Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1258
Peer-review started: May 20, 2021
First decision: June 22, 2021
Revised: July 1, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: October 27, 2021
Processing time: 158 Days and 20.1 Hours
Deep vein thrombosis (DVT) may cause pulmonary embolus, leading to late deaths. The systemic inflammatory and hypercoagulable state of moderate and severe acute pancreatitis (non-mild acute pancreatitis, NMAP) patients may contribute to the development of venous thromboembolism. Accurate prediction of DVT is conducive to clinical decisions.
To develop and validate a potential new prediction nomogram model for the occurrence of DVT in NMAP.
NMAP patient admission between 2013.1.1 and 2018.12.31 at the West China Hospital of Sichuan University was collected. A total of 220 patients formed the training set for nomogram development, and a validation set was constructed using bootstrapping with 100 resamplings. Univariate and multivariate logistic regression analyses were used to estimate independent risk factors associated with DVT. The independent risk factors were included in the nomogram. The accuracy and utility of the nomogram were evaluated by calibration curve and decision curve analysis, respectively.
A total of 220 NMAP patients over 60 years old were enrolled for this analysis. DVT was detected in 80 (36.4%) patients. The final nomogram included age, sex, surgery times, D-dimer, neutrophils, any organ failure, blood culture, and classification. This model achieved good concordance indexes of 0.827 (95%CI: 0.769-0.885) and 0.803 (95%CI: 0.743-0.860) in the training and validation sets, respectively.
We developed and validated a prediction nomogram model for DVT in older patients with NMAP. This may help guide doctors in making sound decisions regarding the administration of DVT prophylaxis.
Core Tip: Deep vein thrombosis (DVT) may cause pulmonary embolus, leading to late death. Few studies have focused on DVT in moderate and severe acute pancreatitis. We identified eight predictors and developed and established a prediction nomogram model for DVT in older patients with moderate and severe acute pancreatitis. This model achieved good concordance indexes and may help guide doctors in the administration of DVT prophylaxis.
- Citation: Yang DJ, Li M, Yue C, Hu WM, Lu HM. Development and validation of a prediction model for deep vein thrombosis in older non-mild acute pancreatitis patients. World J Gastrointest Surg 2021; 13(10): 1258-1266
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1258.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1258
Acute pancreatitis (AP) is a common and potentially lethal disease with a rising incidence. The incidence of AP is 34 cases per 100,000 people in the general population per year worldwide[1]. Among gastrointestinal diseases, AP is one of the most common reasons for hospitalization in the United States, and the disease accounts for $2.6 billion health care dollars per year[2-4]. According to the 2012 Atlanta classification, most AP patients have mild acute pancreatitis. However, 20% of patients develop moderate or severe acute pancreatitis (non-mild acute pancreatitis, NMAP). Furthermore, the mortality of NMAP can reach 35%, which is significantly higher than that of mild acute pancreatitis[5]. Researchers usually focus on complications such as organ failure and infection in NMAP[6,7]. However, few of studies have paid attention to venous thromboembolism in NMAP. A previous study showed that the incidence of venous thromboembolism in hospitalized patients was approximately 0.4% to 1.3%[8]. NMAP usually requires a long hospital stay. The systemic inflammatory and hypercoagulable state of NMAP patients may contribute to the development of venous thromboembolism[9-11]. Deep vein thrombosis (DVT), a kind of venous thromboembolism, commonly develops in the lower extremities. It can cause acute pulmonary embolism (PE) when it falls and flows to the lung[12,13]. A recent study showed that the rate of DVT in AP patients could reach 38%[14]. Older patients more easily develop venous thromboembolism. This may increase the difficulty of treatment in older NMAP patients. However, there is a lack of a scoring model for predicting develop of DVT in NMAP patients. The existing scores for DVT are not suitable for critically ill patients[15-17]. In the past, nomograms were used as a graphical calculation to help solve engineering problems. As a statistical tool, nomograms have a unique advantage in visualizing the relationships of involved parameters. This approach enables users to calculate the overall probability of clinical outcome for an individual patient[18,19]. Recently, it has been widely used in clinical prediction models[20,21]. Thus, the aim of this study was to develop a prediction model for DVT in older NMAP patients.
Medical records of older NMAP patients admitted to West China Hospital from 2013.1.1 to 2018.12.31 were retrospectively collected. Included criteria were as follows: 1. AP was diagnosed in West China Hospital and classified as moderate or severe; 2. More than 60 years old. Pancreatic tumors are one of the causes of AP and are also a risk factor for DVT development[22]. Thrombosis development in other places may be a confounding factor in this study. Thus, patients who had the following diagnoses were excluded from this study: (1) Pancreatic tumor; and (2) Thromboses in other locations. This study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines.
AP was diagnosed through a combination of clinical manifestations and signs (i.e., sudden onset of upper abdominal pain), laboratory tests (amylase or lipase levels were three times higher than normal limits), and imaging examinations (abdominal ultrasound, abdominal computed tomography (CT), and magnetic resonance imaging)[23]. AP was classified according to the revised Atlanta Classification[23]. The definition of non-mild acute pancreatitis (NMAP) is acute pancreatitis classified as moderate or severe. Acute pancreatitis patients aged over 60 years old were defined as older acute pancreatitis patients. The diagnosis of DVT was based on the results of color Doppler ultrasonography when patients presented swelling or pitting edema, redness, and leg tenderness. Organ failure (OF) was defined as a patient who had at least one failure of respiratory function, cardiovascular function, or renal function. Respiratory failure was defined as PaO2 < 60 mmHg, despite FiO2 of 0.30, or a need for mechanical ventilation. Cardiovascular failure was defined based on circulatory systolic blood pressure < 90 mmHg, despite adequate fluid resuscitation, or a need for inotropic catecholamine support. Renal failure was defined as creatinine level > 177 μmol/L after rehydration or the need for hemofiltration or hemodialysis.
The following variables were recorded for the study population: Age, sex, etiology, smoking, drinking, surgery times, any organ failure, respiratory failure, renal failure, cardiovascular failure, severity classification, onset time to diagnosis of DVT, and blood index. In DVT patients, all variables were collected until the time of DVT diagnosis. In NDVT patients, all variables were collected throughout the whole hospital stay. Repeated measurements of continuous variables are shown on average.
Continuous variables are described as the mean (SD) or median and binary variables are expressed as counts (%). Statistical analysis was performed using R software. (Version 3.6.1)
Relevant predictors included age, sex, surgery times, any organ failure, respiratory failure, cardiovascular failure, renal failure, blood culture, C-reactive protein, neutrophils, serum albumin, D-dimer, severity classification of DVT in patients with AP identified from a previous study[24,25] and advice of pancreatologists. Patients with more than 30% of the preselected predictors missing were excluded from model development.
In this study, 80 patients were identified with DVT, and more than ten times patients with NDVT were identified. Due to the imbalance between the DVT and NDVT groups, undersampling was performed to adjust the number between the two groups. A total of 10% NDVT patients were randomly selected compared with DVT patients. Finally, 140 NDVT patients were selected. Thus, training data included 80 DVT and 140 NDVT patients.
Logistic regression was used to identify the variables that were significantly correlated with DVT in the training group. Variables with a P-value less than 0.05 and more than 0.05 but suggested by pancreatologists were fed to a multivariate logistic regression model. Stepwise selection was used to further eliminate redundant variables. The resulting multivariate logistic regression model was used to build the prediction model.
The bootstrap method was used to evaluate the performance of the prediction model. In the bootstrap method, 100 random samples were drawn with replacement from the original data set and the coefficients were recalculated.
To validate the prediction model, two criteria were used to evaluate the prediction performance. On the one hand, the concordance index (c-index) was calculated to estimate the discrimination of the prediction model. On the other hand, calibration curves were plotted to evaluate the consistency between predicted DVT probability and actual DVT proportion. Values of 1 and 0.5 indicate perfect discrimination and no discrimination, respectively. The C-index and calibration results presented are an average of the bootstrapped samples.
Medical records of NMAP patients over 60 years admitted to West China Hospital from 2013.1.1 to 2018.12.31 were collected. DVT was diagnosed in 80 patients. Due to the imbalance of the data, undersampling was performed on selected NDVT patients. Finally, 140 NDVT patients were randomly selected for analysis. The baseline characteristics of the patients, including demographics, clinical indexes, and blood indexes, in the two groups are shown in Table 1. There are 81 and 49 females in the NDVT group and DVT group, respectively. The DVT group included patients aged between 60 and 88 years (mean age: 70.16 years), and the NDVT group included patients aged 69.81 years. Biliary was the most common etiology in both groups. There were 37 (26.4%) and 33 (23.6%) NDVT patients who smoked and drank, respectively. Seventeen (21.2%) and 15 (18.8%) DVT patients smoked and drank, respectively. All organ failure in the DVT group was significantly higher than that in the NDVT group. Respiratory failure accounted for the largest proportion in OF. In total, 65% of patients were classified as severe. However, 67% of patients in the NDVT group were classified as moderate. Blood culture, D-dimer, and serum albumin in the DVT group were significantly different between the two groups. Table 2 shows the thrombus location of the DVT patients. The most common location of vein thrombosis was both lower limbs, which were detected in 31 (38.8%) patients. Only 3 (3.7%) patients were found to have vein thrombosis in the left upper limb, and 12 (15%) patients had vein thrombosis detected in more than two locations.
| Variables | NDVT | DVT | P value |
| N | 140 | 80 | |
| Age | 69.81 (7.52) | 70.16 (7.84) | 0.745 |
| Gender: Female | 81 (57.9) | 49 (61.3) | 0.726 |
| Etiology | |||
| Biliary | 65 (47.4) | 39 (48.8) | |
| Alcohol | 3 (0.21) | 5 (0.62) | |
| Hyperlipidemia | 23 (16.4) | 10 (12.5) | |
| Others | 49 (35.0) | 26 (32.5) | |
| Smoking | 37 (26.4) | 17 (21.2) | 0.507 |
| Drink | 33 (23.6) | 15 (18.8) | 0.487 |
| Surgery times | < 0.001 | ||
| 0 | 114 (81.4) | 47 (58.8) | |
| 1 | 24 (17.1) | 23 (28.7) | |
| 2 | 2 (1.4) | 6 (7.5) | |
| 3 | 0 (0.0) | 4 (5.0) | |
| Any organ failure | 72 (51.4) | 67 (83.8) | < 0.001 |
| Respiratory failure | 61 (43.6) | 58 (72.5) | < 0.001 |
| Renal failure | 15 (10.7) | 31 (38.8) | < 0.001 |
| Cardiovascular failure | 23 (16.4) | 37 (46.2) | < 0.001 |
| Classification | < 0.001 | ||
| Moderate to severe | 95 (67.9) | 28 (35.0) | |
| Severe | 45 (32.1) | 52 (65.0) | |
| Blood index | |||
| Blood culture positive | 8 (5.7) | 20 (25.0) | < 0.001 |
| D-dimer | 5.87 (5.48) | 8.78 (6.47) | 0.002 |
| CRP | 120.77 (84.66) | 124.51 (86.79) | 0.796 |
| WBC | 11.09 (4.37) | 11.74 (4.63) | 0.312 |
| Neutrophils | 9.05 (4.08) | 9.87 (4.48) | 0.177 |
| Serum albumin | 32.90 (4.13) | 31.25 (4.31) | 0.006 |
| Location of the thrombosis | Number of patients (n = 80) |
| Left upper limb isolated | 3 (3.7%) |
| Right upper limb isolated | 6 (7.5%) |
| Both upper limbs | 4 (5.0%) |
| Left lower limb isolated | 14 (17.5%) |
| Right lower limb isolated | 10 (12.5%) |
| Both lower limbs | 31 (38.8%) |
| More than two locations | 12 (15.0%) |
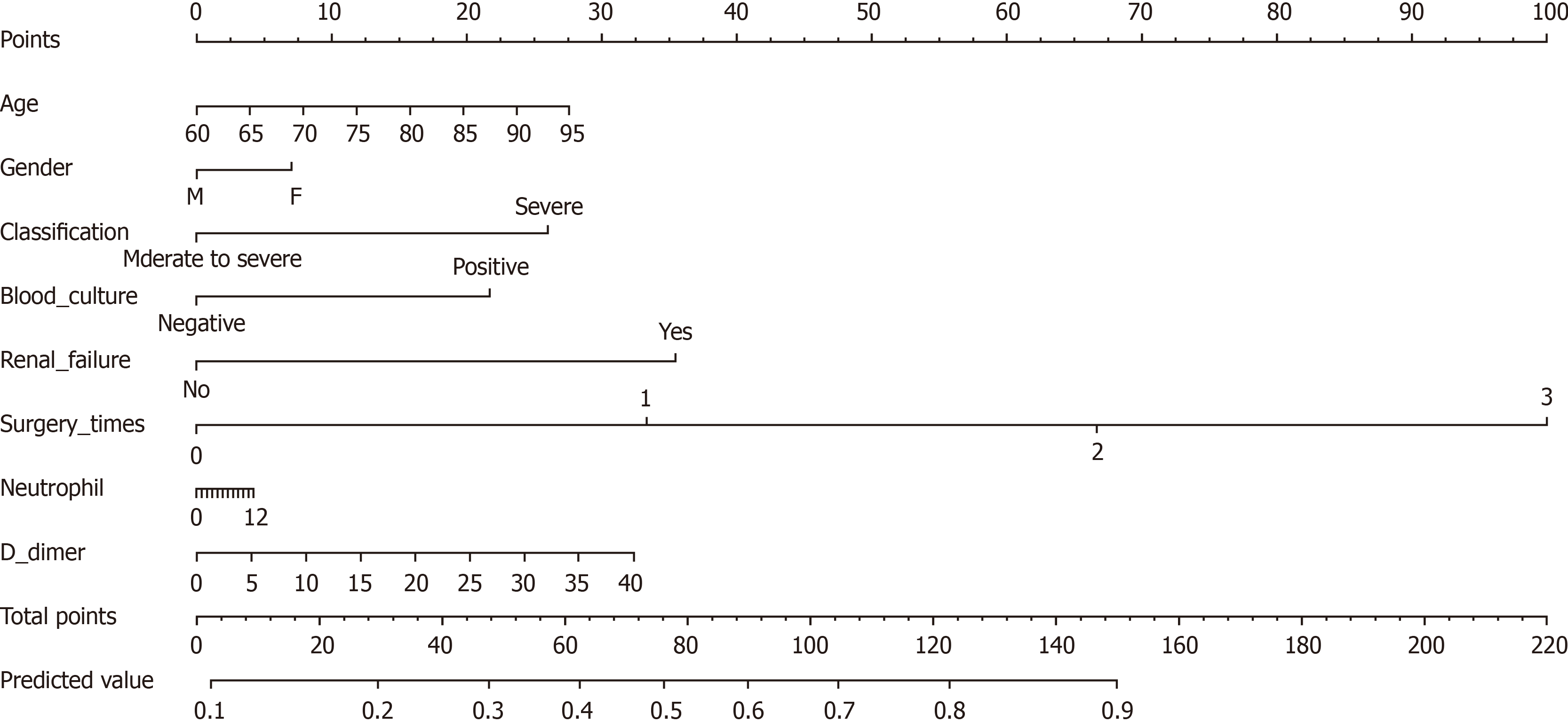
Univariate and multivariate analyses were performed to select potential predictors. A nomogram model was constructed based on the results of the multivariate logistics regression analysis and the suggestions of pancreatologists. Finally, 8 potential predictors based on 220 patients were selected. These features included sex, age, surgery times, renal failure, classification, D-dimer, blood culture, and neutrophils. Figure 1 shows the nomogram in which sex, age, surgery times, renal failure, classification, D-dimer, blood culture, and neutrophils defined the individual risk of DVT in NMAP patients. In this nomogram, D-dimer is a continuous variable and every 5 unit increase in D-dimer results in an approximately 0.8-point increase in risk points. The nomogram maps the predicted probability of DVT on a scale of 0 to 220. For each covariate, a vertical line is drawn upwards, and the corresponding points are noted. This is repeated for each covariate ending with a total score that corresponds to a predicted probability of morbidity at the bottom of the nomogram. The odds ratios of the nomogram variables are summarized in Table 3.
| Variables | OR (95%CI) | P value |
| Age | 1.02 (0.983-1.066) | 0.257 |
| Gender | 1.23 (0.625-2.431) | 0.546 |
| Surgery times | 2.70 (1.566-4.651) | 0.000 |
| Renal failure | 0.35 (0.166-0.728) | 0.005 |
| Classification | 2.17 (1.133-4.164) | 0.020 |
| D-dimer | 1.02 (0.971-1.081) | 0.382 |
| Blood culture | 0.53 (0.218-1.267) | 0.152 |
| Neutrophils | 1.01 (0.930-1.087) | 0.887 |
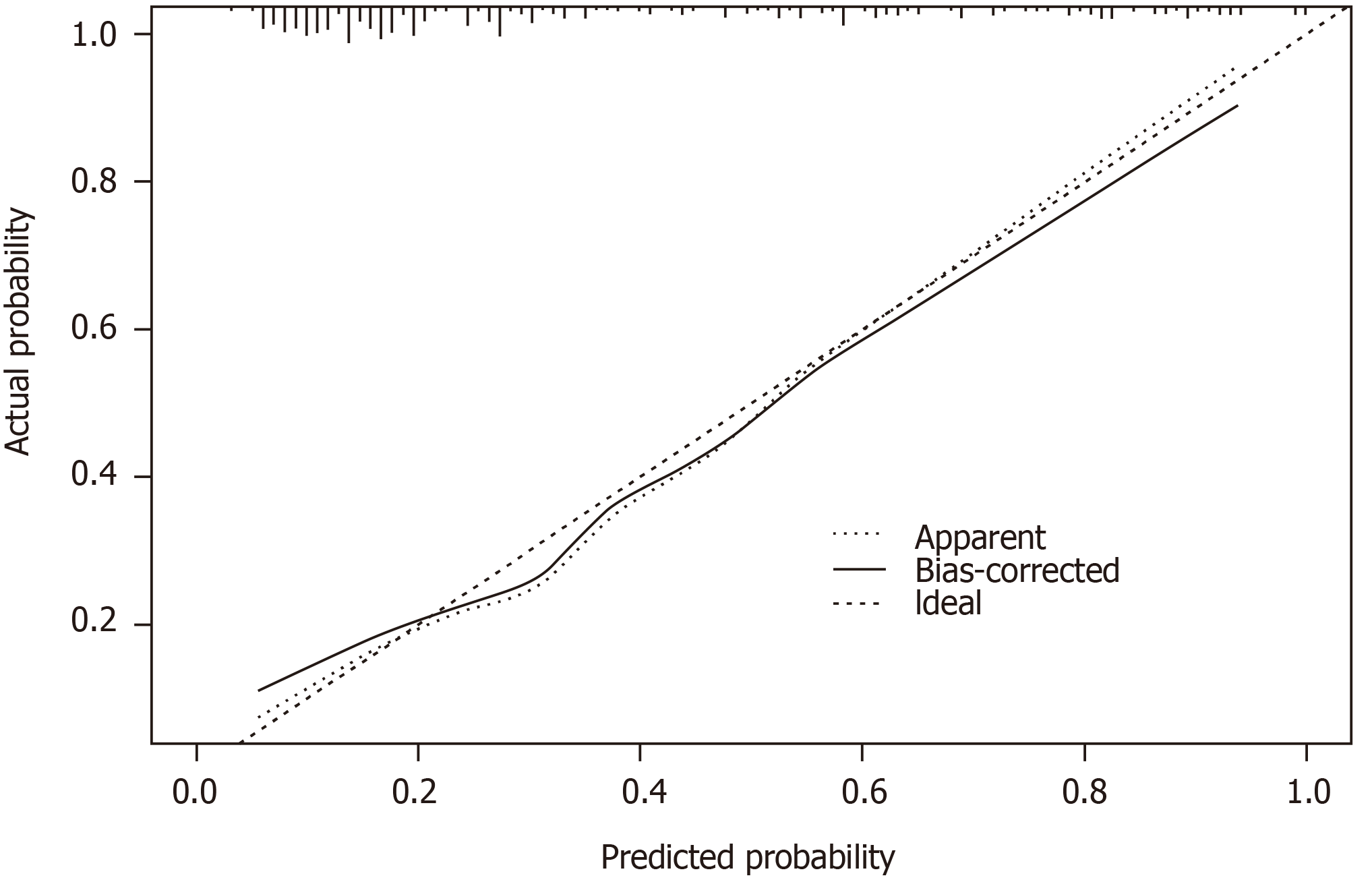
The C-index for the prediction nomogram was 0.827 (95%CI: 0.769-0.885). It was confirmed to be 0.803 (95%CI: 0.743-0.860) through bootstrapping validation, which suggested the model’s good discrimination. The calibration curve in Figure 2 shows good concordance between the estimated risk of DVT and the actual presence of DVT.
Using data from a retrospective study including older NMAP patients with DVT, we developed and internally validated a potential new prediction model for DVT. This is the first model for predicting DVT in older NMAP patients. The performance of the prediction model was adequate. This nomogram, based on routinely available demographic and blood indexes, predicts the probability of DVT in NMAP patients.
The recent criteria of AP classification were put forward in 2012. Non-mild acute pancreatitis patients have a poorer prognosis, and they stay in the hospital for a long time. Hospitalization has been considered a significant risk factor for VTE[26]. Furthermore, older patients usually have slow blood flow. These factors all contribute to DVT development. However, doctors usually pay attention to DVT when patients have clinical manifestations, such as calf swelling. In trauma patients, occult DVT may cause pulmonary embolus, leading to late deaths due to fatality[27]. Early detection of DVT results in decreased rates of pulmonary embolus and mortality[28]. Therefore, accurate prediction of DVT is invaluable to provide treatment for each NMAP patient.
Our findings are essentially in line with previous venous thromboembolism studies. In the present study, we found that DVT mostly develops in both lower limbs at the same time. However, isolated left upper limbs only accounted for 3.7% of patients. A previous study showed that upper limb DVT is less than 10% of all DVT[22]. In this study, more than 16.2% of patients had upper limb DVT.
Some predictors were already confirmed in other studies. D-dimer is the most well validated and widely used biomarker of venous thromboembolism excluded[25]. It is usually combined with the Wells score in practice. In this study, D-dimer was an important predictor of DVT. OF is regarded as one of the most important parameters of AP patients in the course of the early phase[23]. The main causes of OF are cytokine cascades resulting in systemic inflammatory response syndrome (SIRS)[29]. Respiratory failure, renal failure, and cardiovascular failure commonly take place in the clinical. OF can lead to long term bed rest and immobilization. Both of these contribute to DVT development[14]. However, in this study, only renal failure was in the final prediction model for DVT development. This factor was validated in a previous study[30]. Vascular endothelium is activated by proinflammatory cytokines in severe acute pancreatitis. This promotes the activation of coagulation cascades and circulating neutrophils[31]. Furthermore, neutrophils promote coagulation by inhibiting anticoagulant factors and releasing neutrophil extracellular traps[32]. These further promote thrombogenesis. Currently, mechanistic research shows that neutrophil extracellular traps hold promise for novel clinical treatment of DVT[32]. Patients with positive blood cultures have more severe inflammation than others. Additionally, infection has been thought to be a risk factor for venous thromboembolism[24]. Surgery is also a risk factor for venous thromboembolism[24]. Some NMAP patients need reoperation several times. More surgery times mean patients experience more frequent and longer bed rest.
Overall, our study first focused on the development of DVT in older NMAP patients, which has never been studied before. We analyzed the risk factors for DVT and built a nomogram model to predict the probability of developing DVT for NMAP patients. Proper use of this model can help physicians identify patients with a high risk of developing DVT.
There are several limitations in this study. First, this was a retrospective study, and the examination of DVT was not performed routinely. Thus, the diagnosis of DVT may have been missed in some patients. In addition, this was a single-center study, and validation was only performed in internal data. The results could be more convincing if external validation is performed. Moreover, due to the limitation of the sample size, potential bias may exist in the present study.
In this study, a nomogram model was built by combining eight independent risk factors for DVT. This nomogram score is a reliable and effective tool that can predict DVT in older patients with NMAP. This may help guide doctors in making sound decisions regarding the administration of DVT prophylaxis.
Deep vein thrombosis (DVT) may cause pulmonary embolus leading to late deaths. The systemic inflammatory and hypercoagulable state of moderate and severe acute pancreatitis (non-mild acute pancreatitis, NMAP) patients may contribute to the development of venous thromboembolism. Accurate prediction of DVT is conducive to clinical decisions.
There is a lack of a scoring model for predicting the development of DVT in NMAP patients.
We aimed to develop a prediction model for DVT in old NMAP patients.
Univariate and multivariate logistic regression analyses were used to select independent risk factors associated with DVT. The selected risk factors were included in the nomogram. A validation set was constructed using bootstrapping with 100 resamplings. The accuracy and utility of the nomogram were evaluated by calibration curve and decision curve analysis, respectively.
Eighty DVT patients and 140 non-DVT patients were included in this study. Eight factors including age, sex, surgery times, D-dimer, neutrophils, any organ failure, blood culture, and classification constitute the prediction model. This model achieved good concordance indexes of 0.827 (95%CI: 0.769-0.885) and 0.803 (95%CI: 0.743-0.860) in the training and validation set, respectively.
A reliable and effective nomogram model that can predict DVT in old patients with NMAP was constructed.
The usability of the new model needs further validation by other center data.
Manuscript source: Unsolicited manuscript
Specialty type: Peripheral vascular disease
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H S-Editor: Gong ZM L-Editor: A P-Editor: Wu RR
| 1. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 513] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 2. | Gapp J, Hall AG, Walters RW, Jahann D, Kassim T, Reddymasu S. Trends and Outcomes of Hospitalizations Related to Acute Pancreatitis: Epidemiology From 2001 to 2014 in the United States. Pancreas. 2019;48:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1071] [Article Influence: 178.5] [Reference Citation Analysis (1)] |
| 4. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, DiBonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-1187.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1464] [Article Influence: 112.6] [Reference Citation Analysis (1)] |
| 5. | van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, Besselink MG; Dutch Pancreatitis Study Group. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66:2024-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 294] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 6. | Xu F, Chen X, Li C, Liu J, Qiu Q, He M, Xiao J, Liu Z, Ji B, Chen D, Liu K. Prediction of Multiple Organ Failure Complicated by Moderately Severe or Severe Acute Pancreatitis Based on Machine Learning: A Multicenter Cohort Study. Mediators Inflamm. 2021;2021:5525118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Husu HL, Valkonen MM, Leppäniemi AK, Mentula PJ. Occurrence and Risk Factors of Infected Pancreatic Necrosis in Intensive Care Unit-Treated Patients with Necrotizing Severe Acute Pancreatitis. J Gastrointest Surg. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Stein PD, Beemath A, Olson RE. Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients. Am J Cardiol. 2005;95:1525-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 573] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 10. | Mendelson RM, Anderson J, Marshall M, Ramsay D. Vascular complications of pancreatitis. ANZ J Surg. 2005;75:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 11. | Deiss R, Young P, Yeh J, Reicher S. Pulmonary embolism and acute pancreatitis: case series and review. Turk J Gastroenterol. 2014;25:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Geerts W, Selby R. Prevention of venous thromboembolism in the ICU. Chest. 2003;124:357S-363S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | McLeod AG, Geerts W. Venous thromboembolism prophylaxis in critically ill patients. Crit Care Clin. 2011;27:765-780, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Maatman TK, McGuire SP, Lewellen KA, McGreevy KA, Ceppa EP, House MG, Nakeeb A, Nguyen TK, Schmidt CM, Zyromski NJ. Prospective Analysis of the Mechanisms Underlying Ineffective Deep Vein Thrombosis Prophylaxis in Necrotizing Pancreatitis. J Am Coll Surg. 2021;232:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, De Bon E, Tormene D, Pagnan A, Prandoni P. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8:2450-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 804] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 16. | Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol. 2001;38:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Eastham JA, Kattan MW, Scardino PT. Nomograms as predictive models. Semin Urol Oncol. 2002;20:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Kattan MW, Giri D, Panageas KS, Hummer A, Cranor M, Van Zee KJ, Hudis CA, Norton L, Borgen PI, Tan LK. A tool for predicting breast carcinoma mortality in women who do not receive adjuvant therapy. Cancer. 2004;101:2509-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Park SY. Nomogram: An analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155:1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 361] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 21. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2363] [Article Influence: 236.3] [Reference Citation Analysis (0)] |
| 22. | Stubbs MJ, Mouyis M, Thomas M. Deep vein thrombosis. BMJ. 2018;360:k351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4295] [Article Influence: 357.9] [Reference Citation Analysis (44)] |
| 24. | Streiff MB, Agnelli G, Connors JM, Crowther M, Eichinger S, Lopes R, McBane RD, Moll S, Ansell J. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombolysis. 2016;41:32-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 25. | Audu CO, Gordon AE, Obi AT, Wakefield TW, Henke PK. Inflammatory biomarkers in deep venous thrombosis organization, resolution, and post-thrombotic syndrome. J Vasc Surg Venous Lymphat Disord. 2020;8:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1600] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 27. | Rogers FB. Venous thromboembolism in trauma patients. Surg Clin North Am. 1995;75:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Allen CJ, Murray CR, Meizoso JP, Ginzburg E, Schulman CI, Lineen EB, Namias N, Proctor KG. Surveillance and Early Management of Deep Vein Thrombosis Decreases Rate of Pulmonary Embolism in High-Risk Trauma Patients. J Am Coll Surg. 2016;222:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 409] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 30. | Roch AM, Maatman TK, Carr RA, Colgate CL, Ceppa EP, House MG, Lopes J, Nakeeb A, Schmidt CM, Zyromski NJ. Venous Thromboembolism in Necrotizing Pancreatitis: an Underappreciated Risk. J Gastrointest Surg. 2019;23:2430-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Kylänpää ML, Repo H, Puolakkainen PA. Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol. 2010;16:2867-2872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 32. | Metz AK, Diaz JA, Obi AT, Wakefield TW, Myers DD, Henke PK. Venous Thrombosis and Post-Thrombotic Syndrome: From Novel Biomarkers to Biology. Methodist Debakey Cardiovasc J. 2018;14:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |