Copyright
©The Author(s) 2022.
World J Clin Cases. Apr 16, 2022; 10(11): 3601-3608
Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3601
Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3601
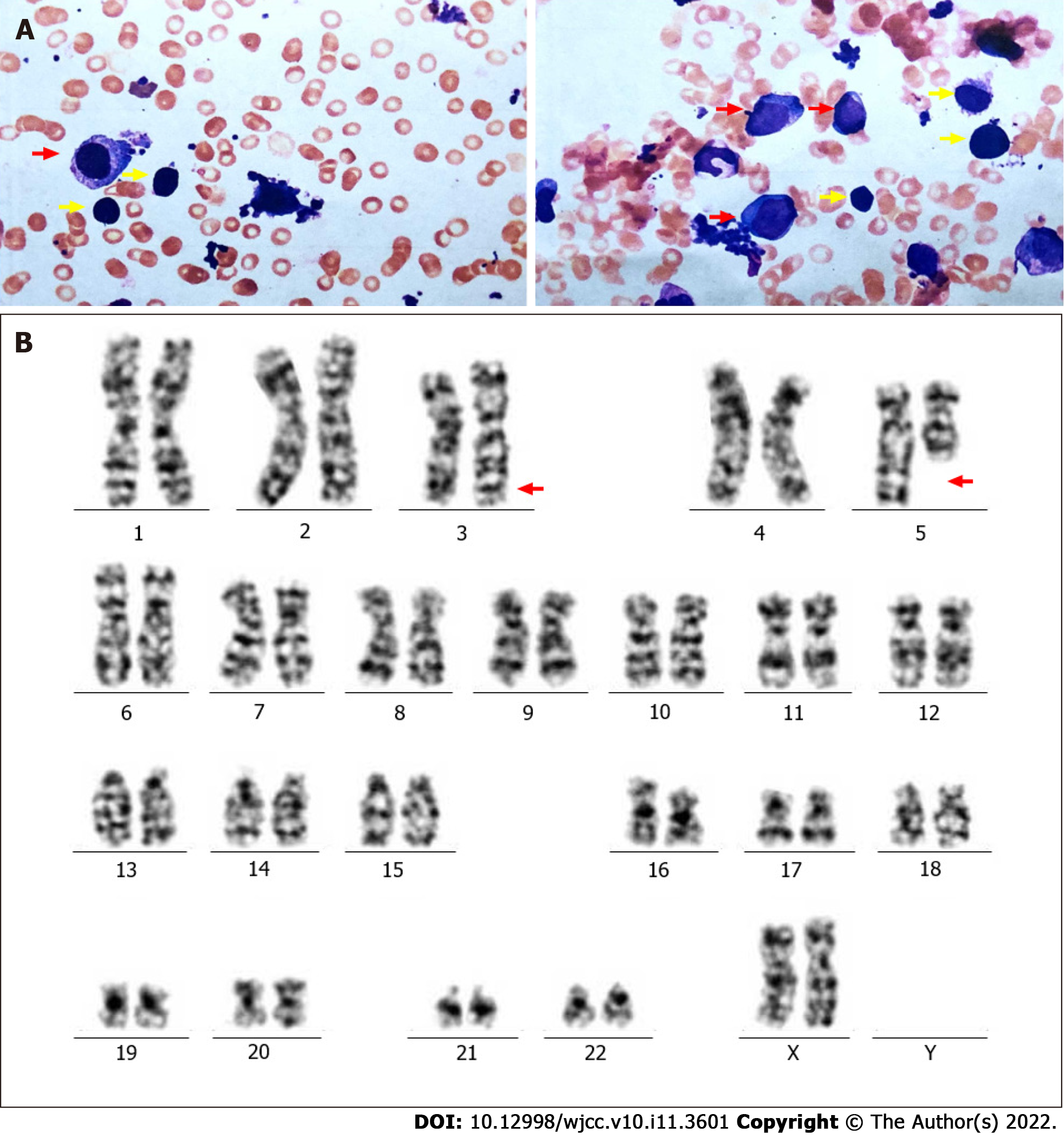
Figure 1 Bone marrow aspiration biopsy, karyotype and fluorescence in situ hybridization assay at first diagnosis.
A: Representative image of May Grunwald-Giemsa staining of bone marrow specimen. It is showed that more than 5% blast cell and more than 10% abnormal megakaryocytes, including unicellular megakaryocytes (panel A red arrow) and lymphatic-like small megakaryocytes (yellow arrow). B: EGR(5q31) gene was detected by fluorescence in situ hybridization. Each signal mode was detected as follows :2G1R 50%,2G2R 50%. EGR(5q31) probe was labeled with red fluorescence, D5S23 and D5S721(5p15.2) probe was labeled with green fluorescence. C: G-band bone marrow karyotype. Arrows indicate the del(5)(q13q31).
Figure 2 Bone marrow aspiration biopsy and karyotype analysis after lenalidomide resistances.
A: Bone marrow aspiration smear showing blast cell counted 16% and Hypercellularity with marked myeloid and erythroid hypoplasia. Abnormal megakaryocytes including unicellular megakaryocytes (red arrow) and lymphatic-like small megakaryocytes (yellow arrow) could still be observed. B: karyotype analysis depicting 46, XX, inv(3)(q21q26),del(5)(q13q31) (Red arrow).
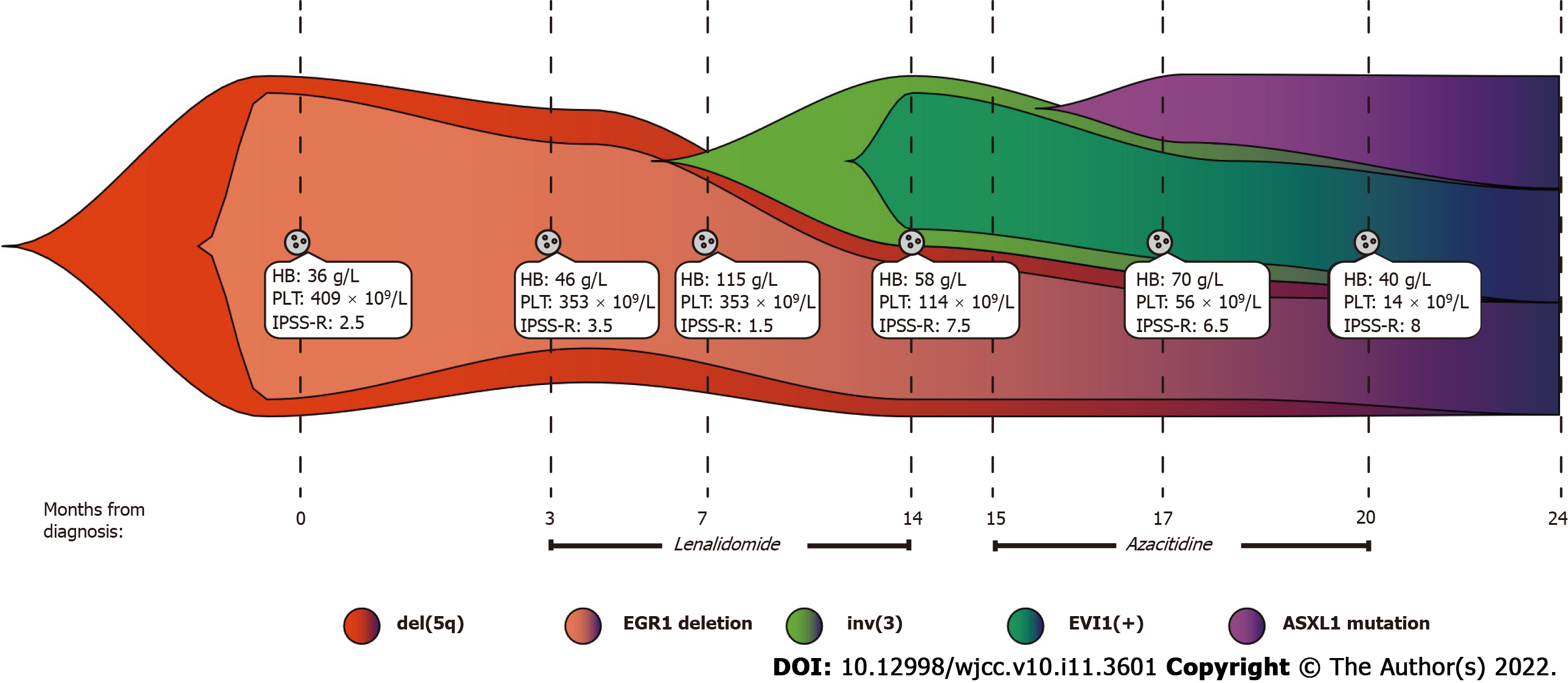
Figure 3 Clonal evolution architecture of the patient.
The patient was initially found to have del(5q) with EGR1 gene deletion, so she was diagnosed with MDS (low risk). Following lenalidomide treatment, the patient developed inv(3) with overexpression of EVI1. At that time, the patient's diagnosis was revised to MDS (very high risk). In addition, the patient also had ASXL1 mutations. The revised international prognostic scoring system (IPSS-R) for myelodysplastic syndrome was calculated according to a previously reported method[24], and the details can be found in Supplementary Table 1. HB: Haemoglobin; PLT: Platelet; IPSS-R: Revised International Prognostic Scoring System.
- Citation: Liang HP, Luo XC, Zhang YL, Liu B. Del(5q) and inv(3) in myelodysplastic syndrome: A rare case report. World J Clin Cases 2022; 10(11): 3601-3608
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3601.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3601