Published online Nov 15, 2016. doi: 10.4251/wjgo.v8.i11.801
Peer-review started: June 1, 2016
First decision: August 10, 2016
Revised: August 25, 2016
Accepted: September 13, 2016
Article in press: September 18, 2016
Published online: November 15, 2016
Processing time: 166 Days and 20.8 Hours
A 48-year-old woman presented with bilateral enlarged ovaries, ascites, bilateral pleural effusion, and advanced gastric cancer. Pleural fluid cytology did not reveal malignant cells. Oophorectomy, performed as a palliative procedure, was followed by rapid resolution of the pleural effusion and ascites. The patient was diagnosed with pseudo-Meigs’ syndrome, and underwent chemotherapy followed by partial gastrectomy. At the last follow-up, 84 mo following oophorectomy, she was alive, and free of disease recurrence, despite not receiving any further treatment. Pseudo-Meigs’ syndrome should be considered in patients with bilateral ovarian tumors, ascites and pleural effusion, and treatment such as oophorectomy may result in symptomatic improvement and better prognosis in similar patients.
Core tip: In general, the prognosis of gastric cancer with distant metastases is poor. On the other hand, oophorectomy for gastric cancer-related metastatic ovarian tumors may improve survival, especially in the absence of metastasis to other organs. We here report a long-term survival case of pseudo-Meigs’ syndrome caused by gastric cancer following oophorectomy. We conclude that pseudo-Meigs’ syndrome should be considered in patients with gastric cancer with enlarged ovaries, pleural effusion, and ascites.
- Citation: Okamoto M, Maeda K, Yanagitani A, Tanaka K. Case of pseudo-Meigs' syndrome caused by gastric cancer-related metastatic ovarian tumor with prolonged survival. World J Gastrointest Oncol 2016; 8(11): 801-804
- URL: https://www.wjgnet.com/1948-5204/full/v8/i11/801.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i11.801
Meigs’ syndrome is defined as the presence of pleural effusion and ascites in association with benign ovarian tumors such as fibromas. In these patients, pleural effusion and ascites typically disappear following oophorectomy. Pseudo-Meigs’ syndrome is similar to Meigs’ syndrome, except that the ovarian tumor may be malignant rather than benign. Pseudo-Meigs’ syndrome caused by gastric cancer with metastatic ovarian tumors is very rare. Herein, we report a case of pseudo-Meigs’ syndrome caused by gastric cancer with metastatic ovarian tumors, with prolonged survival following oophorectomy.
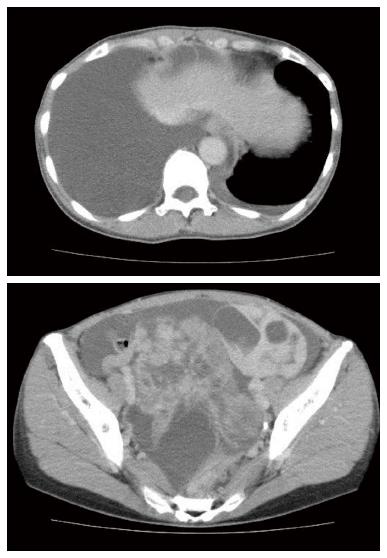
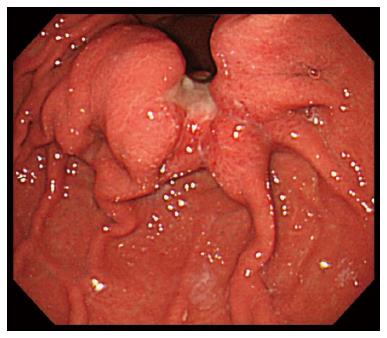
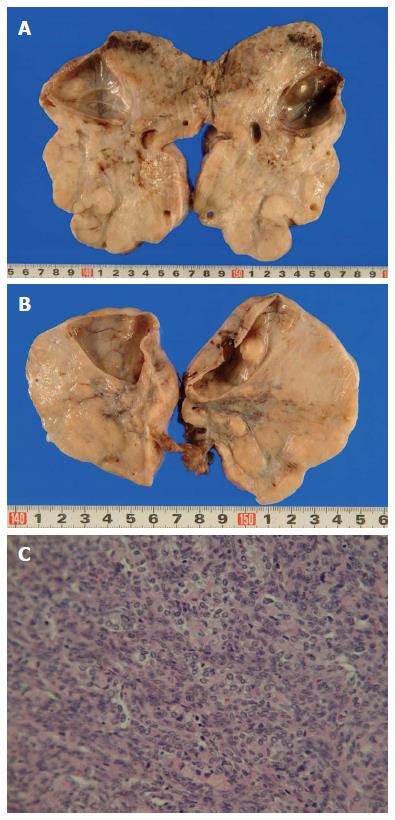
A 48-year-old woman was admitted to our hospital with a 3-mo history of lower abdominal fullness. She had no significant medical history. She smoked half a pack of cigarettes and consumed 1 L of beer a day for 28 years. On physical examination, her height and weight were 161 cm and 49 kg, respectively. Her blood pressure (103/60 mmHg), pulse rate (92 beats/min), body temperature (37.3 °C), and arterial oxygen saturation at room air (96%) were almost normal. The right chest breath sounds were decreased, her abdomen was distended, and a hard fist-sized mass was palpable in her lower abdomen. Her serum levels of carbohydrate antigen 19-9 and carbohydrate antigen 125 (CA 125) were elevated at 170.4 U/mL (normal, < 35 U/mL) and 897 U/mL (normal, < 37 U/mL), respectively. Computed tomography demonstrated bilateral enlarged ovaries, ascites, and bilateral pleural effusion (Figure 1). Upper gastrointestinal endoscopy revealed an ulcerated lesion with raised margins on the greater curvature of the body of the stomach (Figure 2). Biopsy of this lesion confirmed the diagnosis of poorly differentiated adenocarcinoma. Based on the above findings, a diagnosis of gastric cancer with metastatic ovarian tumors, malignant ascites, and pleural effusion, was made, although pleural fluid cytology failed to identify any malignant cells. Consequently, bilateral oophorectomy was performed as a palliative procedure. The resected ovarian tumors measured 15 cm (right) and 8 cm (left) in diameter. The tumors were solid with multiple mucus-containing cysts (Figure 3A and B). There was no evidence of tumor dissemination in the abdomen and the ascitic fluid was negative for cancer cells on cytology. Histological examination of the resected ovarian specimens confirmed that the tumors comprised poorly differentiated adenocarcinoma similar to the gastric tumor (Figure 3C), suggesting that the bilateral ovarian tumors were secondary to the gastric cancer (Krukenberg tumors).
The patient’s pleural effusion and ascites resolved completely within 2 wk of her surgery. In view of these findings, we considered a diagnosis of pseudo-Meigs’ syndrome. Following the oophorectomy, she received chemotherapy with docetaxel and S-1, with one course comprising docetaxel 40 mg/m2 as an intravenous infusion on day 1 and oral S-1 80 mg/m2 on days 1-14 of a 21-d cycle. After 10 cycles of chemotherapy, approximately 9 mo after her first hospital visit, since no further metastases were detected, she underwent distal gastrectomy. The final pathological diagnosis was Stage IV [pT3, pN1, cM1(Ovary)] according to the TNM classification of gastric carcinoma (UICC fifth edition). At the last follow-up, 84 mo following oophorectomy, she was alive and free of disease recurrence, despite not receiving any further treatment.
In 1937, Meigs and Cass[1] first reported a case series of seven patients with ovarian fibroma with pleural effusion and ascites, in whom the pleural effusion and ascites disappeared following removal of the ovarian tumors. Subsequently, Rhoads et al. reported similar cases and coined the term “Meigs’ syndrome”[1,2]. A similar presentation associated with primary malignant or metastatic ovarian tumors, instead of benign ovarian tumors, is referred to as pseudo-Meigs’ syndrome[3].
Ascites may be secondary to ovarian tumors or fluid secretion from the peritoneum, develop as a result of tumor stimulation or in response to cytokines, or may be secondary to tumor-related lymphatic obstruction[4]. Pleural effusion is explained as occurring secondary to the movement of ascitic fluid to the pleural cavity via transdiaphragmatic lymphatics and diaphragmatic foramen[5]. It is uncommon for malignant tumor cells to be identified in the pleural or ascitic fluid in patients with pseudo-Meigs’ syndrome.
Metastatic ovarian cancer comprises 6%-30% of all ovarian malignancies. The most common primary sites are the gastrointestinal tract, breast, and reproductive organs. In Japan, gastric cancer is the most common primary site because of its relatively high prevalence[6]. Pseudo-Meigs’ syndrome is more frequently caused by primary ovarian malignant tumors than ovarian tumors metastases from gastrointestinal cancer, and gastric cancer as the primary source for pseudo-Meigs’ syndrome is particularly rare. In fact, to our knowledge, only 10 cases have been described so far, including five in the literature[5,7-10] and five in Japanese conference proceedings. The mean age of the patients in the published reports was 51.8 (range, 32-76) years. Two and three cases had unilateral and bilateral ovarian metastases, respectively, and the ovarian tumor size ranged from 10 to 25 cm in diameter. Pleural effusion was bilateral in three cases and unilateral in two. Moreover, the serum CA 125 levels are often elevated in patients with Meigs’ syndrome and decrease following oophorectomy, as in the present case. Benjapibal et al[11] explained that CA 125 may be produced from the peritoneal epithelium by a biomedical factor, secondary to mechanical irritation by a large tumor, or owing to an increase in the intraperitoneal pressure related to the large volume of ascites.
The prognosis of gastric cancer with distant metastasis is poor, with a median survival time of 8.6-13.8 mo when treated by chemotherapy alone or in combination with molecular targeted therapy[12]. On the other hand, Lu et al[13] reported that oophorectomy for gastric cancer-related metastatic ovarian tumors may improve survival, especially in the absence of metastasis to other organs. The overall survival times of patients who did and did not undergo metastasectomy were reported as 14.1 and 8 mo, respectively, in their study. Furthermore, Brieau et al[14] reported that oophorectomy along with current chemotherapy regimens, such as taxane- or platinum-based therapy, improved survival even if the patients had extra-ovarian metastatic sites. In the present case, we selected a docetaxel and S-1 combination regimen with the expectation of efficacy for non-measurable lesions other than in the ovaries[15,16]. The increased survival of the patient reported herein may be owing to her oophorectomy in conjunction with chemotherapy. Accordingly, this case emphasizes the need to be aware of pseudo-Meigs’ syndrome, and supports the recommendation of oophorectomy in cases where metastases are limited to the ovaries.
A 48-year-old woman with a 3-mo history of lower abdominal fullness.
Gastric cancer with metastatic ovarian tumors, malignant ascites, and pleural effusion.
Gastric cancer with primary ovarian cancer, reactive ascites and, pleural effusion.
The serum levels of carbohydrate antigen 19-9 and carbohydrate antigen 125 were elevated, at 170.4 U/mL (normal, < 35 U/mL) and 897 U/mL (normal, < 37 U/mL), respectively.
Computed tomography demonstrated bilateral enlarged ovaries, ascites, and bilateral pleural effusion.
The resected ovarian specimens comprised poorly differentiated adenocarcinoma, similar to the gastric tumor, suggesting that the bilateral ovarian tumors were secondary to the gastric cancer; there was no evidence of tumor dissemination in the abdomen and the ascitic fluid was negative for cancer cells on cytology.
Oophorectomy and gastrectomy in conjunction with chemotherapy.
Gastric cancer, as the primary source for pseudo-Meigs’ syndrome, is particularly rare. This case is one of only a few reports of pseudo-Meigs’ syndrome caused by gastric cancer with prolonged survival.
Meigs’ syndrome is defined as the presence of pleural effusion and ascites in association with benign ovarian tumors such as fibromas. In these patients, pleural effusion and ascites typically disappear following oophorectomy. Pseudo-Meigs’ syndrome is similar to Meigs’ syndrome, except that the ovarian tumor may be malignant rather than benign.
The authors experienced a long-term survival case of pseudo-Meigs’ syndrome caused by gastric cancer. If the metastases are limited to the ovaries, it is important not to automatically assume that the tumor is unresectable.
This is an interesting case report in terms of the disappearance of ascites and pleural effusion after oophorectomy, and prolonged survival despite of the extent of the disease at diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Zaanan A S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Meigs JV, Cass JW. Fibroma of the ovary with ascites and hydrothorax, with a report of seven cases. Am J Obstet Gynecol. 1937;33:249-267. [RCA] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 212] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Rhoads JE, Terrell AW. Ovarian fibromas with ascites and hydrothorax (Meigs’ syndrome). JAMA. 1937;109:1684-1687. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | MEIGS JV. Pelvic tumors other than fibromas of the ovary with ascites and hydrothorax. Obstet Gynecol. 1954;3:471-486. [PubMed] |
| 4. | Abramov Y, Anteby SO, Fasouliotis SJ, Barak V. The role of inflammatory cytokines in Meigs’ syndrome. Obstet Gynecol. 2002;99:917-919. [PubMed] |
| 5. | Cetin B, Aslan S, Akinci M, Atalay C, Cetin A. A long surviving case of Pseudomeigs’ syndrome caused by Krukenberg tumor of the stomach. Jpn J Clin Oncol. 2005;35:221-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | de Waal YR, Thomas CM, Oei AL, Sweep FC, Massuger LF. Secondary ovarian malignancies: frequency, origin, and characteristics. Int J Gynecol Cancer. 2009;19:1160-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Dick HJ, Spire LJ, Worboys CS. The association of Meigs’s syndrome with Krukenberg tumors. N Y State J Med. 1950;50:1842-1843. [PubMed] |
| 8. | Miyata R, Hosoda Y, Hashimoto M, Ko J. A case of gastric adenocarcinoma presenting pseudo-Meigs’ syndrome. J Jpn Surg Assoc. 2004;65:508-513. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Okazaki Y, Yonezawa K, Shimomatsuya T, Komai Y, Yukioka N. A case of pseudo-Meigs’ syndrome caused by metastatic ovarian tumors from gastric cancer. Nihon Shokakibyo Gakkai Zasshi. 2009;106:529-535. [PubMed] |
| 10. | Horimatsu T, Miyamoto S, Mashimo Y, Okabe H, Mikami Y, Chiba T, Muto M. Pseudo-Meigs’ syndrome caused by a Krukenberg tumour of gastric cancer. Intern Med. 2015;54:2595-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Benjapibal M, Sangkarat S, Laiwejpithaya S, Viriyapak B, Chaopotong P, Jaishuen A. Meigs’ Syndrome with Elevated Serum CA125: Case Report and Review of the Literature. Case Rep Oncol. 2009;2:61-66. [PubMed] |
| 12. | Jou E, Rajdev L. Current and emerging therapies in unresectable and recurrent gastric cancer. World J Gastroenterol. 2016;22:4812-4823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Lu LC, Shao YY, Hsu CH, Hsu C, Cheng WF, Lin YL, Cheng AL, Yeh KH. Metastasectomy of Krukenberg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res. 2012;32:3397-3401. [PubMed] |
| 14. | Brieau B, Auzolle C, Pozet A, Tougeron D, Bouché O, Soibinet P, Coriat R, Prieux C, Lecomte T, Goujon G. Efficacy of modern chemotherapy and prognostic factors in patients with ovarian metastases from gastric cancer: A retrospective AGEO multicentre study. Dig Liver Dis. 2016;48:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Yoshida K, Ninomiya M, Takakura N, Hirabayashi N, Takiyama W, Sato Y, Todo S, Terashima M, Gotoh M, Sakamoto J. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res. 2006;12:3402-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |