SDC-1 in patients with American Joint Committee on Cancer (AJCC) stage II was 11.29%, which was significantly lower than that for AJCC stage I.
Low SDC-1 expression in breast cancer lesions is related to AJCC staging.
We need further multicenter studies with large samples to confirm this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mazurek A, Prat A S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Sun SX, Moseley TW, Kuerer HM, Yang WT. Imaging-Based Approach to Axillary Lymph Node Staging and Sentinel Lymph Node Biopsy in Patients With Breast Cancer. AJR Am J Roentgenol. 2020;214:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Mok CW, Tan SM, Zheng Q, Shi L. Network meta-analysis of novel and conventional sentinel lymph node biopsy techniques in breast cancer. BJS Open. 2019;3:445-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Cykowska A, Marano L, D'Ignazio A, Marrelli D, Swierblewski M, Jaskiewicz J, Roviello F, Polom K. New technologies in breast cancer sentinel lymph node biopsy; from the current gold standard to artificial intelligence. Surg Oncol. 2020;34:324-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Shojaee L, Abedinnegad S, Nafisi N, Naghshvar F, Godazandeh G, Moradi S, Shakeri Astani K, Godazandeh Y. Sentinel Node Biopsy in Early Breast Cancer Patients with Palpable Axillary Node. Asian Pac J Cancer Prev. 2020;21:1631-1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Cui Q, Dai L, Li J, Xue J. Accuracy of CEUS-guided sentinel lymph node biopsy in early-stage breast cancer: a study review and meta-analysis. World J Surg Oncol. 2020;18:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Parimon T, Yao C, Habiel DM, Ge L, Bora SA, Brauer R, Evans CM, Xie T, Alonso-Valenteen F, Medina-Kauwe LK, Jiang D, Noble PW, Hogaboam CM, Deng N, Burgy O, Antes TJ, Königshoff M, Stripp BR, Gharib SA, Chen P. Syndecan-1 promotes lung fibrosis by regulating epithelial reprogramming through extracellular vesicles. JCI Insight. 2019;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Man V, Wong TT, Co M, Suen D, Kwong A. Sentinel Lymph Node Biopsy in Early Breast Cancer: Magnetic Tracer as the Only Localizing Agent. World J Surg. 2019;43:1991-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Garcia-Etienne CA, Ferrari A, Della Valle A, Lucioni M, Ferraris E, Di Giulio G, Squillace L, Bonzano E, Lasagna A, Rizzo G, Tancredi R, Scotti Foglieni A, Dionigi F, Grasso M, Arbustini E, Cavenaghi G, Pedrazzoli P, Filippi AR, Dionigi P, Sgarella A. Management of the axilla in patients with breast cancer and positive sentinel lymph node biopsy: An evidence-based update in a European breast center. Eur J Surg Oncol. 2020;46:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Sávolt Á, Cserni G, Lázár G, Maráz R, Kelemen P, Kovács E, Győrffy B, Udvarhelyi N, Vörös A, Ormándi K, Mátrai Z. Sentinel lymph node biopsy following previous axillary surgery in recurrent breast cancer. Eur J Surg Oncol. 2019;45:1835-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Zhang J, Wang T, Yan C, Huang M, Fan Z, Ling R. Clinical Practice Status of Sentinel Lymph Node Biopsy for Early-Stage Breast Cancer Patients in China: A Multicenter Study. Clin Epidemiol. 2020;12:917-924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Giuliano AE. The evolution of sentinel node biopsy for breast cancer: Personal experience. Breast J. 2020;26:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Fregatti P, Gipponi M, Diaz R, DE Rosa R, Murelli F, Depaoli F, Pitto F, Baldelli I, Zoppoli G, Ceppi M, Friedman D. The Role of Sentinel Lymph Node Biopsy in Patients With B5c Breast Cancer Diagnosis. In Vivo. 2020;34:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Falco M, Masojć B, Kram A. Locoregional relapse is a strong prognostic indicator of distant metastatic progression in breast cancer patients after negative sentinel lymph node biopsy. Breast J. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Yao W, Rose JL, Wang W, Seth S, Jiang H, Taguchi A, Liu J, Yan L, Kapoor A, Hou P, Chen Z, Wang Q, Nezi L, Xu Z, Yao J, Hu B, Pettazzoni PF, Ho IL, Feng N, Ramamoorthy V, Jiang S, Deng P, Ma GJ, Den P, Tan Z, Zhang SX, Wang H, Wang YA, Deem AK, Fleming JB, Carugo A, Heffernan TP, Maitra A, Viale A, Ying H, Hanash S, DePinho RA, Draetta GF. Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer. Nature. 2019;568:410-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 15. | Alghandour R, Ebrahim MA, Ghazy H, Shamaa S, Emarah Z, Al-Gayyar MM. Evaluation of the Diagnostic and Prognostic Value of Syndecan-1 in Acute Leukemia Patients. Cureus. 2020;12:e10594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Szarvas T, Sevcenco S, Módos O, Keresztes D, Nyirády P, Kubik A, Romics M, Kovalszky I, Reis H, Hadaschik B, Shariat SF, Kramer G. Circulating syndecan-1 is associated with chemotherapy-resistance in castration-resistant prostate cancer. Urol Oncol. 2018;36:312.e9-312.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Qiao W, Liu H, Guo W, Li P, Deng M. Prognostic and clinical significance of syndecan-1 expression in breast cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2019;45:1132-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Yoon CI, Ahn SG, Kim D, Choi JE, Bae SJ, Cha CH, Park S, Jeong J. Repeat Sentinel Lymph Node Biopsy for Ipsilateral Breast Tumor Recurrence After Breast Conserving Surgery With Sentinel Lymph Node Biopsy: Pooled Analysis Using Data From a Systematic Review and Two Institutions. Front Oncol. 2020;10:518568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Lovasik BP, Seidel RL, Novello M, Torres MA, Losken A, Rizzo M. Single incision for oncologic breast conserving surgery and sentinel node biopsy in early stage breast cancer: A minimally invasive approach. Breast J. 2019;25:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Okur O, Sagiroglu J, Kir G, Bulut N, Alimoglu O. Diagnostic accuracy of sentinel lymph node biopsy in determining the axillary lymph node metastasis. J Cancer Res Ther. 2020;16:1265-1268. [PubMed] |