Copyright
©The Author(s) 2020.
World J Clin Cases. Jun 26, 2020; 8(12): 2448-2463
Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2448
Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2448
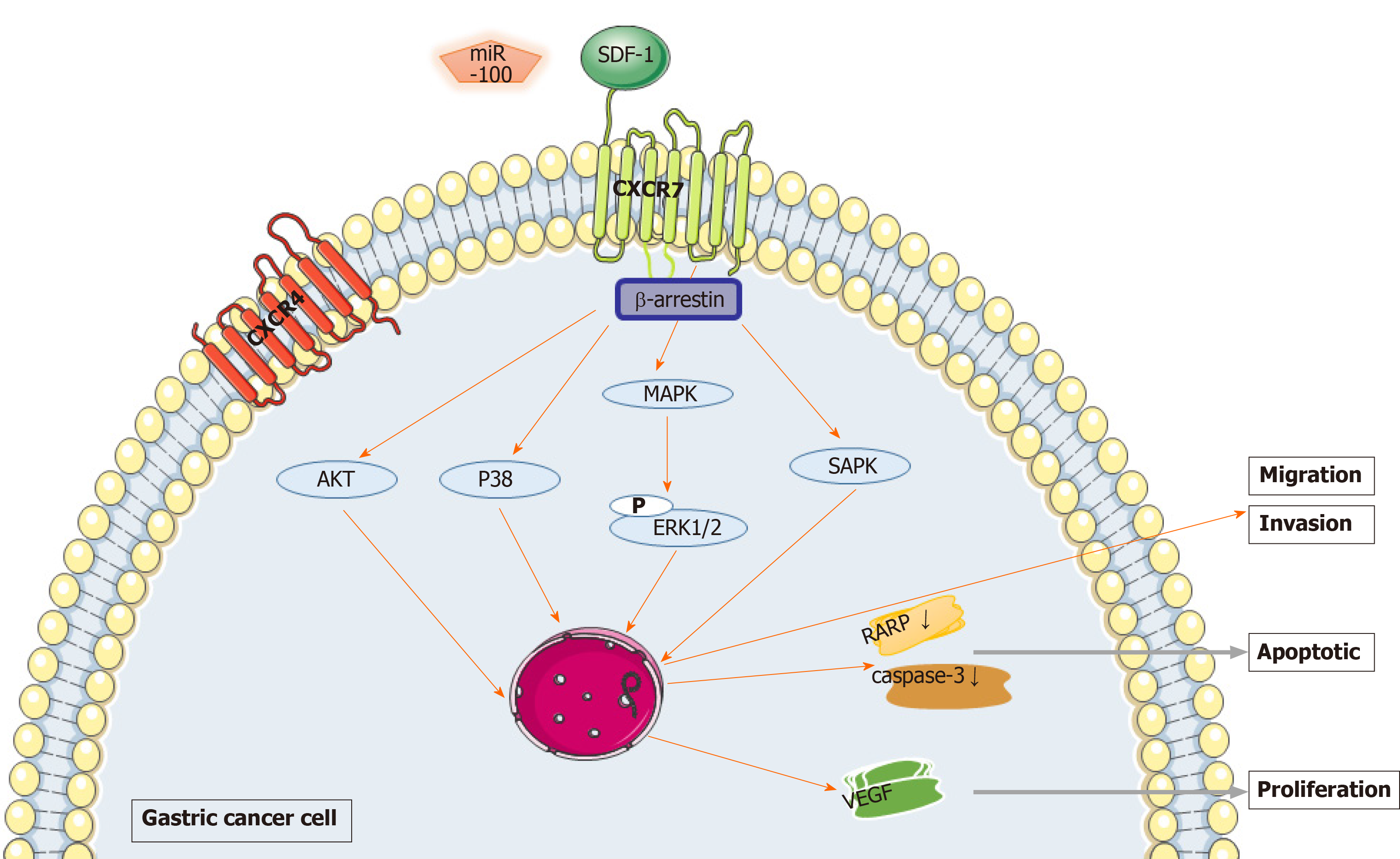
Figure 1 Mechanism of chemokine (C-X-C motif) receptor 7 promoting the growth and metastasis of gastric cancer.
Chemokine (C-X-C motif) receptor 7 (CXCR7) affects disease progression by stimulating proliferation, invasion, migration and adhesion of gastric cancer through β-arrestin dependent downstream signaling, including also known as Protein Kinase B, extracellular regulated protein kinases 1/2, p38 mitogen-activated protein kinases, just another kinase 2/ serial advanced technology attachment 3/ cellular-myelocytomatosis, and stress-activated protein kinase pathways by combining with the stromal cell-derived factor-1. CXCR7 inhibits the processing of poly ADP-ribose polymerase and caspase-3 and induces an anti-apoptotic effect. Stromal cell-derived factor-1 promotes the secretion of vascular endothelial growth factor in gastric cancer cells by binding CXCR7, thus causing angiogenesis in gastric cancer. CXCR7: Chemokine (C-X-C motif) receptor 7; Akt: Also known as Protein Kinase B, PKB; ERK1/2: Extracellular regulated protein kinases 1/2; MAPK: Mitogen-activated protein kinases; JAK2: Just another kinase 2; STAT3: Serial advanced technology attachment 3; c-Myc: Cellular-myelocytomatosis; SAPK: Stress-activated protein kinase; SDF-1: Stromal cell-derived factor-1; PARP: Poly ADP-ribose polymerase; VEGF: Vascular endothelial growth factor.
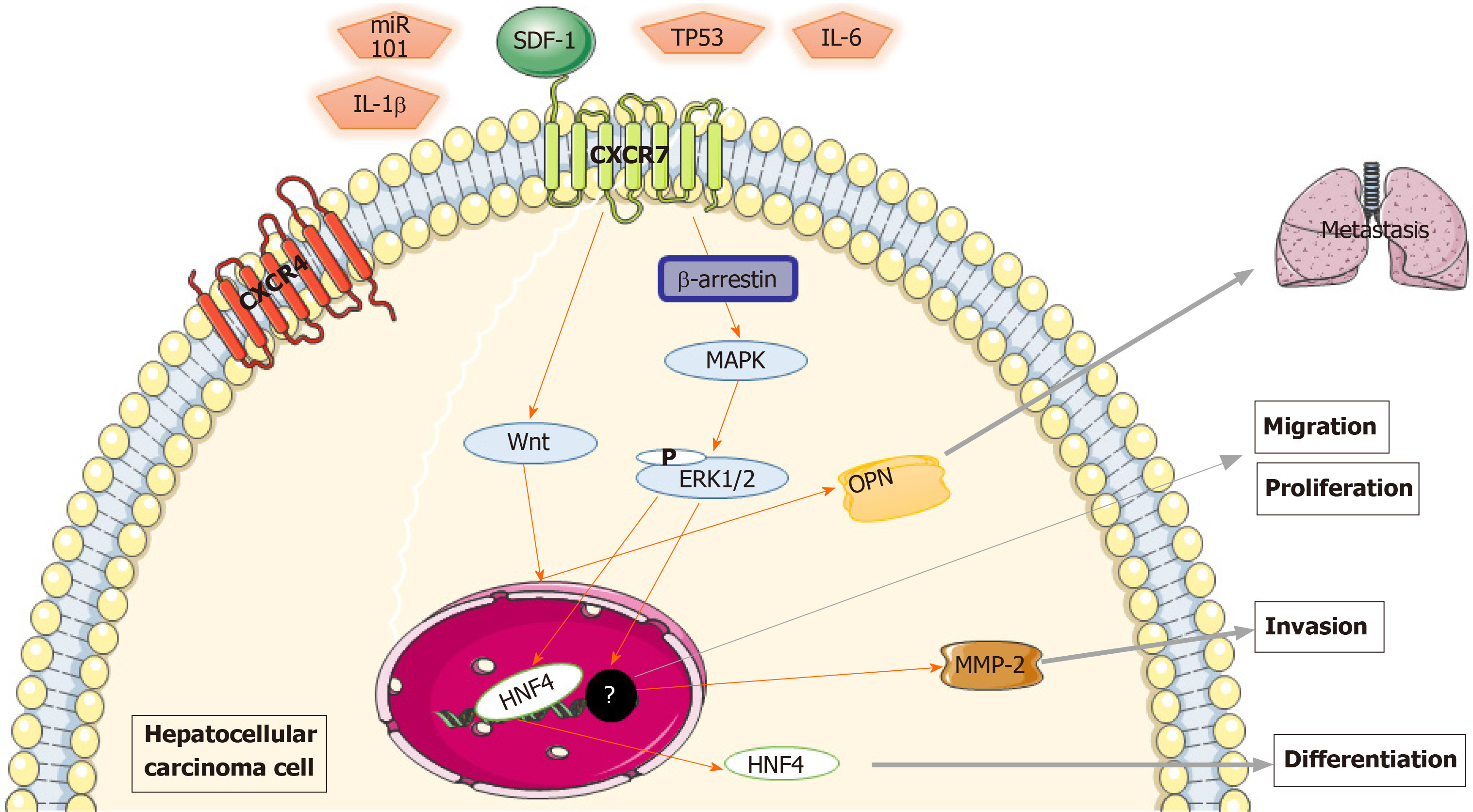
Figure 2 Mechanism of chemokine (C-X-C motif) receptor 7 promoting the growth and metastasis of hepatocellular carcinoma.
Chemokine (C-X-C motif) receptor 7 (CXCR7) contributed to hepatocellular carcinoma growth, including extracellular regulated protein kinases 1/2, and invasiveness via the promotion of matrix metalloproteinase 2 expression; CXCR7- mitogen-activated protein kinases-hepatocyte nuclear factor 4α cascade is the general pathway in the differentiation of hepatocellular carcinomas. Activated transmembrane CXCR7 activates the Wnt signaling pathway through β-arrestin, causing osteopontin expression, which in turn stimulates the ability of tumor cells to metastasize. Several regulators, including tumor protein 53, interleukin-6 and interleukin-1β, are potential upstream regulators of CXCR7-induced signaling. CXCR7: Chemokine (C-X-C motif) receptor 7; ERK1/2: Extracellular regulated protein kinases 1/2; MMP-2: Matrix metalloproteinase 2; MAPK: Mitogen-activated protein kinases; HNF4α: Hepatocyte nuclear factor 4α; OPN: Osteopontin; TP53: Tumor protein 53; IL-6: Interleukin-6; IL-1β: Interleukin-1β.
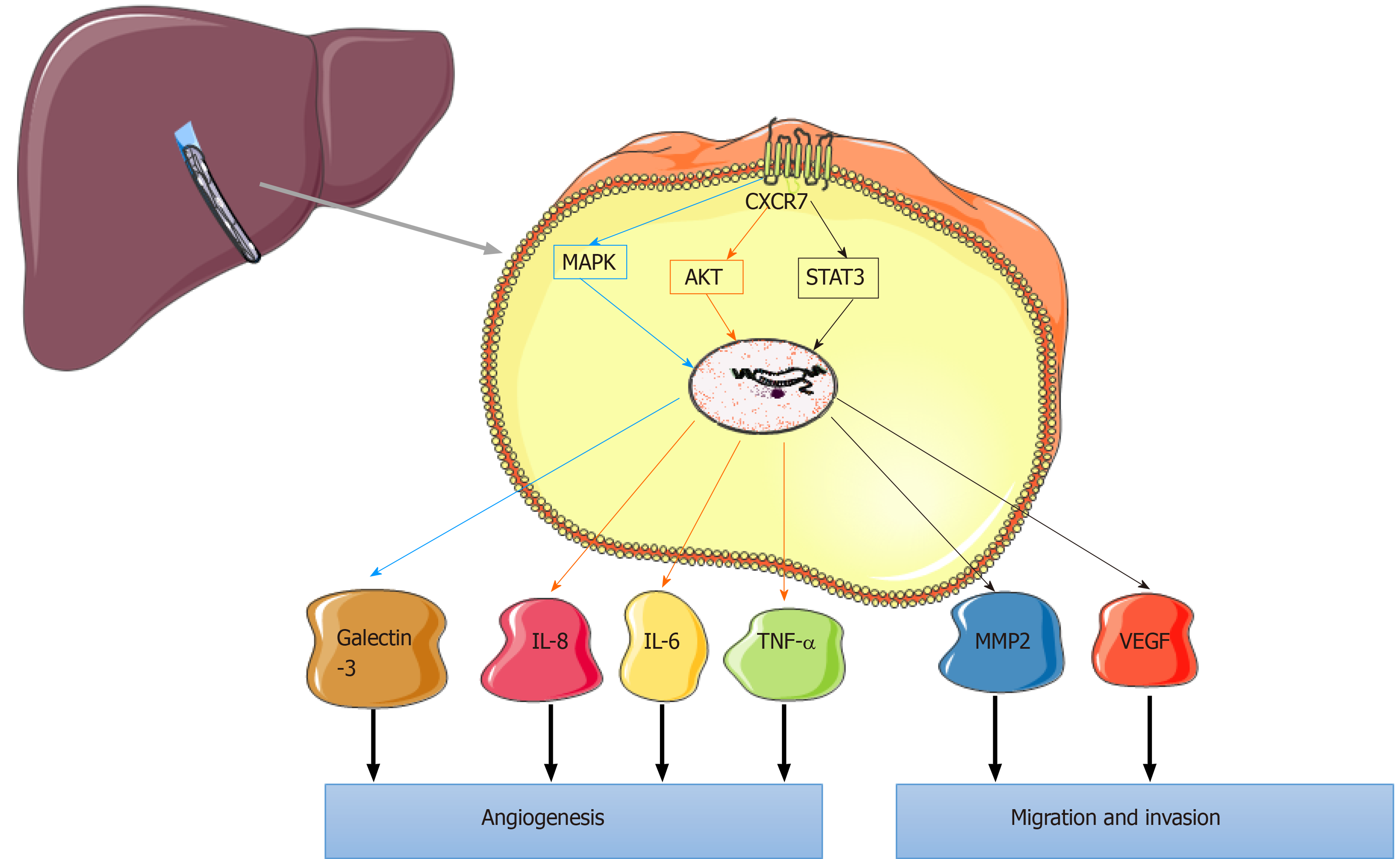
Figure 3 Mechanism of chemokine (C-X-C motif) receptor 7 in angiogenesis of hepatocellular carcinoma.
Chemokine (C-X-C motif) receptor 7 (CXCR7) activation promotes angiogenesis by regulating vascular endothelial growth factor and galectin-3 in hepatocellular carcinoma via the mitogen-activated protein kinases signaling pathway. This process occurs through activating the also known as Protein Kinase B signaling pathway, and the expression of also known as Protein Kinase B target proteins, including human tumor necrosis factor-alpha, interleukin-6, and interleukin-8, is also induced by CXCR7; CXCR7 can promote tumor endothelial cells migration and invasion in hepatocellular carcinomas by phosphorylated serial advanced technology attachment 3 at Tyr705 to influence the signaling pathway and its downstream genes matrix metalloproteinase 2 and vascular endothelial growth factor. CXCR7: Chemokine (C-X-C motif) receptor 7; VEGF: Vascular endothelial growth factor; MAPK: Mitogen-activated protein kinases; Akt: Also known as Protein Kinase B, PKB; TNFα: Human tumor necrosis factor-alpha; IL-6: Interleukin-6; IL-8: Interleukin-8; TEC: Tumor endothelial cells; STAT3: Serial advanced technology attachment 3; MMP-2: Matrix metalloproteinase 2; VEGF: Vascular endothelial growth factor.
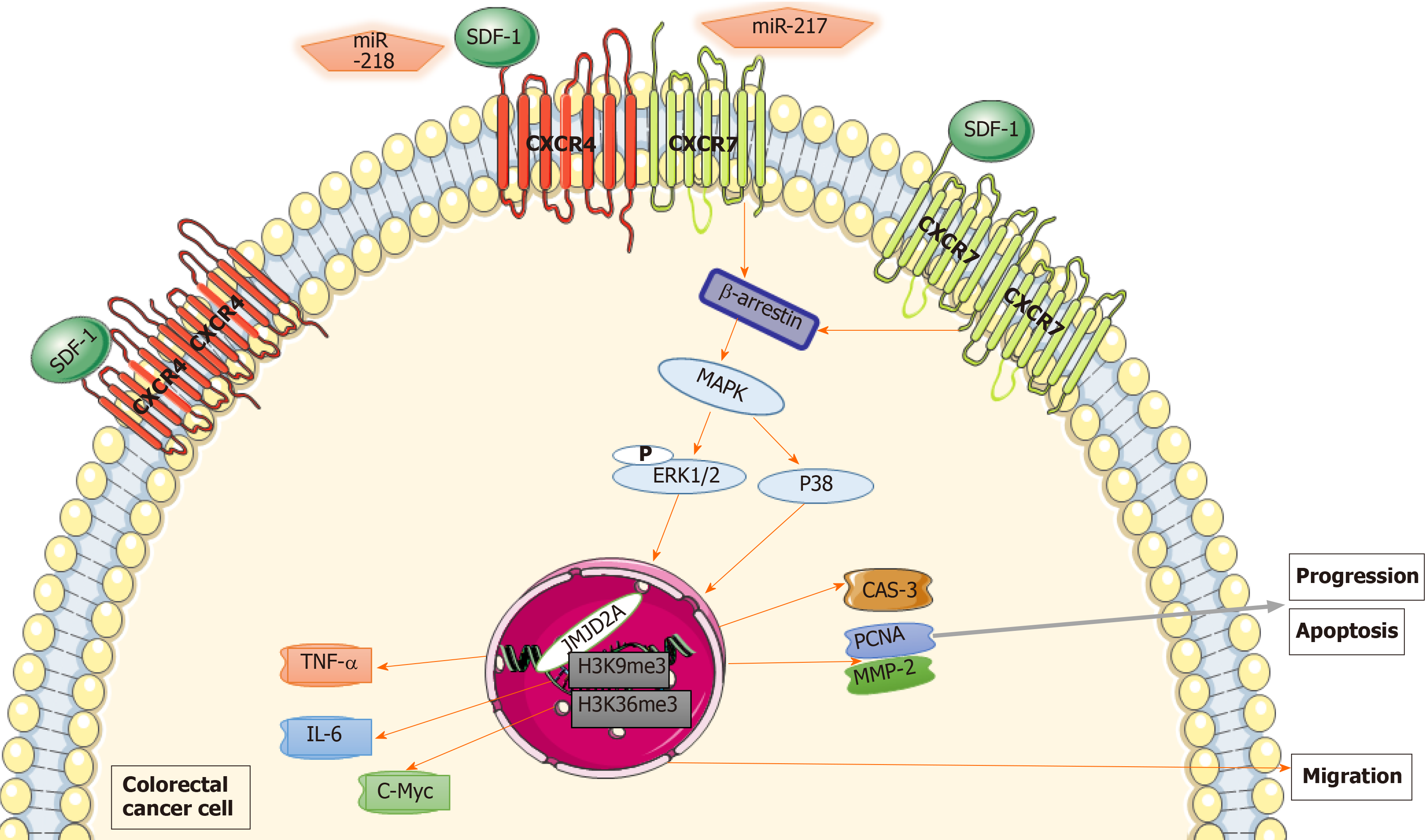
Figure 4 Mechanism of chemokine (C-X-C motif) receptor 7 promoting colorectal cancer growth and metastasis.
Chemokine (C-X-C motif) receptor 7 (CXCR7) gene induced cell anti-apoptosis and promoted colorectal cancer progression in colorectal cancer cells through extracellular regulated protein kinases 1/2 and β-arrestin pathway-mediated regulation of proliferating cell nuclear antigen, matrix metalloproteinase 2 and caspase-3 expression. miR-217, miR-218 and their targets may be regulatory molecules of Chemokine (C-X-C motif) receptor 4 (CXCR4) or CXCR7. The CXCR7/CXCR4 heterodimer translocated much more β-arrestin 1 to the nucleus and then activated histone lysine demethylase to regulate JMJD2A expression via multiprotein complexes, selectively enriching the promoters of human tumor necrosis factor-alpha, interleukin-6, and cellular-myelocytomatosis, which might facilitate the recruitment of histone demethylases, resulting in enhanced local histone demethylation and transcription of these genes. The CXCR7/CXCR4 heterodimer induced high levels of JMJD2A, leading to demethylation of histones H3K9me3 and H3K36me3 compared with the CXCR7 monomer and CXCR4 monomer. CXCR7: Chemokine (C-X-C motif) receptor 7; ERK1/2: Extracellular regulated protein kinases 1/2; PCNA: Proliferating cell nuclear antigen; MMP-2: Matrix metalloproteinase 2; CAS-3: Caspase-3; CXCR4: Chemokine (C-X-C motif) receptor 4; TNFα: Human tumor necrosis factor-alpha; IL-6: Interleukin-6; c-Myc: Cellular-myelocytomatosis.
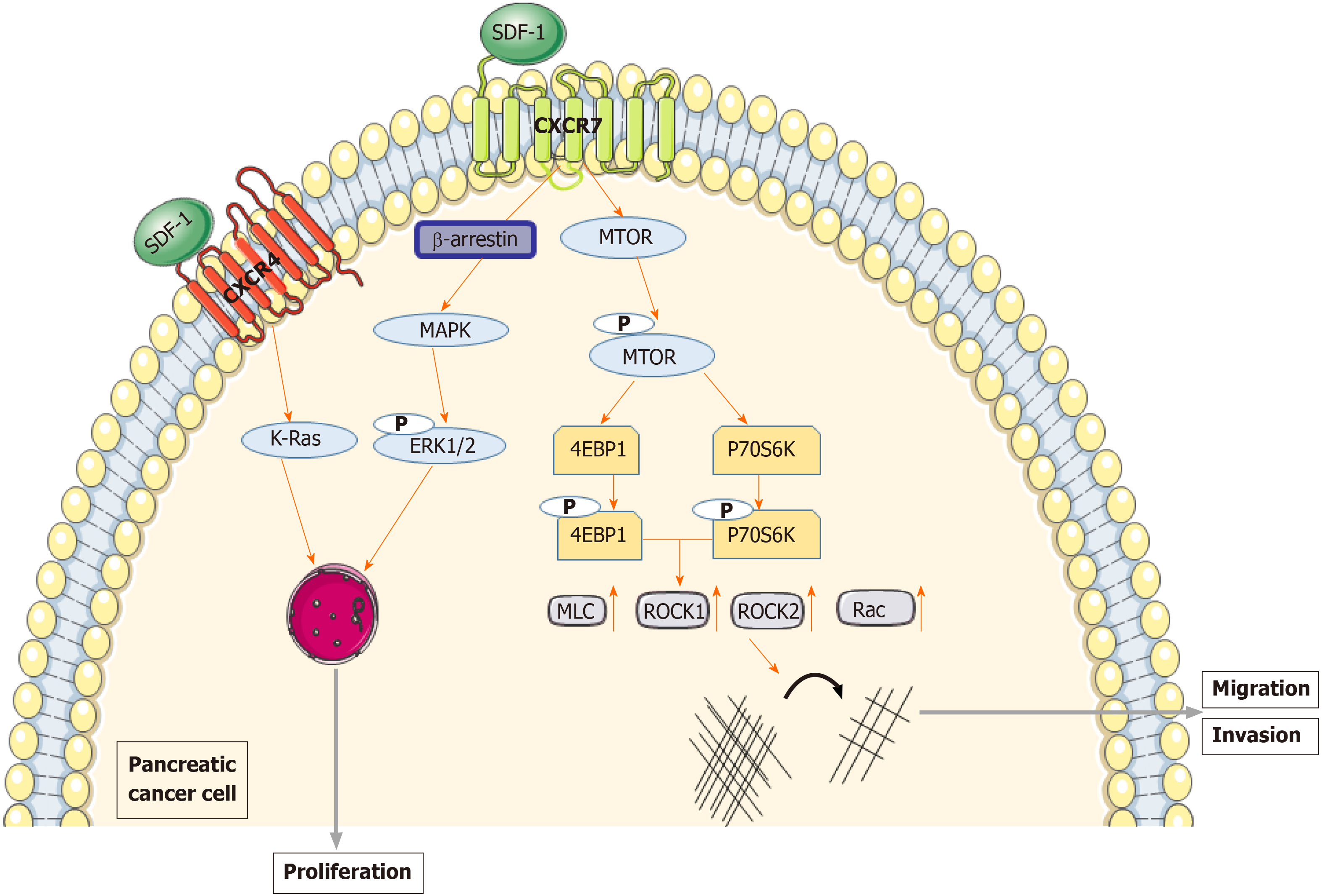
Figure 5 Mechanism of chemokine (C-X-C motif) receptor 7 promoting the growth and metastasis of pancreatic cancer.
After chemokine (C-X-C motif) receptor 7 and stromal cell-derived factor-1 activation, β-arrestin 2 induces extracellular regulated protein kinases 1/2 phosphorylation, leading to pancreatic cancer cell proliferation. Chemokine (C-X-C motif) receptor 7 binds to stromal cell-derived factor-1 to enhance the migration and invasion of pancreatic cancer cells by activating the mammalian target of rapamycin and Rho/Rho associated coiled-coil forming protein kinase signaling pathways. CXCR7: Chemokine (C-X-C motif) receptor 7; SDF-1: Stromal cell-derived factor-1; ERK1/2: Extracellular regulated protein kinases 1/2; mTOR: Mammalian target of rapamycin; Rho/ROCK: Rho/Rho associated coiled-coil forming protein kinase.
- Citation: Xin Q, Sun Q, Zhang CS, Zhang Q, Li CJ. Functions and mechanisms of chemokine receptor 7 in tumors of the digestive system. World J Clin Cases 2020; 8(12): 2448-2463
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2448.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2448