Published online Apr 15, 2024. doi: 10.4239/wjd.v15.i4.664
Peer-review started: November 26, 2023
First decision: December 17, 2023
Revised: January 8, 2024
Accepted: March 7, 2024
Article in press: March 7, 2024
Published online: April 15, 2024
Processing time: 137 Days and 21.9 Hours
Nutrition recommendations in patients with type 2 diabetes mellitus (T2DM) are to consume rye or integral bread instead of white bread. A positive effect on glucoregulation has been achieved by enriching food with various biologically active substances of herbal origin, so we formulated an herbal mixture that can be used as a supplement for a special type of bread (STB) to achieve better effects on postprandial glucose and insulin levels in patients with T2DM.
To compare organoleptic characteristics and effects of two types of bread on postprandial glucose and insulin levels in T2DM patients.
This trial included 97 patients with T2DM. A parallel group of 16 healthy subjects was also investigated. All participants were given 50 g of rye bread and the same amount of a STB with an herbal mixture on 2 consecutive days. Postprandial blood glucose and insulin levels were compared at the 30th, 60th, 90th and 120th min. A questionnaire was used for subjective estimation of the organoleptic and satiety features of the two types of bread.
Compared to patients who consumed rye bread, significantly lower postprandial blood glucose and insulin concentrations were found in T2DM patients who consumed STB. No relevant differences were found among the healthy subjects. Subjectively estimated organoleptic and satiety characteristics are better for STB than for rye bread.
STB have better effects than rye bread on postprandial glucoregulation in T2DM patients. Subjectively estimated organoleptic and satiety characteristics are better for STB than for rye bread. Therefore, STB can be recommended for nutrition in T2DM patients.
Core Tip: We are testing special types of bread in populations with compromised glucoregulation. It is novel, tasty, and very effective in postprandial glucoregulation. In this study, we compared novel bread (TopiGluk) with standard hospital rye bread and obtained significant differences regarding glucose metabolism of TopiGluk compared to rye bread. This research might be interesting for readers because TopiGluk could become a standard supplement in bread for patients with compromised glucoregulation and those at high risk for diabetes.
- Citation: Gostiljac DM, Popovic SS, Dimitrijevic-Sreckovic V, Ilic SM, Jevtovic JA, Nikolic DM, Soldatovic IA. Effect of special types of bread with select herbal components on postprandial glucose levels in diabetic patients. World J Diabetes 2024; 15(4): 664-674
- URL: https://www.wjgnet.com/1948-9358/full/v15/i4/664.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i4.664
Diabetes mellitus (DM) is one of the main health issues worldwide, and its incidence has been increasing for several decades[1,2]. Additionally, many people have reduced glucose tolerance[3]. A Global Report on Diabetes showed that the number of adult DM patients increased almost fourfold between 1980 and 2014, going from 108 million patients to 422 million patients[4]. This dramatic increase is mostly due to type 2 DM (T2DM) and its risk factors, especially obesity[5].
Dietary interventions are the main economic and effective strategies aimed at reducing blood glucose and insulin levels in the population[3,6]. Physical activity also improves glucose tolerance as a supplemental treatment for obesity but not as a replacement for dietary measures[7]. Controlled nutrition improves glucose tolerance by reducing endogenous glucose production and improving sensitivity to insulin[8]. The consumption of foods with a low glycemic index (GI) decreases postprandial blood glucose and insulin levels and their fluctuations[9,10].
One of the basic foodstuffs worldwide is bread. Recommendations for this population include the consumption of rye or whole grain bread with higher dietary fiber (DF) content instead of white bread[11]. Several trials have shown that a positive effect on glucoregulation may also be achieved by enriching food with various biologically active substances of herbal origin[12].
TopiGluk bread was patented by the National Intellectual Property Office (registration No. 73932) as a supplementary treatment for people with disease. TopiGluk bread is made by adding a mixture of nutrients to whole wheat, oat and buckwheat flour.
We hypothesized that TopiGluk bread would significantly improve glucose tolerance in T2DM patients. The aim of this study was to evaluate the effects of TopiGluk bread on postprandial glucose levels in T2DM patients. Additionally, the insulin levels and organoleptic properties of the examined bread were evaluated as secondary objectives.
A prospective study was conducted at the Clinic of Endocrinology, Diabetes and Metabolic Disorders, Clinical Center of Serbia, Belgrade. The study was carried out from 20 May 2016 to 25 March 2017.
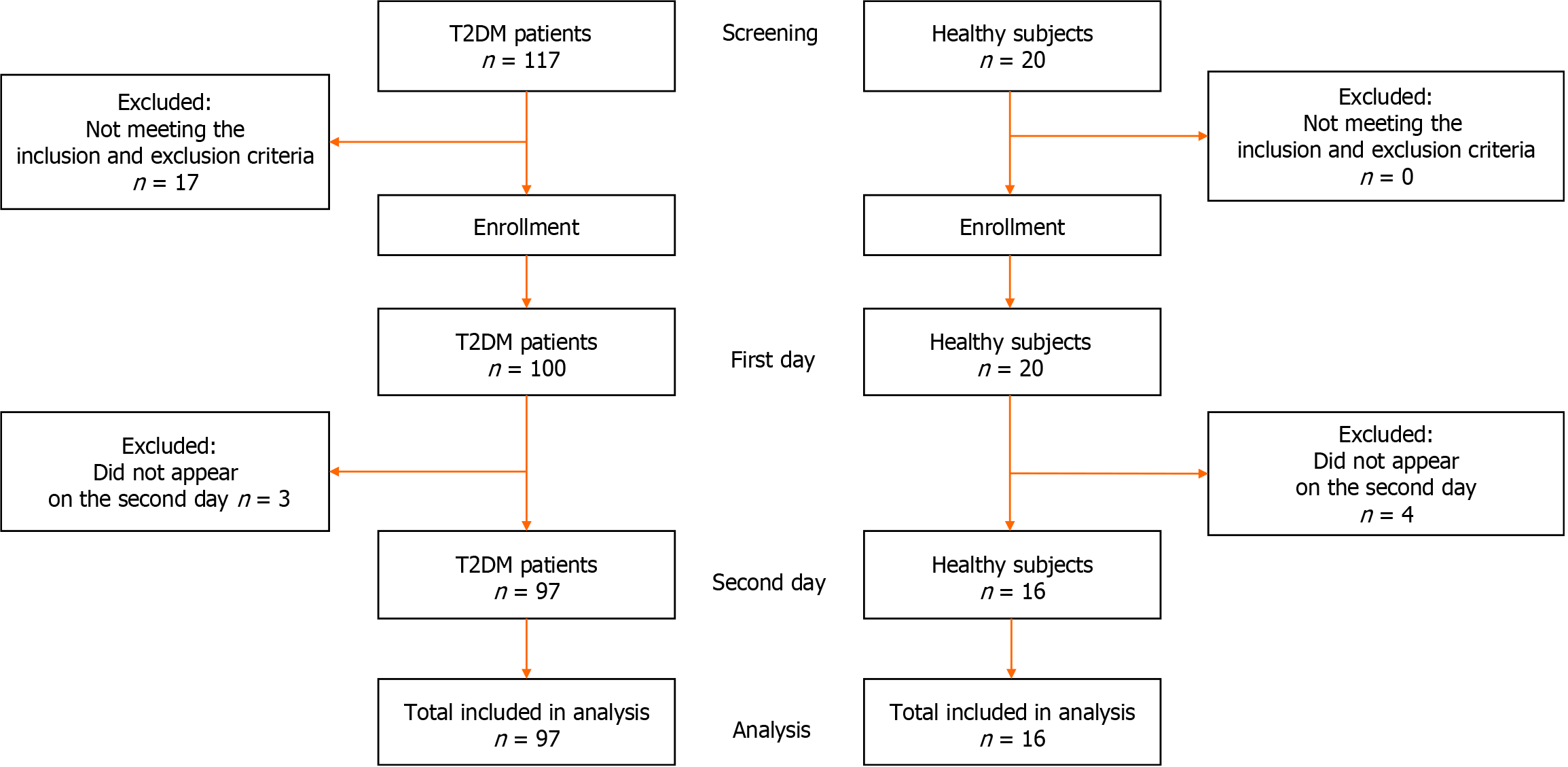
The trial included 97 patients with T2DM who were treated as outpatients at the Clinic of Endocrinology, Diabetes and Metabolic Disorders, Faculty of Medicine, University of Belgrade. The inclusion criteria were 18 years or older and confirmed T2DM. The exclusion criteria were acute T2DM complications; acute inflammatory conditions; chronic diseases of the liver or gastrointestinal tract; malignant diseases; immunodeficiency; narcotic or alcoholic addictions; and pregnancy (Figure 1).
A parallel group of 16 healthy controls were analyzed; they had no history of metabolic disorders (including DM) and had preserved glucose homeostasis based on their medical history.
All of the subjects provided informed consent for inclusion in the study. The investigation was carried out in accordance with the Declaration of Helsinki and was revised in 2013. The study was carried out in accordance with the EU Directive 20/2001/EC, the Commission Directive 2005/28/EC, and the European Parliament Declaration on Good Clinical Practice, as well as the International Conference on Harmonization ICH-GCP (E6); approval for this research was given by the Ethics Committee, Clinical Center of Serbia, No. 105/39 on 19 May 2016. The study is registered in the German Clinical Trials Register DRKS00023611. The clinical trial was registered after completion because the law of the Republic of Serbia does not require registration in the international registry. The authors confirm that all ongoing and related trials for this food supplement have been registered.
Prior to the trial, a detailed history was taken, which included data on physical activity and lifestyle, the duration of T2DM and therapy, chronic complications of T2DM and nutritional habits. Data on anthropometric characteristics, weight and height were obtained from medical records if no significant changes were observed in the last 6 mo. Body mass index was calculated by dividing the body weight in kilograms by the squared body height in meters (kg/m2). All participants were instructed to fast for at least 12 h prior to blood sampling.
A slice of bread weighing 50 g was provided to each participant, which is the usual portion for T2DM patients. The nutritional content of the bread is presented in Table 1. One day, all participants were given a piece of rye bread, and on another day, they were given a piece of special bread. Patients were advised to perform similar activities on both days. Furthermore, patients were required to fast for 12 h prior to each blood sampling.
| Name of nutrient (unit) | Rye bread | TopiGluk bread | RDI, % |
| Energy (kJ) | 1072 | 1088 | 12.95 |
| Energy (kcal) | 253 | 259 | 12.95 |
| Fat (g) | 1.6 | 6.6 | 9.43 |
| Saturated fatty acids (g) | 0.6 | 1.2 | 6 |
| Carbohydrates (g) | 48.6 | 37.5 | 14.42 |
| Sugars (g) | 0.1 | 3.7 | 4.11 |
| Dietary fibers (g) | 3.8 | 8.6 | 143.3 |
| Proteins (g) | 9.2 | 8.1 | 16.2 |
| Salt (g) | 1.4 | 1.1 | 18.3 |
| Magnesium (mg) | - | 72.6 | |
| Zinc (mg) | - | 1.6 | |
| Chromium (µg) | - | 20.1 | |
| Selenium (µg) | - | 5.3 |
Participants were instructed to complete a questionnaire about the look, smell, taste, quantity, and satiety of the bread. Answers were given on a numerical scale from 1 (worst) to 5 (best). Upon arrival at the clinic at approximately 08:00 in the morning, the responsible researcher accommodated each participant in the laboratory. A venous cannula was inserted in the arm of the patient. The first blood sample was taken before the meal. Next, four blood samples were taken every 30 min after the meal. Healthy subjects were with evaluated with a glucose meter, and blood samples were collected using finger sticks (capillary blood sampling) in the infirmary.
The laboratory analyses of T2DM patients included hemoglobin A1c (HbA1c) and C-peptide levels (at baseline only) and glucose and insulin levels (at baseline and 30 min, 60 min, 90 min and 120 min after the meal) on both examination days. Blood glucose measurements were performed using the spectrophotometric (hexokinase) method with a COBAS 6000 (Roche Diagnostics, Basil, Switzerland) with a reference range of 3.9-6.1 mmol/L. Insulin and C-peptide levels were measured using an immunoradiometric assay method with a gamma counter for in vitro diagnosis (LKB-WALLAC ChinGamma Model 1272).
Laboratory analysis of healthy controls included only glucose levels at baseline (before the meal) and at 30 min, 60 min, 90 min and 120 min after the meal. Blood measurements from capillary blood were performed with an electrochemical method using a Biosen C-Line machine (EKF Diagnostics, Cardiff, United Kingdom) with a reference range of 3.9-6.1 mmol/L.
Due to differences in sampling methods, diabetic and healthy subjects were not compared, except for blood sugar changes from baseline to 120 min (the sampling method did not affect the change).
Differences between 30 min and baseline, 60 min and baseline, 90 min and baseline and 120 min and baseline levels were calculated (delta glucose and delta insulin). The area under the curve (AUC) was calculated using a trapezoidal model[13].
The bread was a brand of the Delhaize Serbia distributor in cooperation with partners for product development: The international company Puratos and the national bakery products manufacturer Alimpije-and with a partner for product improvement-ZZ Zdravlje (Čačak, Serbia), the inventor of TopiGluk®.
The ingredients of TopiGluk® bread are as follows: Basil (Ocimum basilicum), garlic (Allium sativum), Greek seed (Trigonella foenum graecum), ginger (Zingiber officinale), oat (Avena sativa), Jerusalem artichoke (Helianthus tuberosus), and cinnamon (Cinnamomum verum). Sunflower and linen seeds are also among the supplements. The nutritional content of 100 g of the special type of bread (STB) with the TopiGluk mixture is presented in Table 1. Approximately one-third of the mentioned mixture contains active principles (TopiGluk), while the remaining two-thirds are equivalent parts buckwheat, oat and whole wheat flour. The representation of the components in TopiGluk was 0.5% garlic, 0.5% ginger, 2% basil, 3% Greek seed, 5% cinnamon, 8% oat, and 13% Jerusalem artichoke.
A sample size of 97 T2DM patients with achieved 90% power to detect a mean difference of 1.0 ± 3.0 mmol/L with a sig
The results are presented as counts (%) or means ± SD depending on the data type and distribution. Measurements were compared using parametric (paired sample t test) and nonparametric (Wilcoxon signed rank test) tests. Glucose changes (delta) between healthy subjects and patients were compared using an independent samples t test. Deltas were calculated as the difference between the 30 min, 60 min, 90 min and 120 min time points and baseline. The AUC was calculated using the trapezoidal rule. All P values less than 0.05 were considered significant. All the data were analyzed using R 3.4.2. R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org/).
The majority of participants were males in the 6th to 7th decade of life, preobese to obese individuals, and physically active individuals. The majority of patients were receiving oral antidiabetic therapy, while one-third were receiving insulin. Coronary disease and neuropathy were present in one-third of patients. The average duration of diabetes was shorter than 10 years. Most of the patients consumed rye bread, for which the median daily intake was 1 slice, while other bread types (whole wheat bread and white bread) were less represented in terms of nutritional status (both medians were 0) (Table 2).
| Characteristic | Value |
| Age in yr | 61.1 ± 9.3 |
| Sex as male | 62 (62.9) |
| BMI in kg/m2 | 29.2 ± 4.7 |
| Physically active | 83 (85.6) |
| Sedentary job | 60 (61.94) |
| Oral antidiabetic therapy | 86 (88.7) |
| Insulin therapy | 28 (28.8) |
| Combined | 15 (50.0) |
| Conventional | 4 (13.3) |
| Intensive | 11 (36.7) |
| Duration of DM in yr | 7 (3-12) |
| HbA1c | 7.6 ± 1.5 |
| C peptide in ng/mL | 1.23 ± 0.78 |
| Complications of DM | |
| Coronary disease | 34 (35.1) |
| Cerebrovascular disease | 7 (7.2) |
| Peripheral vascular disease | 21 (21.6) |
| Retinopathy | 25 (25.87) |
| Neuropathy | 35 (36.1) |
| Nephropathy | 12 (12.4) |
| Diabetic foot | 4 (4.1) |
| Main bread in nutrition | |
| Rye | 52 (53.6) |
| Whole wheat | 40 (41.2) |
| White wheat | 35 (36.1) |
All the evaluated characteristics were greater for the STB than for rye bread, as presented in Table 3.
| Characteristic | Bread | P value1 | |
| Rye | TopiGluk | ||
| Appearance | 3.42 ± 1.02 | 4.25 ± 1.01 | < 0.001 |
| Aroma | 3.33 ± 1.08 | 4.28 ± 1.06 | < 0.001 |
| Taste | 3.37 ± 1.05 | 4.34 ± 1.06 | < 0.001 |
| Satisfaction with the amount | 4.10 ± 1.16 | 4.33 ± 1.05 | 0.057 |
| Satiety level | 3.91 ± 1.04 | 4.45 ± 0.84 | < 0.001 |
| Duration of satiety | 3.67 ± 1.12 | 4.43 ± 0.84 | < 0.001 |
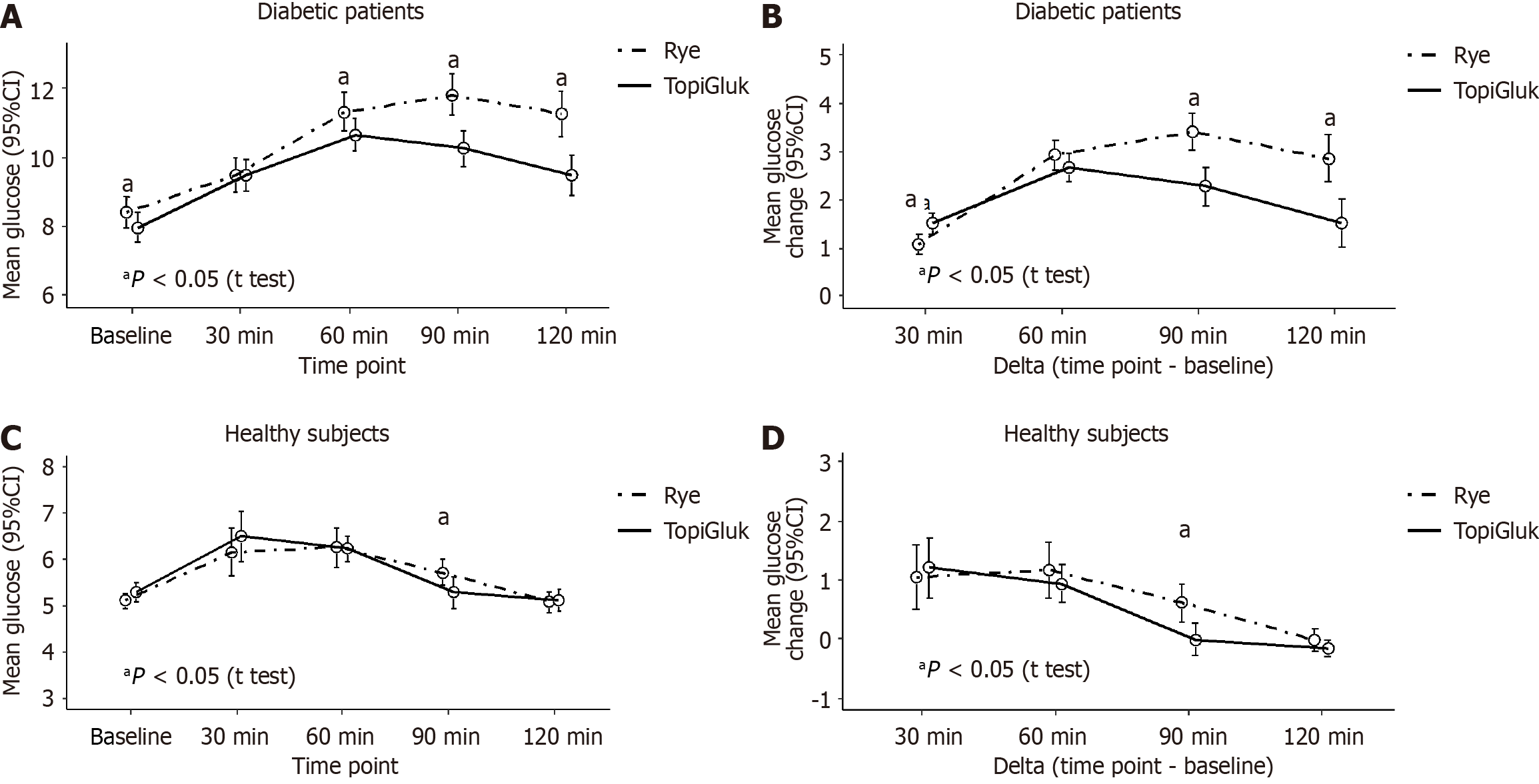
In T2DM patients, we found better glucoregulation after the STB portion than after the rye bread portion. Significantly lower delta values of blood glucose were observed after the STB portion at the 90th and 120th min than after the rye bread portion. In healthy subjects, no significant differences in blood glucose delta values were observed, except at the 90th min. Concerning the AUC of blood glucose, a significant difference was found only at the 90th min. In T2DM patients, the AUC was 21.2% ± 5.1% after the rye bread portion and 19.6% ± 4.5% after the STB with TopiGluk portion (P < 0.001). In healthy subjects, no significant differences in the AUC were found after the two types of bread were consumed (P = 0.924) (Figure 2).
When comparing patients and healthy subjects regarding delta values, all differences were significant at the 0.001 level for both the rye bread and the STB with the TopiGluk® mixture, except for the change from baseline to 30 minutes after the rye (P = 0.919) and STB with TopiGluk® (P = 0.313) portions (Figure 2).
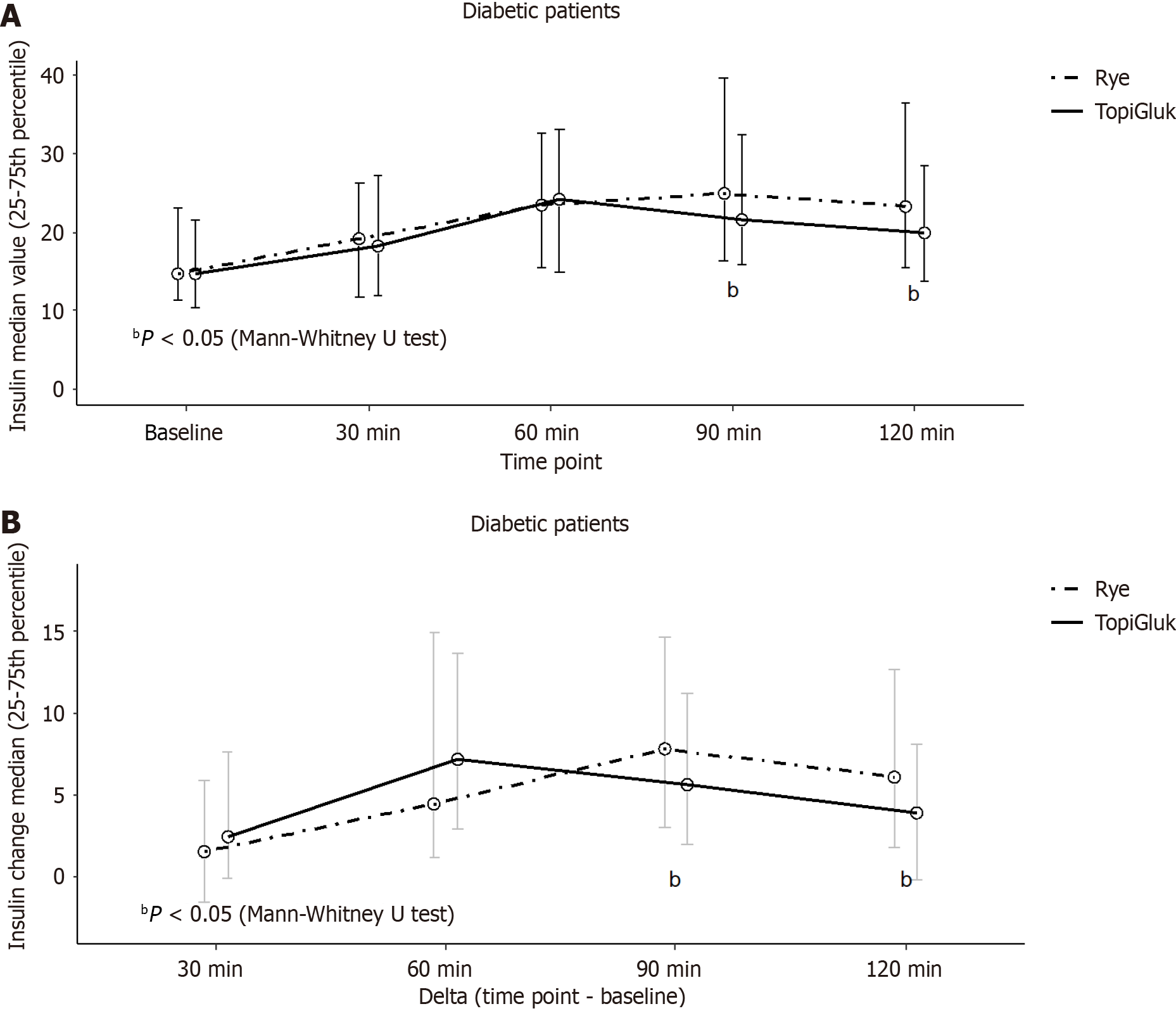
The insulin levels of the patients were analyzed at each time point (0, 30, 60, 90 and 120 min) and for each type of bread (Figure 3). Initially, the median insulin concentrations were identical. However, significant differences in insulin levels were observed at the 90th min and the 120th min after the two types of bread were consumed. After the STB with TopiGluk® was consumed, we found significantly lower median insulin concentrations compared to after rye bread was consumed. A comparison of the deltas revealed significant differences at the same time points. The median value and interquartile range of the AUC of insulin in T2DM patients with was 46.7 (29.7-61.1) after consuming rye bread and 39.9 (30.1-57.0) after consuming the STB with TopiGluk® (P = 0.035).
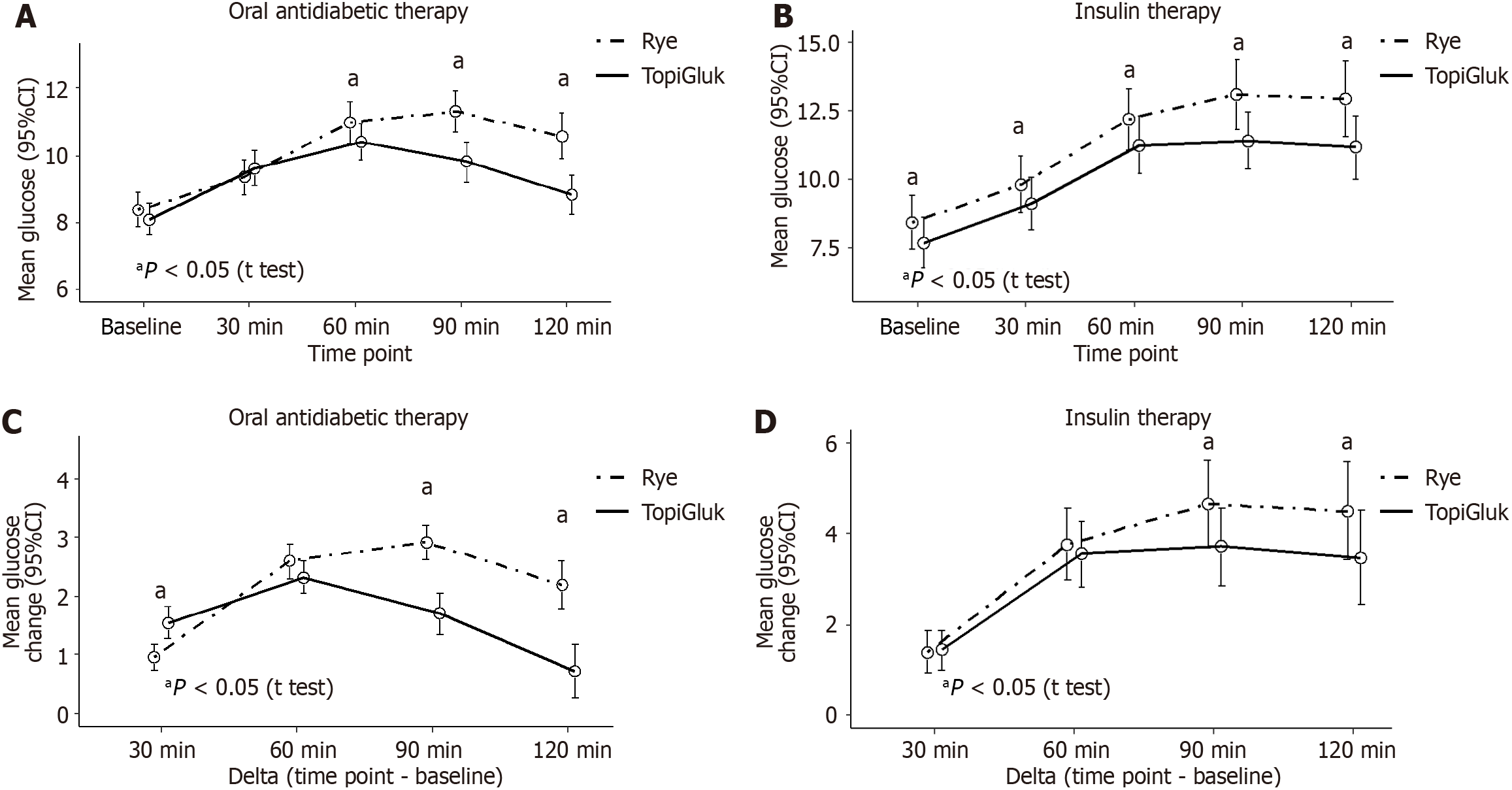
Diabetic patients were divided into two subgroups: patients receiving oral antidiabetic therapy and patients receiving insulin therapy (Figure 4). In patients on oral antidiabetic therapy, significantly higher blood glucose levels were observed at the 60th, 90th and 120th min after the rye bread was consumed than after the STB with TopiGluk® was consumed. Differences in blood glucose changes from baseline were significant at the 90th min and at the 120th min. In patients receiving insulin therapy, all mean glucose levels were significantly greater after rye bread was consumed than after the STB with TopiGluk® was consumed, but the changes (deltas) were significantly different only at the 90th min and at the 120th min (Figure 4).
This study compared the effects of rye bread and (STB) with TopiGluk® on glucoregulation. Patients subjectively assessed the STB as better than the rye bread regarding appearance, aroma, taste, amount, satiety level and duration of satiety. We observed lower glycemic and insulin levels from baseline to the 120th min in diabetic patients after the STB was consumed than after the rye bread was consumed. In healthy subjects, this difference was not observed, as expected.
The STB with TopiGluk® can be described as a “functional food” because it may have the following positive effects on glucose metabolism: decreased glucose absorption from the intestine to blood, increased glucose utilization, increased cell sensitivity to insulin, reduced insulin resistance, increased endogenous insulin production and increased glycogen in the liver[14].
Glucose absorption has been evaluated in several studies. Glucose absorption can be reduced using foods rich in DFs. DFs include lignin and a range of polysaccharides derived from cell walls that are poorly digested in the upper intestine[15]. DM patients are usually instructed to eat rye bread, which is rich in DF. Viscous and gel-forming soluble DFs inhibit macronutrient absorption and reduce the postprandial glucose response. However, in prospective cohort studies, insoluble cereal DF and whole grains, but not soluble DF, are consistently associated with reduced DM risk, suggesting that further unknown mechanisms are likely involved[16,17]. TopiGluk® contains whole wheat flour, buckwheat and oatmeal, which are rich in DF, especially beta glucans. TopiGluk is also rich in the soluble DF inulin from Jerusalem artichoke, which binds water and forms a viscous solution that delays gastric emptying and intestinal transit, thus reducing glucose absorption. This leads to a decreased blood glucose response[16,17]. DF also decreases insulin secretion and reduces the chance of reactive hypoglycemia during the postprandial period. On the other hand, this promotes satiety and satiation, increases fat oxidation and decreases fat storage[18].
Several authors have examined the effect of whole-meal and whole-kernel rye breads on glucose metabolism compared to that of white wheat bread. Leinonen et al[19] concluded that whole kernel rye bread has no effect on the glucose response but has an effect on the postprandial insulin response. In healthy subjects, we found no differences in blood glucose levels after consumption of the STB with TopiGluk® or after consumption of rye bread. This is probably due to preserved regulatory mechanisms of glucose metabolism in healthy subjects.
However, in T2DM patients, we found lower blood glucose and insulin levels after the consumption of the STB with TopiGluk® than after the consumption of the rye bread. This approach is very important for individuals with impaired glucose tolerance or an increased risk of diabetes. Starchin bread decomposes during digestion to simple sugars, which affect glucoregulation. TopiGluk is a mixture of natural metabolically active ingredients that play a proven role in me
A comparison of the organoleptic properties of two kinds of bread, an STB with TopiGluk® and rye bread, revealed that the former was better than the latter. A number of respondents emphasized the sweet taste of the STB with TopiGluk®, which may be particularly important for DM patients because they are usually not allowed to consume sweet food.
The results of our research are consistent with the latest data from the literature indicating that the plants added to the TopiGluk® bread (Ocimum basilicum, Allium sativum, Trigonella foenum graecum, Zingiber officinale, Avena sativa, Helianthus tuberosus, and Cinnamomum verum) have antidiabetic properties[30-35].
Based on the present results, we can conclude that postprandial blood glucose levels in T2DM patients are lower after consuming TopiGluk bread than after consuming the same amount of rye bread. Improved glucoregulation was noted in T2DM patients at 90 and 120 min, both in patients who were taking oral antidiabetic drugs and in patients receiving insulin therapy. An STB made with TopiGluk® has better subjectively assessed organoleptic and fine characteristics than rye bread. The STB with TopiGluk® can be recommended as part of the diet in T2DM patients.
Bread that we are testing is novel, tasty and very effective in populations with compromised glycoregulation. In our study, we compared novel bread with standard hospital rye bread and obtained significant differences regarding glucose metabolism of special type of bread (STB) with TopiGluk compared to rye bread.
All participants were given 50 g of rye bread or STB with herbal mixture on 2 consecutive days. In the continuation of these studies, it would be interesting to increase the amount of tested bread and see how it would affect postprandial glycemia.
To compare organoleptic characteristics of two sorts of bread and their effects on postprandial glucose and insulin levels in type 2 diabetes mellitus (T2DM) patients.
Postprandial blood glucose and insulin levels were examined on 2 consecutive days after the consumption of rye bread and a special type of bread with an herbal mixture. A questionnaire was used for comparison of the organoleptic properties of two kinds of bread.
A special type of bread with an herbal mixture caused significantly lower postprandial blood glucose in T2DM patients than rye bread, and it showed better organoleptic and satiety characteristics.
Our study showed a significant difference in postprandial blood glucose and insulin levels between patients that consumed rye bread and those that consumed a special type of bread with herbal mixture. This special type of bread has better effects on postprandial glucoregulation in T2DM patients.
The results of this research can be the basis and incentive for future research that would determine which biochemical substances from plant components added to STB are responsible for the effect of postprandial glycemia.
The authors wish to thank the Puratos and Delhaize companies for the administrative and technical support.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh; Horowitz M, Australia; Zeng Y, China S-Editor: Qu XL L-Editor: Filipodia P-Editor: Zhao S
| 1. | Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep. 2019;21:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 401] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 2. | International Diabetes Federation (IDF). IDF Diabetes Atlas Eighth Edition. 2017. Available from: https://diabetesatlas.org/. |
| 3. | Kelley DE. Sugars and starch in the nutritional management of diabetes mellitus. Am J Clin Nutr. 2003;78:858S-864S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2533] [Article Influence: 281.4] [Reference Citation Analysis (0)] |
| 5. | Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2454] [Article Influence: 175.3] [Reference Citation Analysis (0)] |
| 6. | Levesque C. Therapeutic Lifestyle Changes for Diabetes Mellitus. Nurs Clin North Am. 2017;52:679-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;12:CD003054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163:437-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 9. | Stevenson EJ, Williams C, Mash LE, Phillips B, Nute ML. Influence of high-carbohydrate mixed meals with different glycemic indexes on substrate utilization during subsequent exercise in women. Am J Clin Nutr. 2006;84:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 10. | Stevenson E, Williams C, Nute M. The influence of the glycaemic index of breakfast and lunch on substrate utilisation during the postprandial periods and subsequent exercise. Br J Nutr. 2005;93:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Johansson DP, Gutiérrez JLV, Landberg R, Alminger M, Langton M. Impact of food processing on rye product properties and their in vitro digestion. Eur J Nutr. 2018;57:1651-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ota A, Ulrih NP. An Overview of Herbal Products and Secondary Metabolites Used for Management of Type Two Diabetes. Front Pharmacol. 2017;8:436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Yeh ST. Using Trapezoidal Rule for the Area Under a Curve Calculation. Proceedings of the 27th Annual SAS® User Group International (SUGI’02). 2002. Available from: https://support.sas.com/resources/papers/proceedings/proceedings/sugi27/p229-27.pdf. |
| 14. | United States Department of Agriculture Agricultural Research Service. Functional Foods Research in ARS. 2010. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/00000000/NPS/FinalFunctionalFoodsPDFReadVersion6-25-10.pdf. |
| 15. | Dahl WJ, Stewart ML. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J Acad Nutr Diet. 2015;115:1861-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 16. | Liatis S, Tsapogas P, Chala E, Dimosthenopoulos C, Kyriakopoulos K, Kapantais E, Katsilambros N. The consumption of bread enriched with betaglucan reduces LDL-cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabetes Metab. 2009;35:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Weickert MO, Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. 2008;138:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 458] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 18. | Burton-Freeman BM, Keim NL. Glycemic index, cholecystokinin, satiety and disinhibition: is there an unappreciated paradox for overweight women? Int J Obes (Lond). 2008;32:1647-1654. [PubMed] [DOI] [Full Text] |
| 19. | Leinonen K, Liukkonen K, Poutanen K, Uusitupa M, Mykkänen H. Rye bread decreases postprandial insulin response but does not alter glucose response in healthy Finnish subjects. Eur J Clin Nutr. 1999;53:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Ezeani C, Ezenyi I, Okoye T, Okoli C. Ocimum basilicum extract exhibits antidiabetic effects via inhibition of hepatic glucose mobilization and carbohydrate metabolizing enzymes. J Intercult Ethnopharmacol. 2017;6:22-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 724] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 22. | Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res. 2017;61:1377571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Neelakantan N, Narayanan M, de Souza RJ, van Dam RM. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: a meta-analysis of clinical trials. Nutr J. 2014;13:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Huang FY, Deng T, Meng LX, Ma XL. Dietary ginger as a traditional therapy for blood sugar control in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e15054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Hou Q, Li Y, Li L, Cheng G, Sun X, Li S, Tian H. The Metabolic Effects of Oats Intake in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients. 2015;7:10369-10387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Chang WC, Jia H, Aw W, Saito K, Hasegawa S, Kato H. Beneficial effects of soluble dietary Jerusalem artichoke (Helianthus tuberosus) in the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease in high-fructose diet-fed rats. Br J Nutr. 2014;112:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Ahn HY, Kim M, Seo CR, Yoo HJ, Lee SH, Lee JH. The effects of Jerusalem artichoke and fermented soybean powder mixture supplementation on blood glucose and oxidative stress in subjects with prediabetes or newly diagnosed type 2 diabetes. Nutr Diabetes. 2018;8:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Medagama AB. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Xu L, Li Y, Dai Y, Peng J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol Res. 2018;130:451-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 30. | Eid AM, Jaradat N, Shraim N, Hawash M, Issa L, Shakhsher M, Nawahda N, Hanbali A, Barahmeh N, Taha B, Mousa A. Assessment of anticancer, antimicrobial, antidiabetic, anti-obesity and antioxidant activity of Ocimum Basilicum seeds essential oil from Palestine. BMC Complement Med Ther. 2023;23:221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 31. | Pandey KP, Dewangan J, Tripathi SS, Singh R, Jamal F, Rath SK. Garlic (Allium sativum): A Potential Antidiabetic Agent. 1st ed. 2022; 247-275. [DOI] [Full Text] |
| 32. | Geberemeskel GA, Debebe YG, Nguse NA. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella foenum-graecum L.) on Hyperlipidemia in Diabetic Patients. J Diabetes Res. 2019;2019:8507453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Van B, Abdalla AN, Algarni AS, Khalid A, Zengin G, Aumeeruddy MZ, Mahomoodally MF. Zingiber officinale Roscoe (Ginger) and its Bioactive Compounds in Diabetes: A Systematic Review of Clinical Studies and Insight of Mechanism of Action. Curr Med Chem. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Takahashi H, Nakajima A, Matsumoto Y, Mori H, Inoue K, Yamanouchi H, Tanaka K, Tomiga Y, Miyahara M, Yada T, Iba Y, Matsuda Y, Watanabe K, Anzai K. Administration of Jerusalem artichoke reduces the postprandial plasma glucose and glucose-dependent insulinotropic polypeptide (GIP) concentrations in humans. Food Nutr Res. 2022;66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Stevens N, Allred K. Antidiabetic Potential of Volatile Cinnamon Oil: A Review and Exploration of Mechanisms Using In Silico Molecular Docking Simulations. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |