Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2200
Peer-review started: January 19, 2024
First decision: January 27, 2024
Revised: February 8, 2024
Accepted: March 11, 2024
Article in press: March 11, 2024
Published online: May 15, 2024
Processing time: 111 Days and 6.1 Hours
The lack of specific symptoms of gastric cancer (GC) causes great challenges in its early diagnosis. Thus it is essential to identify the risk factors for early diagnosis and treatment of GC and to improve the survival rates.
To assist physicians in identifying changes in the output of publications and research hotspots related to risk factors for GC, constructing a list of key risk factors, and providing a reference for early identification of patients at high risk for GC.
Research articles on risk factors for GC were searched in the Web of Science core collection, and relevant information was extracted after screening. The literature was analyzed using Microsoft Excel 2019, CiteSpace V, and VOSviewer 1.6.18.
A total of 2514 papers from 72 countries and 2507 research institutions were retrieved. China (n = 1061), National Cancer Center (n = 138), and Shoichiro Tsugane (n = 36) were the most productive country, institution, or author, respec
In this study, we found that H. pylori infection is the most significant risk factor for GC; single-nucleotide polymorphism (SNP) is the most dominant genetic factor for GC; bio-diagnostic markers are the most promising diagnostic modality for GC. GC risk prediction models are the latest current research hotspot. We conclude that the most important risk factors for the development of GC are H. pylori infection, SNP, smoking, diet, and alcohol.
Core Tip: In this study, we searched and organized relevant articles in the field of risk factors for gastric cancer (GC) and carried out a bibliometric analysis. In this manuscript, we summarize the current state of development in the field, produce a table of key risk factors in GC, and achieve a prediction of future research trends.
- Citation: Li M, Gao N, Wang SL, Guo YF, Liu Z. Hotspots and trends of risk factors in gastric cancer: A visualization and bibliometric analysis. World J Gastrointest Oncol 2024; 16(5): 2200-2218
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2200.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2200
Gastric cancer (GC) is a prevalent cancer worldwide, with over 1 million new cases and 783000 new deaths worldwide in the year 2018 alone[1]. According to the World Health Organization (WHO), GC is the fifth most prevalent cancer and the fourth leading cause of cancer-related deaths worldwide as of 2020[2]. In China, GC ranks as the third leading cause of cancer-related deaths[3]. Although surgery is the standard treatment for GC, early detection, diagnosis, and treatment are pivotal in improving patient outcomes[4]. The early symptoms of GC patients are not obvious, leading to diagnosis at advanced stages[5]. It has been noted that patients diagnosed with early-stage GC can achieve a 5-year survival rate of up to 90%[6], while for those diagnosed with advanced stage GC, the rate is only 10%[7]. In East Asia, population-based screening programs have been implemented in countries with high GC morbidity and mortality. For example, opportunistic screening has been conducted in China, Japan, Korea, and Singapore[8], and in particular, Japan and Korea have implemented national GC screening programs[9]. The Chinese Ministry of Health launched a program to promote early diagnosis and treatment of cancer in rural areas with a high prevalence of malignancies in 2005, of which GC is a key target[10]. Evidence from a nested case-control study in Korea demonstrated that endoscopic screening program can reduce GC-related mortality by 47%[11]. These screening programs increased the detection rate of early GC and significantly reduced the mortality rate of GC patients[4]. However, despite advancements in the assessment and management of gastric precancerous lesions, significant uncertainty persists regarding risk factors indicating tumor progression[12]. Therefore, the identification of risk factors is of great importance for GC screening. For example, compared to non-smoking men, those with a smoking history had 1.13 and 2.43 times higher risks of GC when presenting with normal and below-normal serum pepsinogen I (PGI) levels, respectively[13]. Adamu et al[14] found that advanced age was a key risk factor for the development and progression of chronic atrophic gastritis. In addition, factors such as alcohol consumption[15] and a family history of tumors[16] also increase the risk of GC development.
In order to better understand the field, the following questions need to be addressed first: (1) What are the annual trends in articles? (2) What are the differences in nationals, institutionals and authors contributions? (3) What are the most popular risk factors in research? And (4) In what area is future research likely to emerge? Traditional literature reviews are time-consuming and labor-intensive, and there are limitations to the research[17], making it difficult to provide a reasonable description of the aforementioned issues. In contrast, bibliometrics, through a multidisciplinary approach[18], can achieve qualitative and quantitative analyses of publications in a specific field[19] so as to sort out the research lineages and grasp the research trends and directions[20].
There is no research to date that has sorted and analyzed risk factors for GC-related research results from a bibliometric perspective. This paper provides a detailed analysis of risk factors for GC field in terms of countries, institutions, authors, sources and keywords, allowing physicians to stay up-to-date with the latest research evidence in key clinical areas and make increasingly informed decisions.
Data were retrieved using the Science Citation Index-Expanded in the Web of Science Core Collection (WOSCC). In order to keep the proportion of articles that were not relevant to the topic within a reasonable range, the search method of title search was utilized[21-23]. The search formula was set as: TI = (“Stomach Neoplasm” OR “Gastric Neoplasm” OR “Cancer of Stomach” OR “Stomach Cancer” OR “Gastric Cancer” OR “Cancer of the Stomach”) AND TI = (“Risk Factor” OR “Social Risk Factor” OR “Health Correlates” OR “Population at Risk” OR “Risk Score” OR “Risk Factor Score” OR “Predictor” OR “Risk”).
The screening criteria were: (1) Publication time range: from Jan. 01, 2000 to Dec. 04, 2022; (2) publication language in English; and (3) publication type was research article or review. The data retrieval strategy and screening process of this study are shown in Figure 1.
In this study, Microsoft Excel 2019, CiteSpace V, and VOSviewer 1.6.18 software were used for data analysis. Microsoft Excel software allows descriptive statistics on the number of publications and citations by countries, institutions, and authors[24], where Impact factor (IF), and Journal Citation Report (JCR) category[25] were obtained using JCR Science Edition (2021). VOSviewer enables the visual analysis of collaborative networks of countries, institutions and authors as well as keyword clusters. CiteSpace allows for keyword burst detection, which leads to the analysis of research hotspots and trends in the time dimension[26]. The software parameters were set as time slice (1990-2022), years per slice (1), and selection strategy (g-index, k = 25)[27].
Two investigators (Li M and Gao N) performed the data search independently and discussed any discrepancies. Data retrieval and export were completed on the same day (December 04, 2022), minimizing bias caused by database updates. This study did not include any animals or experiments and therefore did not require ethics committee approval.
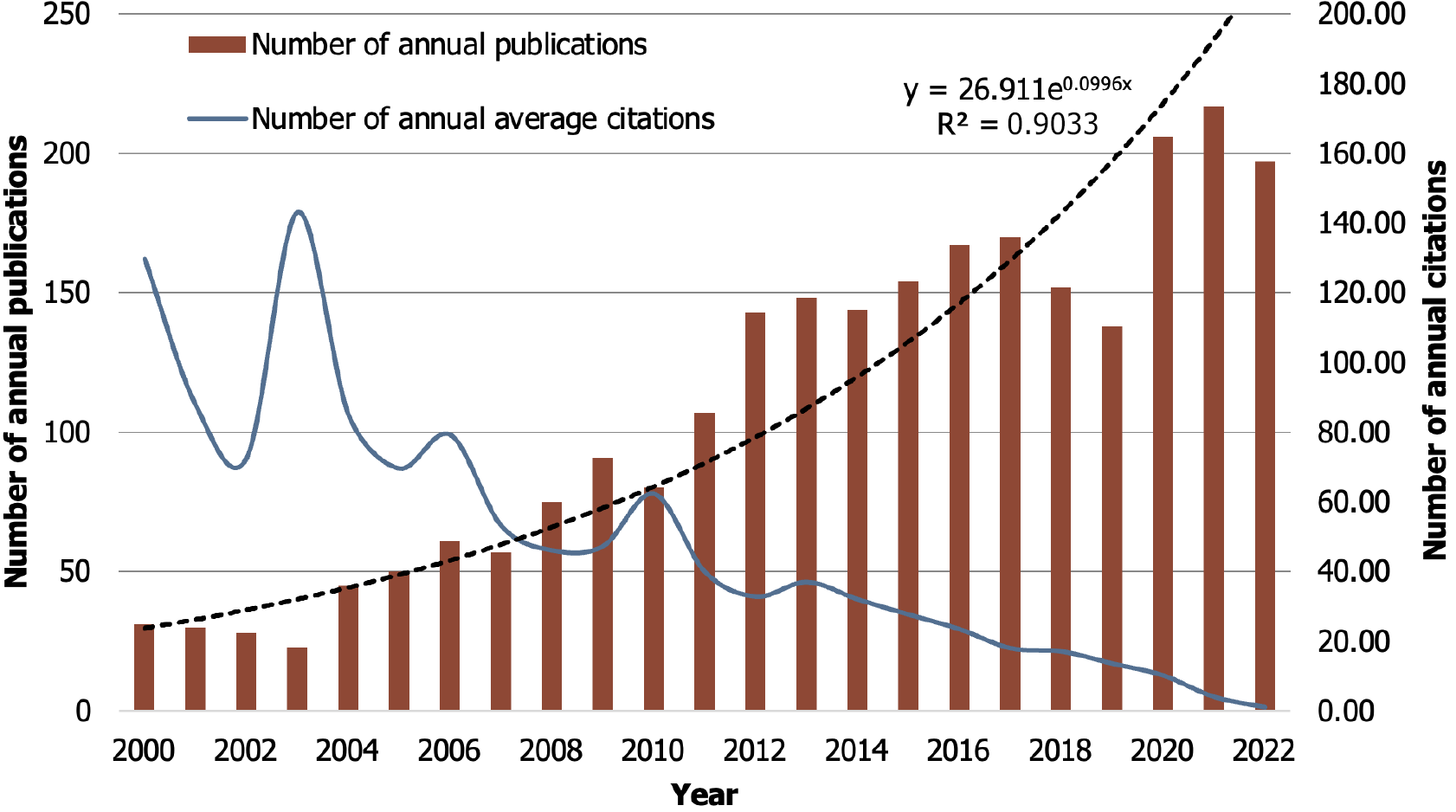
Between 2000 and 2022, WOSCC published 2514 papers on risk factors for GC, including 2352 articles and 162 reviews. Figure 2 illustrates the annual growth trend of publication volume and citations, which showed an overall upward trend in the past 20 years and reached its peak in 2021 (n = 217). The exception to this trend was a small decrease in volume in 2018 and 2019. The annual growth curve of publications (y = 26.911e0.0996x) was fitted by an exponential function, where R2 = 0.9033, suggesting a strong correlation between the number of publications and the year. For the average annual citations of publications, there were two peaks in 2000 and 2003, respectively, after which the average citations decreased year by year. It can be predicted that the field will continue to attract the attention of scholars in the coming years, meanwhile the number of annual publications will continue to grow.
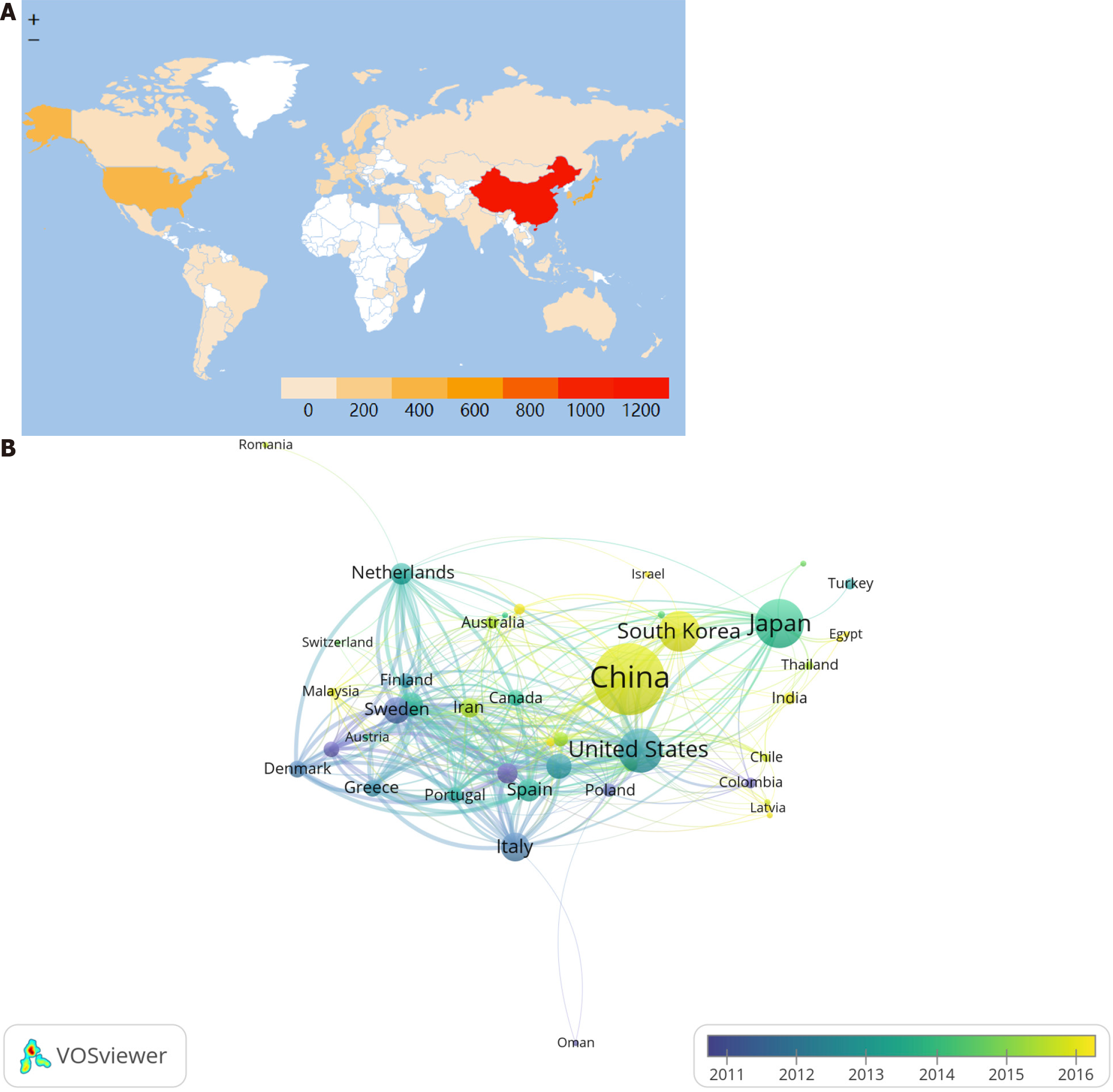
Seventy-two countries were involved in publishing risk factors for GC-related studies, and China was the main contributing country in this field with 1061 publications, accounting for 42.20% of the total publications, followed by Japan (n = 467), United States (n = 383), and South Korea (n = 311). Nine of the top ten contributing countries were developed countries, indicating a strong correlation between a country’s economic development level and its publication output. In terms of citations, the United Kingdom has the highest average number of citations (n = 73.60), followed by Netherlands (n = 57.31), and United States (n = 55.34), respectively. This may be a reflection of the differences in research quality between countries. Although China is a high-output country in risk factors for GC, the quality of research needs to be further improved (Table 1). Worldwide, the participation of East Asian, North American, and Western European countries is high, while the participation of African, Western Asian, and Eastern European countries is low, with some regional differences in participation (Figure 3A). Among the cooperative networks, the United States has the highest total link strength (TLS = 644) and has cooperated with 45 countries, among which the closest cooperation is with China (TLS = 131). Meanwhile, it can be seen by the node color that European countries such as France, Switzerland, and Norway conducted related research around 2011, while China and Korea started later, but developed faster (Figure 3B).
| Rank | Country | TP | Percent (%) | TC | CPP |
| 1 | China | 1061 | 42.20 | 22402 | 21.11 |
| 2 | Japan | 467 | 18.58 | 13585 | 29.09 |
| 3 | United States | 383 | 15.23 | 21194 | 55.34 |
| 4 | South Korea | 311 | 12.37 | 6831 | 21.96 |
| 5 | Italy | 152 | 6.05 | 7327 | 48.20 |
| 6 | Sweden | 122 | 4.85 | 5669 | 46.47 |
| 7 | Germany | 116 | 4.61 | 4919 | 42.41 |
| 8 | United Kingdom | 108 | 4.30 | 7949 | 73.60 |
| 9 | Spain | 89 | 3.54 | 4225 | 47.47 |
| 10 | Netherlands | 83 | 3.30 | 4757 | 57.31 |
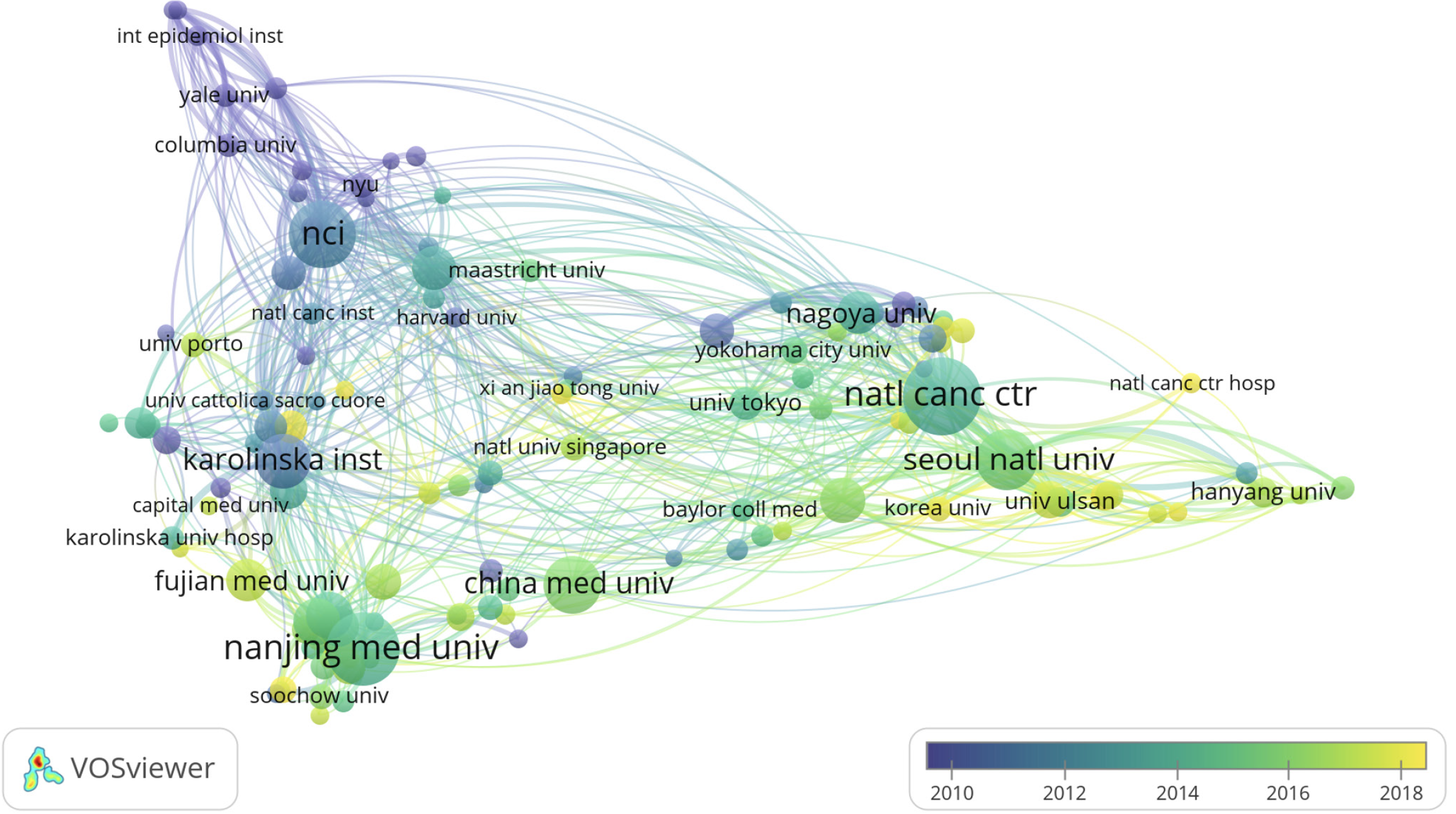
There were 2507 institutions involved in the publication of risk factors for GC-related articles. The highest contributor was the National Cancer Center with 138 publications, followed by Nanjing Medical University (n = 122), National Cancer Institute (n = 104), and Seoul National University (n = 88). 7 of the top 10 contributors are located in Asia, including 4 in China and 3 in Korea. In addition, National Cancer Institute ranks first in terms of total citations and average citations (Table 2). The National Cancer Institute has the highest TLS (TLS = 236) in the collaborative network, having collaborated with 152 institutions, with the closest collaboration with Vanderbilt University (TLS = 25) (Figure 4). Both institutions have carried out extensive research work on the correlation between diet and GC and found a correlation between GC and the folic acid content of food[28], meat intake[29], and fruit intake[30].
| Rank | Institution | Country | TP | TC | CPP |
| 1 | National Cancer Center | Korea | 138 | 5475 | 39.67 |
| 2 | Nanjing Medical University | China | 122 | 2906 | 23.82 |
| 3 | National Cancer Institute | United States | 104 | 9330 | 89.71 |
| 4 | Seoul National University | Korea | 88 | 2336 | 26.55 |
| 5 | China Medical University | China | 81 | 1460 | 18.02 |
| 6 | Karolinska Institute | Sweden | 73 | 3111 | 42.62 |
| 7 | Fudan University | China | 57 | 1435 | 25.18 |
| 8 | Shanghai Jiao Tong University | China | 57 | 1706 | 29.93 |
| 9 | Yonsei University | Korea | 53 | 1628 | 30.72 |
| 10 | Vanderbilt University | United States | 50 | 2843 | 56.86 |
There are 489 journals with publications in the field of risk factors for GC, and Table 3 shows the 10 journals with the highest number of articles. These 10 journals published 604 articles, accounting for 24.03% of the total number of articles. International Journal of Cancer published the most articles with 115, followed by Gastric Cancer, Plos One, and World Journal of Gastroenterology, all with 71 articles. Only two journals had IFs >5, Gastric Cancer (IF = 7.70) and World Journal of Gastroenterology (IF = 5.37). Three journals were temporarily outside the SCI category and two journals were in the Q1 JCR division.
| Rank | Journal | TP | TC | CPP | JCR | IF2021 |
| 1 | International Journal of Cancer | 115 | 6620 | 57.57 | Q2 | 4.37 |
| 2 | Gastric Cancer | 71 | 1751 | 24.66 | Q1 | 7.70 |
| 3 | Plos One | 71 | 1417 | 19.96 | Q2 | 3.75 |
| 4 | World Journal of Gastroenterology | 71 | 2364 | 33.30 | Q2 | 5.37 |
| 5 | Asian Pacific Journal of Cancer Prevention | 56 | 742 | 13.25 | - | - |
| 6 | Cancer Epidemiology Biomarkers & Prevention | 55 | 3968 | 72.15 | - | - |
| 7 | Annals of Surgical Oncology | 42 | 1350 | 32.14 | Q1 | 4.34 |
| 8 | BMC Cancer | 42 | 843 | 20.07 | Q2 | 4.64 |
| 9 | Oncotarget | 41 | 849 | 20.71 | - | - |
| 10 | Medicine | 40 | 605 | 15.13 | Q3 | 1.82 |
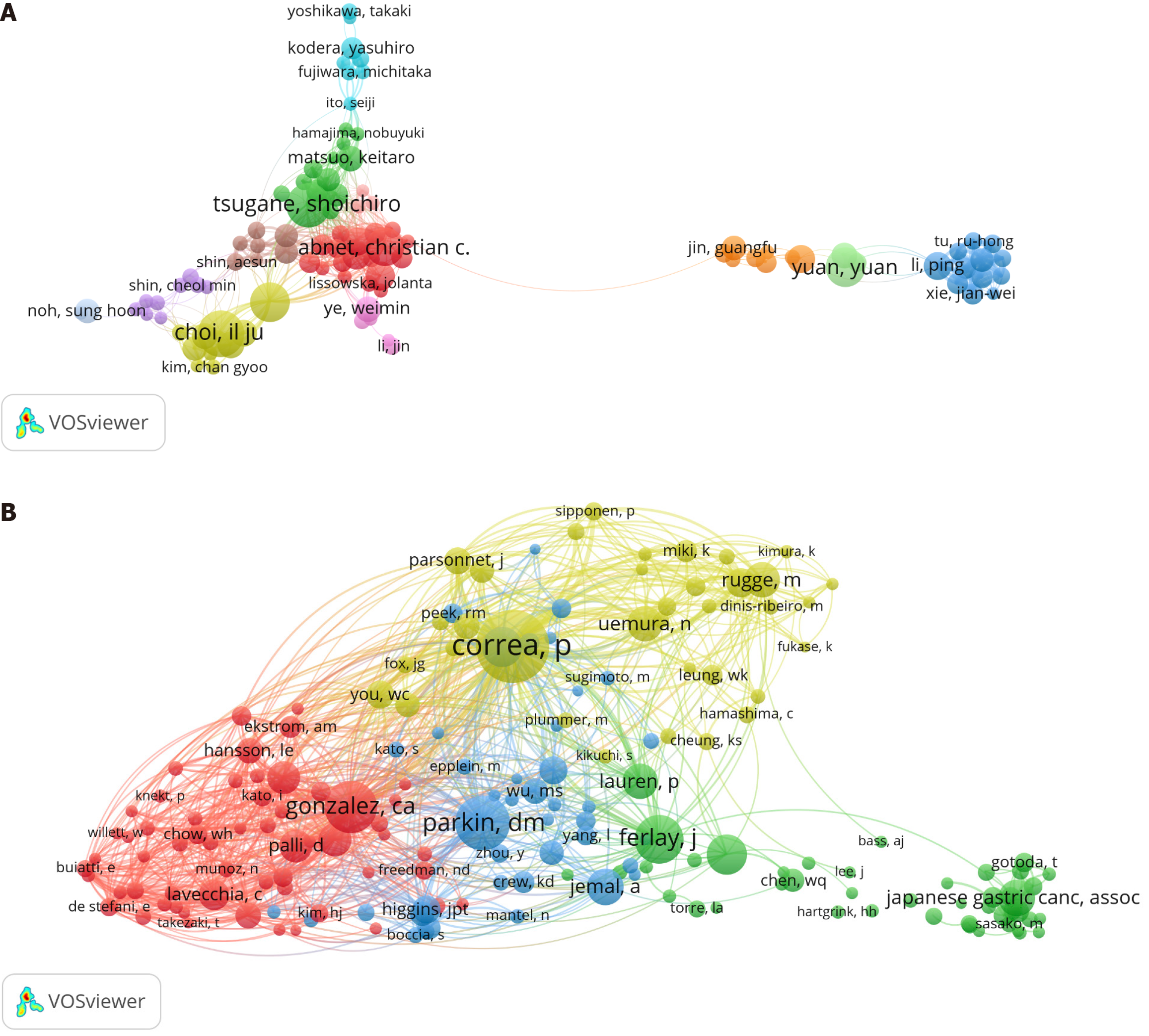
A total of 12569 researchers were involved in publishing risk factors for GC-related articles, with Il Ju Choi publishing the most articles (n = 36), followed by Shoichiro Tsugane (n = 34), Jeongseon Kim (n = 31), and Yuan Yuan (n = 31). Wong-ho Chow has the highest CPP value along with a relatively high study quality (Table 4). A threshold value of 10 was set to construct an author collaboration network, in which Shoichiro Tsugane had the highest TLS (TLS = 169) and has successively collaborated with 70 authors. In addition, it can be seen by the node color that numerous tightly knit research teams are involved in the risk factors for GC field (Figure 5A). A total of 30894 researchers constitute the co-cited authors of publications in the risk factors for GC field, with P Correa ranked first in terms of co-citations (n = 617), followed by Dm Parkin (n = 442) and Ca Gonzalez (n = 393). A threshold value of 50 was set to construct the co-cited authors' network, and the results are presented in Figure 5B.
| Rank | Author | TP | TC | CPP | Co-cited author | Co-citations |
| 1 | Il Ju Choi | 36 | 706 | 19.61 | P Correa | 617 |
| 2 | Shoichiro Tsugane | 34 | 1021 | 30.03 | Dm Parkin | 422 |
| 3 | Jeongseon Kim | 31 | 619 | 19.97 | Ca Gonzalez | 393 |
| 4 | Yuan yuan | 31 | 526 | 16.97 | J Ferlay | 352 |
| 5 | Christian C Abnet | 30 | 1161 | 38.70 | Em El-omar | 271 |
| 6 | Wong-ho Chow | 23 | 1091 | 47.43 | F Bray | 270 |
| 7 | Ping Li | 22 | 336 | 15.27 | A Jemal | 241 |
| 8 | Xiao-ou Shu | 22 | 564 | 25.64 | N Uemura | 233 |
| 9 | Li Yang | 22 | 414 | 18.82 | M Rugge | 230 |
| 10 | Wei Zheng | 22 | 564 | 25.64 | P Lauren | 228 |
Highly cited papers indicate a significant impact in a field[31], reflecting the hot spots and depth of research in the field[32]. Table 5 lists the top 10 cited publications, with Emad M El-Omar's "Interleukin-1 polymorphisms associated with increased risk of gastric cancer" published in 2000 being the most cited. Two of these ten articles are reviews on risk factors for GC and three are related to Helicobacter pylori (H. pylori) infection. Further co-cited reference analysis was performed, and the results are shown in Table 6.
| Rank | Title | First author | Source | Publication year | TC |
| 1 | Interleukin-1 polymorphisms associated with increased risk of gastric cancer | Emad M El-Omar | Nature | 2000 | 1800 |
| 2 | Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial | Benjamin Chun-Yu Wong | Jama | 2004 | 1045 |
| 3 | Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention | Parisa Karimi | Cancer Epidemiol Biomarkers Prev | 2014 | 1015 |
| 4 | Helicobacter pylori and gastric cancer: factors that modulate disease risk | Lydia E Wroblewski | Clin Microbiol Rev | 2010 | 811 |
| 5 | Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms | Emad M El-Omar | Gastroenterology | 2003 | 711 |
| 6 | Population attributable risks of esophageal and gastric cancers | Lawrence S Engel | J Natl Cancer Inst | 2003 | 513 |
| 7 | Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China | Gina D Tran | Int J Cancer | 2005 | 494 |
| 8 | Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands | Annemarie C de Vries | Gastroenterology | 2008 | 468 |
| 9 | Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer | Hiroshi Ohata | Int J Cancer | 2004 | 376 |
| 10 | Gastric cancer epidemiology and risk factors | Douglas E Guggenheim | J Surg Oncol | 2013 | 346 |
| Rank | Title | First author | Source | Publication year | TC |
| 1 | Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries | Freddie Bray | Ca-cancer J Clin | 2018 | 251 |
| 2 | The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. | P Lauren | Acta Pathol Mic Sc | 1965 | 224 |
| 3 | Helicobacter pylori infection and the development of gastric cancer | N Uemura | New Engl J Med | 2001 | 207 |
| 4 | Global cancer statistics, 2002 | D Max Parkin | Ca-cancer J Clin | 2005 | 196 |
| 5 | Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994 | M F Dixon | Am J Surg Pathol | 1996 | 180 |
| 6 | Bias in meta-analysis detected by a simple, graphical test | M Egger | BMJ | 1997 | 159 |
| 7 | Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 | Jacques Ferlay | Int J Cancer | 2015 | 150 |
| 8 | Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts | Helicobacter and Cancer Collaborative Group | Gut | 2001 | 144 |
| 9 | Interleukin-1 polymorphisms associated with increased risk of gastric cancer | M Egger | BMJ | 1997 | 137 |
| 10 | Meta-analysis in clinical trials | R DerSimonian | Control Clin Trials | 1986 | 126 |
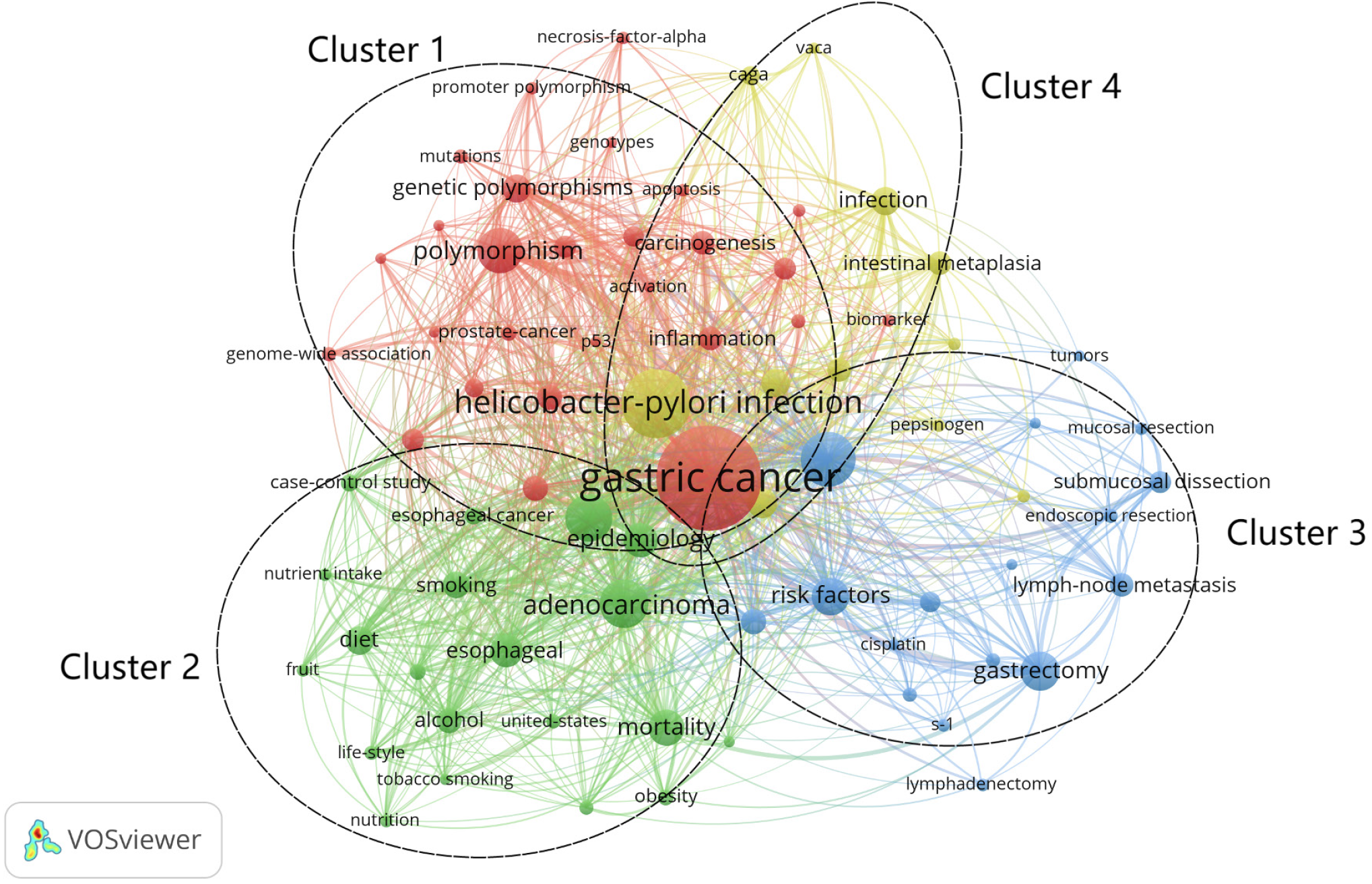
The list of key risk factors for GC: Keywords are high-level summaries and condensations of topics in an article[33]. High-frequency keywords represent hot topics in a research field[34]. In this study, the frequency of research on the risk factors in the keywords represents their visibility in the field. It also reflects the importance of this risk factor in the development of GC. A total of 6412 keywords were included in this study, and the top 20 risk factors in terms of study frequency were further extracted to construct a list of key risk factors for GC by excluding the subject terms (Table 7). Among them, "H. pylori infection" (n = 717), "polymorphism" (n = 326), "smoking" (n = 218), and "diet" (n = 137) were the main risk factors for GC.
| Rank | Keyword | TP | Rank | Keyword | TP |
| 1 | Helicobacter pylori infection | 717 | 11 | Nutrient intake | 62 |
| 2 | Polymorphism | 326 | 12 | DNA methylation | 40 |
| 3 | Smoking | 218 | 13 | Life-style | 36 |
| 4 | Diet | 137 | 14 | Fruit | 33 |
| 5 | Alcohol | 112 | 15 | Pepsinogen | 32 |
| 6 | IM | 99 | 16 | Promoter polymorphism | 31 |
| 7 | Inflammation | 98 | 17 | Lymphadenectomy | 29 |
| 8 | Obesity | 91 | 18 | Necrosis-factor-alpha | 29 |
| 9 | Caga | 63 | 19 | s-1 | 29 |
| 10 | Biomarker | 62 | 20 | p53 | 27 |
Research hotspots (clustering analysis of keywords): In the keyword co-occurrence network (Figure 6), the keywords can be divided into 4 clusters according to their colors, which are: Cluster 1 (red): molecular epidemiology, involving 27 keywords including "apoptosis", "DNA methylation", "genetic polymorphisms", "necrosis-factor-aloha" and 27 other keywords; Cluster 2 (green): lifestyle, including "alcohol", "cigarette-smoking", "diet", and "obesity"; Cluster 3 (blue): postoperative risk factors, involving "chemotherapy", "endoscopic resection", "gastrectomy" and 17 other keywords; Cluster 4 (yellow): GC-related disease screening, containing only "chronic atrophic gastritis", "endoscopy", "helicobacter-pylori infection" and 11 other keywords.
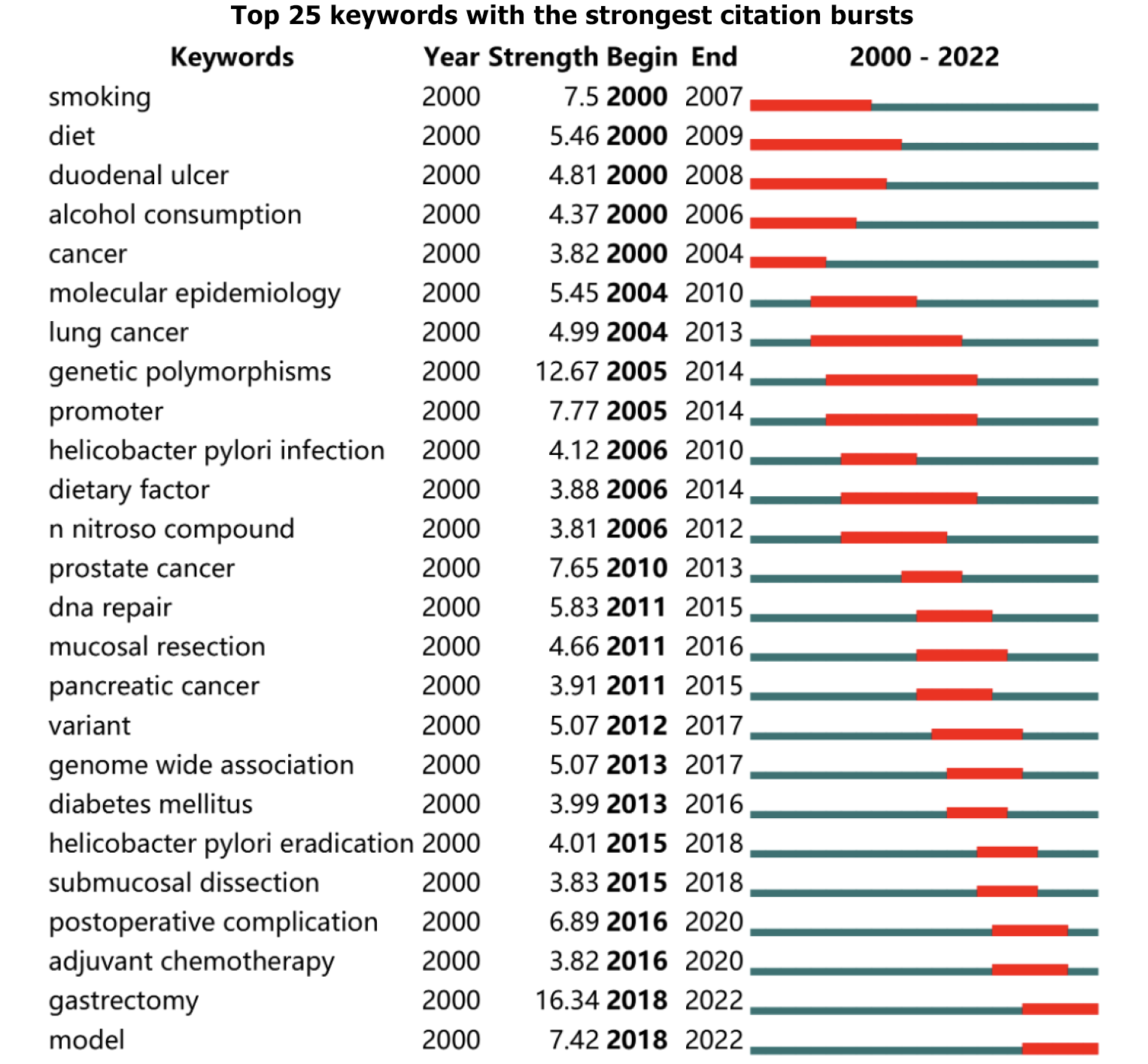
Research fronts (keyword burst analysis): Keyword burst analysis can determine the evolution of research hotspots by analyzing the temporal trends of research hotspots[35]. Figure 7 shows the top 25 keywords in terms of burst intensity between 2000-2022, where the strongest keyword is gastrectomy (16.34) and the keywords with the longest burst duration are diet (2000-2009), lung cancer (2004-2013), genetic polymorphisms (2005-2014), and promoter (2005-2014). Notably, the keywords that continue to explode until 2022 are: gastrectomy (2018-2022), and model (2018-2022), respectively, which are the current issues at the forefront in risk factors for GC.
In this study, we used bibliometric methods to count 2514 risk factors for GC-related articles in the WOSCC database, analyze the contributions of countries, institutions, authors, and journals, and summarize hot topics and predict future research directions. To the best of our knowledge, this study is the first bibliometric analysis of publications on risk factors for GC.
The number of annual publications has increased each year since 2000, with 72 countries, 489 journals, 2507 institutions, and 12569 authors participating in this field of study. This indicates that risk factors for GC is a popular research area at present, and it is foreseeable that more countries and researchers will participate in risk factors for GC research in the future. China, Japan, and the United States are the main contributors to publications in the field, and the United States is an important initiator and promoter of international cooperation, playing an active role in exchanging academic results and sharing academic resources. Asian institutions, such as the National Cancer Center (Goyang-si, Korea) and Nanjing Medical University (Nanjing, China), are the primary contributors in the field of risk factors for GC. Leading researchers such as Il Ju Choi, Shoichiro Tsugane, Jeongseon Kim, and Yuan Yuan have made substantial contributions. International Journal of Cancer, Gastric Cancer, Plos One, and World Journal of Gastroenterology are the four authoritative academic journals in the field of risk factors for GC, which is valuable for understanding the significant academic perspectives.
Through analyses of high-frequency keywords, highly cited literature, keyword clustering, and burst words, we identified four research hotspots: (1) H. pylori infection; (2) single nucleotide polymorphism; (3) bio-diagnostic markers; and (4) GC risk prediction models.
Keyword analysis identified H. pylori infection as the most significant risk factor for GC. Approximately 90% of GC are associated with H. pylori infection. Eradication of H. pylori reduces the inflammatory response, promotes ulcer healing, and reduces the incidence of GC. It was reported that if 30% of GC could be prevented by eradicating H. pylori, then it would be a cost effective cancer prevention strategy[36]. Since 1994, when H. pylori was recognized as a Group I carcinogen by the WHO, research on the pathogenicity, diagnosis, and treatment of H. pylori has been the focus, with new research constantly emerging. Plummer et al[37] found that H. pylori has a higher oncogenic effect than infectious agents such as hepatitis B virus and human papillomavirus, and is by far the most common oncogenic infectious agent. Currently, however, the treatment of H. pylori is facing serious difficulties due to increased antibiotic resistance, and eradication rates of less than 80% in some countries[38]. It is generally accepted that the emergence of antibiotic resistance is associated with mutations in the H. pylori gene[39]. For example, the 23S ribosomal RNA A2143G mutation is associated with clarithromycin resistance[40], the 87th mutation in gyrA is associated with levofloxacin resistance[41], and the tet-1 mutation in 16S rRNA is associated with tetracycline resistance[42]. Currently, the Maastricht guidelines recommend the use of bismuth-containing quadruple therapy as the first-line treatment for H. pylori eradication in areas with high clarithromycin resistance[43]. Xie et al[44] have developed an H. pylori vaccine using chitosan as an adjuvant and found that it was effective in preventing H. pylori infection and inducing Th1 and Th2 immune responses. Currently, vaccine development and application remain challenging due to the weak immune response stimulated by protein antigens on the mucosal surface, the potential toxicity of exotoxin-based mucosal adjuvants, and the limited capacity to control H. pylori infection[45]. Gao et al[46] concluded that risk stratification based solely on the presence or absence of H. pylori infection is of limited value and that serologic markers based on reactions to virulence factors are more necessary. The detection of 15 H. pylori protein serum markers revealed that CagA and GroEL were independent risk predictors for the occurrence of GC.
Keyword analysis identified single-nucleotide polymorphism (SNP) as the predominant genetic factor in GC. The occurrence of GC is the result of the interactions between multiple genetic, environmental, and social factors[47]. Only a small percentage of these individuals exposed to risk factors such as H. pylori develop GC as the genetic risk is low[48], and there are significant differences in genetic susceptibility between individuals[49]. Additionally, studies have shown that up to 10% of GC cases are due to germline pathogenic variants caused by underlying genetic susceptibility[50]. Thus, novel and effective methods for identifying susceptibility genetic factors are expected to provide new insights into the underlying molecular pathways of tumorigenesis[51]. In recent years, genomic analysis of gastric tumors has highlighted the importance of their genetic heterogeneity. Over the last decade, SNP analysis has been widely used to screen candidate genes and detect a variety of complex human diseases, providing an approach to identify genetic loci associated with cancer heterogeneity[52]. The available evidence suggests that most SNPs are functionally neutral, yet there are still some SNPs shown to be associated with GC development and prognosis[53]. Studies have shown that SP4 gene polymorphisms (rs39302, rs7811417)[54], GBAP1 gene polymorphisms (rs140081212, rs1057941 and rs2990220)[55], and Cathepsin B gene polymorphisms (rs9009, rs6731 and rs17814426)[56] are associated with a reduced risk of GC and could provide a protective effect in its development. Although the genetic causes of cancer have been extensively studied, it is becoming increasingly clear that a large proportion of cancer susceptibility cannot be attributed to variations in protein-coding sequences, with one study specifying that more than 80% of cancer-associated SNPs occur in non-coding regions of the genome[57]. Long noncoding RNAs (lncRNA) are universally transcribed non-protein-coding transcripts in the genome that are longer than 200 nucleotides[58]. These lncRNAs play an important role and available evidence has demonstrated that many SNPs in lncRNAs lead to the modification of their interaction partners by interfering with the splicing process and the stability of mRNA conformation[59]. This in turn affects the molecular functions of cells or of tissue development and differentiation, which consequently have an impact on the expression of tumor characteristics[60] and increasing cancer susceptibility[57]. Ge et al[61] performed a clinical study using TaqMan technology on 1536 subjects evaluating three lncRNA PTENP1 genotypes. They found that subjects with the rs7853346 G allele had significantly lower risk of GC compared to those with the C allele. However, there are also some issues that must be addressed in the current research: (1) The relatively small sample size used for stratified analysis may reduce statistical power[62]; (2) limited genotypes were selected and the overall representativeness of the SNP characteristics is questionable[63]; and (3) most studies failed to consider the influence of environmental factors on the results[64].
Bio-diagnostic markers are the most promising diagnostic modality for GC, as identified through keyword analysis. Gastrointestinal endoscopy combined with gastric mucosal biopsy is currently the gold standard for the diagnosis of GC, and its sensitivity and specificity can reach 69% and 96%, respectively[65]. Nevertheless, its long operation time, the invasiveness of the operation, and the partially subjective nature of the diagnosis[66] are still issues that cannot be ignored. The American Society for Gastrointestinal Endoscopy explicitly recommends endoscopic surveillance only for patients with a specific ethnicity and family history[67]. A convenient, noninvasive way to perform mass screening is therefore necessary[68]. Bio-diagnostic markers are the most promising diagnostic modalities currently under investigation. Despite the widespread clinical use of bio-diagnostic markers such as CEA, CA19-9, CA72-4, and CA125, they often failed to meet actual clinical needs due to insufficient sensitivity and specificity[69]. Consequently, the discovery of new bio-diagnostic markers remains a pressing concern to improve the overall survival of GC patients[70]. It has been shown that Apolipoprotein C1 (APOC1)[71], interferon gamma receptor 1 (IFNGR1)[72], and hioredoxin reductase 1 (TXNRD1)[73] differ in serum concentrations between healthy and diseased populations, and reflect, to some extent, the staging, classification and progression of the disease. Thus, APOC1, IFNGR1, and TXNRD1 are potential serum bio
The GC risk prediction model is a recent research hotspot identified by burst word analysis. The reasons for GC risk prediction models to become a hot research topic are as follows: (1) Risk prediction models are a simple and effective method for assessing individual risk by quantifying the incidence of cancer[80]; and (2) risk prediction models can integrate multiple GC causative factors, including environmental, behavioral, biological, and genetic factors, to realize the accuracy of cancer risk assessment[81]. Table 8 summarizes the research characteristics and model features of the GC risk prediction model-related literature[82-94]. For instance, Pei et al[95] constructed a linear prediction model based on H. pylori infection, PGI, G-17, and the number of lesions that showed good predictive value in both the subsequent training cohort and the confirmatory cohort, providing a good basis for patient assessment and individualized treatment. The GC risk prediction model constructed by Iida et al[91] has good discriminative power and calibration scale. Eom et al[80] developed GC risk prediction models based on different genders and showed good accuracy and predictability. Although existing risk prediction models have the desired predictive power, it is evident that many models still have a high risk of bias and also lack validation components[96]. In addition, the lack of calibration evaluation and unclear application settings have hindered the practical application and widespread spread of predictive models[97].
| Research characteristics | Model characteristics | Findings | Ref. | |||||
| Country | Research design | Number of participants | Data types | Model types | AUC | C-index | ||
| China | Prospective cohort study | 435673 | ①②③④ | c | - | 0.736 | The GCRS can be an effective risk assessment tool for tailored endoscopic screening of GC in China. RESCUE, an online tool was developed to aid the use of GCRS | Zhu et al[82] |
| China | Retrospective study | 6005 | ①④⑤⑥⑦ | - | 0.708 | - | Li’s prediction model performs the best for risk stratification in the screening, detection, and diagnosis of GC and precancerous lesions, whereas the overall performance of the other three models is similar | Hu et al[83] |
| South Korea | Retrospective cohort study | 1157 | ①④⑤⑦ | a | 0.894 | - | The 4-point discriminative model may help identify patients with a normal serological test who are nonetheless at risk of developing GC | Cho et al[84] |
| China | Cohort study | 89568 | ①②④⑨ | b | 0.97 | - | This model could enable a potentially more cost- effective endoscopic surveillance program, as well as to exclude very low-risk patients from unnecessary surveillance | Leung et al[85] |
| China | Retrospective cohort study | 2287 | ①②④⑩ | a | 0.684 | - | The present study established a predictive model to assess the risk of GC using high-evidence genetic variants and detected the potential gene-environment interaction, which may be helpful in prevention of the cancer | Qiu et al[86] |
| China | Case-control study | 383 | ①②③④⑤ | a | 0.883 | - | This model is simple, convenient, and economical, has good patient compliance, is easy to implement clinically, is easy to concentrate medical resources, and is expected to identify high-risk groups at an early stage, then to increase the detection rate of GC | Tao et al[87] |
| America | Case-control study | 140 | ①②③④⑦ | a | 0.9495 | - | The addition of ethnic and cultural variables, particularly the immigration/generation, to conventional risk factor variables improved the ability of models to identify individuals at high risk for GC | In et al[88] |
| Japan | Case-control study | 1431 | ①④⑦⑧ | b | 0.899 | - | XGBoost outperformed logistic regression and showed the highest AUC value | Taninaga et al[89] |
| China | Cross-sectional study | 14929 | ①②③④⑤⑥⑦ | a | 0.76 | - | The prediction rule had good performance and showed significantly better discrimination ability to identify a patient with GC than three other alternative prediction methods | Cai et al[90] |
| Japan | Cohort study | 5648 | ①②⑦⑧ | c | 0.790 | - | We developed a risk assessment tool for gastric cancer that provides a useful guide for stratifying an individual’s risk of future gastric cancer | Iida et al[91] |
| China | Cross-sectional study | 12112 | ①②③④⑤⑥⑦ | c | 0.811 | - | A serological biopsy composed of the five stomach-specific circulating biomarkers could be used to identify high-risk individuals for further diagnostic gastroscopy, and to stratify individuals’ risk of developing GC and thus to guide targeted screening and precision prevention | Tu et al[92] |
| Japan | Prospective cohort study | 19028 | ①②③④⑤⑦ | c | - | 0.777 | In this study, the authors developed a model and a simple scoring system to estimate an individual's risk of developing GC, based on factors such as H. pylori antibodies, serum pepsinogen levels, and lifestyle habits | Charvat et al[93] |
| South Korea | Case-control study | 217 | ①②③④ | a | 0.888 | - | This study provides the first predictive model for assessing the risk factors for GC in Korea, where the incidence rate of GC is high. This study has also identified new risk factors for GC, such as drinking tap water | Lee et al[94] |
The list of key risk factors for GC: In this study, we extracted the top 20 risk factors in terms of study frequency and constructed a list of key risk factors for GC based on the frequency magnitudes. The ranking order represents the level of interest in the risk factors for GC field for each risk factor, and, to some extent, reflects its importance in the development of GC. Based on our analysis, we believe that H. pylori infection, SNP, smoking, diet, and alcohol are the most important risk factors for the development of GC. Previous studies also support our findings, for example, H. pylori is thought to cause 65% to 80% of GC cases[98], and smoking contributed to approximatley 11% of GC cases[99]. Currently, GC prevention and treatment strategies have a few limitations, such as the propagation of one single GC prevention and treatment strategy[100]; the reliance on endoscopy, which has difficulty in population dissemination[101]; and the inclusion of a large number of risk factors without prioritization[102]. To dress these issues, prevention and screening efforts should center on a clear list of key risk factors, and the list of risk factors derived from this study may potentially contribute to it. Accordingly, it is important to first control H. pylori infection while avoiding smoking, diet and alcohol consumption habits. Meanwhile, genotyping associated with GC could be screened by SNP microarray detection technology[103], which could effectively determine GC occurrence.
Common risk factors for different tumors: In this study, analysis was conducted to explore the common risk factors of GC with other tumors. For example, Chen et al[104] found an association between the MDM2 promoter rs937283 and lung cancer risk, as well as an association with GC risk in men, population with smoking and alcohol consumption characteristics. Lee et al[105] found that proton pump inhibitor use for ≥ 2 years was not associated with increased risk of gastrointestinal cancers, including gastric and pancreatic cancers. Bidel et al[106] also have not yet found an association between coffee consumption and risk of gastric and/or pancreatic cancer. The exploration of common risk factors can help optimize tumor prevention and treatment strategies and reduce social public health costs.
Gender differences in risk factors for GC: In the study, we also found that there were some gender differences in risk factors for GC. For example, one study found that the prevalence of H. pylori infection is greatly increased in adult males, and this gender difference is one of the factors contributing to the emergence of a male predominance in diseases associated with H. pylori infection, including GC[107]. The same finding was observed by other studies, including Song et al[108] and Verma et al[109]. This may be a key factor contributing to gender differences in the incidence of GC[110] and is closely associated with gender differences in clinical staging[111] and pathological features[112].
To the best of our knowledge, this is the first bibliometric study for risk factors for GC. This study has helped to identify frontier and hot topics and to select appropriate research directions, collaborating institutions, and publishing journals. The key risk factors for GC may provide a reference for orderly clinical intervention. This study also has the following limitations: (1) The literature included in this study is in English, and articles published in other languages may not be included; (2) this study only searches the WOS database, and important literature included in other databases may be missed; and (3) this study's literature search only extends to December 04, 2022, and as some articles are published online first and not yet actually published, there is a certain risk of bias.
The number of publications in the risk factors for GC field has grown steadily over the past two decades. H. pylori infection was the most important risk factor for GC; SNP is the most dominant genetic factor in GC, and bio-diagnostic markers are the most promising diagnostic modality for GC; GC risk prediction model is the latest research hotspot. A list of key risk factors for GC was also constructed, and we identified H. pylori infection, SNP, smoking, diet, and alcohol as the most important risk factors for the development of GC, and proposed corresponding strategies for GC prevention.
The absence of specific symptoms in patients with gastric cancer (GC) causes great difficulties in early diagnosis of the disease, therefore, it is very valuable to clarify the risk factors for early diagnosis and treatment of GC and to improve the survival of GC.
Researchers have conducted a large number of studies on risk factors in GC. However, a summary of the current state of research is lacking.
This study was conducted to enable physicians to keep abreast of the latest research evidence in the clinical field and to make increasingly informed decisions.
The articles covered in this study were obtained from the Web of Science database and the data were processed through CiteSpace and VOSviewer software.
A total of 2514 papers from 72 countries and 2507 research institutions were retrieved. China, National Cancer Center, and Shoichiro Tsugane were the most productive country, institution, or author, respectively. The research hotspots in the study of risk factors for GC are summarized in four areas, namely: Helicobacter pylori (H. pylori) infection, single nucleotide polymorphism, bio-diagnostic markers, and GC risk prediction models.
A list of key risk factors for GC was also constructed, and we identified H. pylori infection, single-nucleotide polymorphism, smoking, diet, and alcohol as the most important risk factors for the development of GC, and proposed corresponding strategies for GC prevention.
GC risk prediction modeling is the latest research hotspot and will likely be the future direction of research in the field.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee KS, South Korea S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 3. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2198] [Article Influence: 732.7] [Reference Citation Analysis (1)] |
| 4. | Zhang X, Li M, Chen S, Hu J, Guo Q, Liu R, Zheng H, Jin Z, Yuan Y, Xi Y, Hua B. Endoscopic Screening in Asian Countries Is Associated With Reduced Gastric Cancer Mortality: A Meta-analysis and Systematic Review. Gastroenterology. 2018;155:347-354.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 225] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 5. | He J, Chen WQ, Li ZS, Li N, Ren JS, Tian JH, Tian WJ, Hu FL, Peng J; Expert Group of China Guideline for the Screening,Early Detection and Early Treatment of Gastric Cancer; ; Working Group of China Guideline for the Screening, Early Detection and Early Treatment of Gastric Cancer. [China guideline for the screening, early detection and early treatment of gastric cancer (2022, Beijing)]. Zhonghua Zhong Liu Za Zhi. 2022;44:634-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Tan Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med Sci Monit. 2019;25:3537-3541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 7. | Layke JC, Lopez PP. Gastric cancer: diagnosis and treatment options. Am Fam Physician. 2004;69:1133-1140. [PubMed] |
| 8. | Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin JT, You WC, Ng EK, Sung JJ; Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 647] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 9. | Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Zhu J, Wang SM, Chen R, Li XQ, Wei WW. [Progress on screening for gastric cancer]. Zhonghua Zhong Liu Za Zhi. 2020;42:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, Jung KW, Lee CW, Choi IJ, Park EC, Lee D. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology. 2017;152:1319-1328.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 369] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 12. | Gawron AJ, Shah SC, Altayar O, Davitkov P, Morgan D, Turner K, Mustafa RA. AGA Technical Review on Gastric Intestinal Metaplasia-Natural History and Clinical Outcomes. Gastroenterology. 2020;158:705-731.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Nieminen AA, Kontto J, Puolakkainen P, Virtamo J, Kokkola A. Long-term gastric cancer risk in male smokers with atrophic corpus gastritis. Scand J Gastroenterol. 2019;54:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Adamu MA, Weck MN, Rothenbacher D, Brenner H. Incidence and risk factors for the development of chronic atrophic gastritis: five year follow-up of a population-based cohort study. Int J Cancer. 2011;128:1652-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Lee HW, Huang D, Shin WK, de la Torre K, Song M, Shin A, Lee JK, Kang D. Frequent low dose alcohol intake increases gastric cancer risk: the Health Examinees-Gem (HEXA-G) study. Cancer Biol Med. 2022;19:1224-1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 16. | Huang X, Tajima K, Hamajima N, Inoue M, Takezaki T, Kuroishi T, Hirose K, Tominaga S, Xiang J, Tokudome S. Effect of life styles on the risk of subsite-specific gastric cancer in those with and without family history. J Epidemiol. 1999;9:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Li D, Zuo M, Hu X. Global Trends in Research of Treatment on Bladder Cancer with Chinese Medicine Monomer from 2000 to 2021: A Bibliometric Analysis. J Oncol. 2022;2022:3382360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS One. 2019;14:e0223994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 520] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 19. | Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101 Suppl 1:5303-5310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1310] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 20. | Hou JH, Yang XC, Chen CM. Emerging trends and new developments in information science: A document Co-citation analysis (2009-2016). Scientometrics. 2018;115:869-892. [RCA] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 21. | Memon AR, Vandelanotte C, Olds T, Duncan MJ, Vincent GE. Research Combining Physical Activity and Sleep: A Bibliometric Analysis. Percept Mot Skills. 2020;127:154-181. [PubMed] [DOI] [Full Text] |
| 22. | Zyoud SH, Shakhshir M, Koni A, Abushanab AS, Shahwan M, Jairoun AA, Al Subu R, Abu Taha A, Al-Jabi SW. Mapping the global research landscape on insulin resistance: Visualization and bibliometric analysis. World J Diabetes. 2022;13:786-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Chen JW, Guan Y, Zheng YL, Zhu K. Research trends and frontiers in exercise for movement disorders: A bibliometric analysis of global research from 2010 to 2021. Front Aging Neurosci. 2022;14:977100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Yu HY, Chang YC. A Bibliometric Analysis of Platelet-Rich Fibrin in Dentistry. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Feng XW, Hadizadeh M, Zheng LH, Li WH. A Bibliometric and Visual Analysis of Exercise Intervention Publications for Alzheimer's Disease (1998-2021). J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 26. | Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724-728. [PubMed] |
| 27. | Shi Y, Wei W, Li L, Wei Q, Jiang F, Xia G, Yu H. The global status of research in breast cancer liver metastasis: a bibliometric and visualized analysis. Bioengineered. 2021;12:12246-12262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Kweon SS, Shu XO, Xiang Y, Yang G, Ji BT, Li H, Gao YT, Zheng W, Shrubsole MJ. One-carbon metabolism dietary factors and distal gastric cancer risk in chinese women. Cancer Epidemiol Biomarkers Prev. 2014;23:1374-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Navarro Silvera SA, Mayne ST, Risch HA, Gammon MD, Vaughan T, Chow WH, Dubin JA, Dubrow R, Schoenberg J, Stanford JL, West AB, Rotterdam H, Blot WJ. Principal component analysis of dietary and lifestyle patterns in relation to risk of subtypes of esophageal and gastric cancer. Ann Epidemiol. 2011;21:543-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Epplein M, Shu XO, Xiang YB, Chow WH, Yang G, Li HL, Ji BT, Cai H, Gao YT, Zheng W. Fruit and vegetable consumption and risk of distal gastric cancer in the Shanghai Women's and Men's Health studies. Am J Epidemiol. 2010;172:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Wu H, Cheng K, Guo Q, Yang W, Tong L, Wang Y, Sun Z. Mapping Knowledge Structure and Themes Trends of Osteoporosis in Rheumatoid Arthritis: A Bibliometric Analysis. Front Med (Lausanne). 2021;8:787228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 32. | Yu X, Yu C, He W. Emerging trends and hot spots of NLRP3 inflammasome in neurological diseases: A bibliometric analysis. Front Pharmacol. 2022;13:952211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 33. | Liu X, Hu X, Yu X, Li P, Gu C, Liu G, Wu Y, Li D, Wang P, Cai J. Frontiers and hotspots of (18)F-FDG PET/CT radiomics: A bibliometric analysis of the published literature. Front Oncol. 2022;12:965773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Gao M, Zhang H, Gao Z, Sun Y, Wang J, Wei F, Gao D. Global hotspots and prospects of perimenopausal depression: A bibliometric analysis via CiteSpace. Front Psychiatry. 2022;13:968629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 35. | Long D, Mao C, Zhang X, Liu Y, Shangguan X, Zou M, Zhu Y, Wang X. Coronary heart disease and gut microbiota: A bibliometric and visual analysis from 2002 to 2022. Front Cardiovasc Med. 2022;9:949859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 36. | Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet. 1996;348:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 209] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609-e616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 1018] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 38. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 735] [Article Influence: 49.0] [Reference Citation Analysis (1)] |
| 39. | Li XH, Huang YY, Lu LM, Zhao LJ, Luo XK, Li RJ, Dai YY, Qin C, Huang YQ, Chen H. Early genetic diagnosis of clarithromycin resistance in Helicobacter pylori. World J Gastroenterol. 2021;27:3595-3608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 681] [Article Influence: 32.4] [Reference Citation Analysis (2)] |
| 41. | Cattoir V, Nectoux J, Lascols C, Deforges L, Delchier JC, Megraud F, Soussy CJ, Cambau E. Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int J Antimicrob Agents. 2007;29:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Gerrits MM, de Zoete MR, Arents NL, Kuipers EJ, Kusters JG. 16S rRNA mutation-mediated tetracycline resistance in Helicobacter pylori. Antimicrob Agents Chemother. 2002;46:2996-3000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ; European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1588] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 44. | Xie Y, Zhou NJ, Gong YF, Zhou XJ, Chen J, Hu SJ, Lu NH, Hou XH. Th immune response induced by H pylori vaccine with chitosan as adjuvant and its relation to immune protection. World J Gastroenterol. 2007;13:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Ikuse T, Blanchard TG, Czinn SJ. Inflammation, Immunity, and Vaccine Development for the Gastric Pathogen Helicobacter pylori. Curr Top Microbiol Immunol. 2019;421:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Gao L, Michel A, Weck MN, Arndt V, Pawlita M, Brenner H. Helicobacter pylori infection and gastric cancer risk: evaluation of 15 H. pylori proteins determined by novel multiplex serology. Cancer Res. 2009;69:6164-6170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Kong X, Yang S, Liu C, Tang H, Chen Y, Zhang X, Zhou Y, Liang G. Relationship between MEG3 gene polymorphism and risk of gastric cancer in Chinese population with high incidence of gastric cancer. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Wang N, Qiao Q, Bao G, Wu T, Li Y, Li J, Lu J, He X. Genetic polymorphisms are associated with the risk of gastric and colorectal cancers in a Han Chinese population. Oncotarget. 2017;8:28805-28811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 49. | Li L, Guo G, Zhang H, Zhou B, Bai L, Chen H, Zhao Y, Yan Y. Association between H19 SNP rs217727 and lung cancer risk in a Chinese population: a case control study. BMC Med Genet. 2018;19:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Zhong G, Luo X, Li J, Liao Y, Gui G, Sheng J. MTRR rs1532268 polymorphism and gastric cancer risk: evidence from a meta-analysis. J Int Med Res. 2022;50:3000605221097486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 51. | Liu Y, Qin W, Zhang F, Wang J, Li X, Li S, Qin X, Lu Y. Association between WNT-1-inducible signaling pathway protein-1 (WISP1) genetic polymorphisms and the risk of gastric cancer in Guangxi Chinese. Cancer Cell Int. 2021;21:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Hu X, Jia J, Yang Z, Chen S, Xue J, Duan S, Yang P, Peng S, Yang L, Yuan L, Bao G. PLCE1 Polymorphisms Are Associated With Gastric Cancer Risk: The Changes in Protein Spatial Structure May Play a Potential Role. Front Genet. 2021;12:714915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 53. | Ye ZM, Hu QY, Zheng JH, Zhang C, Zhu XD, Tang YM. A comprehensive evaluation of single nucleotide polymorphisms associated with gastric cancer risk: A protocol for systematic review and network meta-analysis. Medicine (Baltimore). 2020;99:e20448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 54. | Cui Y, Wang W, Luo P, Feng Y, Mi C, Jia A. The genetic polymorphisms in the SP4 gene and the risk of gastric cancer. Future Oncol. 2022;18:3993-4004. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Duan X, Shan L, Shi S, Xu B, Chen X, Di J, Chen B, Li X, Liu S, Wang Y, Yang W. GBAP1 polymorphisms (rs140081212, rs1057941 and rs2990220) contribute to reduced risk of gastric cancer. Future Oncol. 2022;18:1861-1872. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 56. | Ma X, Wang Y, Fan H, Zhu C, Chen W, Li Z, Xiao J, Ni P, Xu Z, Yang L. Genetic polymorphisms of Cathepsin B are associated with gastric cancer risk and prognosis in a Chinese population. Cancer Biomark. 2021;32:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108:2419-2425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 610] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 58. | Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4004] [Cited by in RCA: 3939] [Article Influence: 246.2] [Reference Citation Analysis (0)] |
| 59. | Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6:e1001233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 625] [Cited by in RCA: 725] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 60. | Park JH, Jin EH, Hong JH, Lee SI, Sung JK. The association between polymorphism of the long noncoding RNA, Plasmacytoma variant translocation 1, and the risk of gastric cancer. Medicine (Baltimore). 2021;100:e27773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 61. | Ge Y, He Y, Jiang M, Luo D, Huan X, Wang W, Zhang D, Yang L, Zhou J. Polymorphisms in lncRNA PTENP1 and the Risk of Gastric Cancer in a Chinese Population. Dis Markers. 2017;2017:6807452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Hong JH, Jin EH, Chang IA, Kang H, Lee SI, Sung JK. Association Between lncRNA HULC rs7763881 Polymorphism and Gastric Cancer Risk. Pharmgenomics Pers Med. 2020;13:121-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 63. | Wang L, Xiao S, Zheng Y, Gao Z. Interaction Between Vascular Endothelial Growth Factor Gene Polymorphism and Smoking on Gastric Cancer Risk in Chinese Han Population. Pathol Oncol Res. 2022;28:1610495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 64. | He BS, Sun HL, Xu T, Pan YQ, Lin K, Gao TY, Zhang ZY, Wang SK. Association of Genetic Polymorphisms in the LncRNAs with Gastric Cancer Risk in a Chinese Population. J Cancer. 2017;8:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Choi KS, Jun JK, Park EC, Park S, Jung KW, Han MA, Choi IJ, Lee HY. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One. 2012;7:e50041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 66. | Liu K, Zhao Q, Li B, Zhao X. Raman Spectroscopy: A Novel Technology for Gastric Cancer Diagnosis. Front Bioeng Biotechnol. 2022;10:856591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 67. | ASGE Standards of Practice Committee; Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, Pasha SF, Sharaf R, Shergill AK, Cash BD, DeWitt JM. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc. 2015;82:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 68. | Zheng P, Liu J. Cost-Effectiveness Analysis of Hp and New Gastric Cancer Screening Scoring System for Screening and Prevention of Gastric Cancer. Curr Oncol. 2023;30:1132-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Jeong S, Oh MJ, Kim U, Lee J, Kim JH, An HJ. Glycosylation of serum haptoglobin as a marker of gastric cancer: an overview for clinicians. Expert Rev Proteomics. 2020;17:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Herrera-Pariente C, Montori S, Llach J, Bofill A, Albeniz E, Moreira L. Biomarkers for Gastric Cancer Screening and Early Diagnosis. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 71. | Yi J, Ren L, Wu J, Li W, Zheng X, Du G, Wang J. Apolipoprotein C1 (APOC1) as a novel diagnostic and prognostic biomarker for gastric cancer. Ann Transl Med. 2019;7:380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 72. | Tong W, Ye F, He L, Cui L, Cui M, Hu Y, Li W, Jiang J, Zhang DY, Suo J. Serum biomarker panels for diagnosis of gastric cancer. Onco Targets Ther. 2016;9:2455-2463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Zhu Y, Hu Y, Zhu X, Zhang J, Yuwen D, Wei X, Tang C, Zhang W. Plasma thioredoxin reductase: a potential diagnostic biomarker for gastric cancer. Carcinogenesis. 2022;43:736-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 74. | Ishimoto T, Baba H, Izumi D, Sugihara H, Kurashige J, Iwatsuki M, Tan P. Current perspectives toward the identification of key players in gastric cancer microRNA dysregulation. Int J Cancer. 2016;138:1337-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, Yoshida K, Sasaki H, Nomura S, Seto Y, Kaminishi M, Calin GA, Croce CM. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 676] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 76. | Manterola L, Guruceaga E, Gállego Pérez-Larraya J, González-Huarriz M, Jauregui P, Tejada S, Diez-Valle R, Segura V, Samprón N, Barrena C, Ruiz I, Agirre A, Ayuso A, Rodríguez J, González A, Xipell E, Matheu A, López de Munain A, Tuñón T, Zazpe I, García-Foncillas J, Paris S, Delattre JY, Alonso MM. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro Oncol. 2014;16:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 77. | Tang S, Cheng J, Yao Y, Lou C, Wang L, Huang X, Zhang Y. Combination of Four Serum Exosomal MiRNAs as Novel Diagnostic Biomarkers for Early-Stage Gastric Cancer. Front Genet. 2020;11:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 78. | Li Y, Sun H, Guan J, Ji T, Wang X. Serum microRNA-381: A Potential Marker for Early Diagnosis of Gastric Cancer. Yonsei Med J. 2019;60:720-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 79. | Zheng GD, Xu ZY, Hu C, Lv H, Xie HX, Huang T, Zhang YQ, Chen GP, Fu YF, Cheng XD. Exosomal miR-590-5p in Serum as a Biomarker for the Diagnosis and Prognosis of Gastric Cancer. Front Mol Biosci. 2021;8:636566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 80. | Eom BW, Joo J, Kim S, Shin A, Yang HR, Park J, Choi IJ, Kim YW, Kim J, Nam BH. Prediction Model for Gastric Cancer Incidence in Korean Population. PLoS One. 2015;10:e0132613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Freedman AN, Seminara D, Gail MH, Hartge P, Colditz GA, Ballard-Barbash R, Pfeiffer RM. Cancer risk prediction models: a workshop on development, evaluation, and application. J Natl Cancer Inst. 2005;97:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 82. | Zhu X, Lv J, Zhu M, Yan C, Deng B, Yu C, Guo Y, Ni J, She Q, Wang T, Wang J, Jiang Y, Chen J, Hang D, Song C, Gao X, Wu J, Dai J, Ma H, Yang L, Chen Y, Song M, Wei Q, Chen Z, Hu Z, Shen H, Ding Y, Li L, Jin G. Development, validation, and evaluation of a risk assessment tool for personalized screening of gastric cancer in Chinese populations. BMC Med. 2023;21:159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 83. | Hu Y, Bao H, Jin H, Zhao J, Xu Y, Huang X, Liu S, Lu B. Performance evaluation of four prediction models for risk stratification in gastric cancer screening among a high-risk population in China. Gastric Cancer. 2021;24:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Cho JH, Jin SY, Park S. Scoring model for discriminating gastric cancer risk in patients with negative serum pepsinogen and anti-Helicobacter pylori antibody results. J Gastroenterol Hepatol. 2021;36:3345-3353. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 85. | Leung WK, Cheung KS, Li B, Law SYK, Lui TKL. Applications of machine learning models in the prediction of gastric cancer risk in patients after Helicobacter pylori eradication. Aliment Pharmacol Ther. 2021;53:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 86. | Qiu L, Qu X, He J, Cheng L, Zhang R, Sun M, Yang Y, Wang J, Wang M, Zhu X, Guo W. Predictive model for risk of gastric cancer using genetic variants from genome-wide association studies and high-evidence meta-analysis. Cancer Med. 2020;9:7310-7316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Tao W, Wang HX, Guo YF, Yang L, Li P. Establish a Scoring Model for High-Risk Population of Gastric Cancer and Study on the Pattern of Opportunistic Screening. Gastroenterol Res Pract. 2020;2020:5609623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | In H, Solsky I, Castle PE, Schechter CB, Parides M, Friedmann P, Wylie-Rosett J, Kemeny MM, Rapkin BD. Utilizing Cultural and Ethnic Variables in Screening Models to Identify Individuals at High Risk for Gastric Cancer: A Pilot Study. Cancer Prev Res (Phila). 2020;13:687-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Taninaga J, Nishiyama Y, Fujibayashi K, Gunji T, Sasabe N, Iijima K, Naito T. Prediction of future gastric cancer risk using a machine learning algorithm and comprehensive medical check-up data: A case-control study. Sci Rep. 2019;9:12384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 90. | Cai Q, Zhu C, Yuan Y, Feng Q, Feng Y, Hao Y, Li J, Zhang K, Ye G, Ye L, Lv N, Zhang S, Liu C, Li M, Liu Q, Li R, Pan J, Yang X, Zhu X, Li Y, Lao B, Ling A, Chen H, Li X, Xu P, Zhou J, Liu B, Du Z, Du Y, Li Z; Gastrointestinal Early Cancer Prevention & Treatment Alliance of China (GECA). Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: a nationwide multicentre study. Gut. 2019;68:1576-1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 91. | Iida M, Ikeda F, Hata J, Hirakawa Y, Ohara T, Mukai N, Yoshida D, Yonemoto K, Esaki M, Kitazono T, Kiyohara Y, Ninomiya T. Development and validation of a risk assessment tool for gastric cancer in a general Japanese population. Gastric Cancer. 2018;21:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Tu H, Sun L, Dong X, Gong Y, Xu Q, Jing J, Bostick RM, Wu X, Yuan Y. A Serological Biopsy Using Five Stomach-Specific Circulating Biomarkers for Gastric Cancer Risk Assessment: A Multi-Phase Study. Am J Gastroenterol. 2017;112:704-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 93. | Charvat H, Sasazuki S, Inoue M, Iwasaki M, Sawada N, Shimazu T, Yamaji T, Tsugane S; JPHC Study Group. Prediction of the 10-year probability of gastric cancer occurrence in the Japanese population: the JPHC study cohort II. Int J Cancer. 2016;138:320-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 94. | Lee DS, Yang HK, Kim JW, Yook JW, Jeon SH, Kang SH, Kim YJ. Identifying the risk factors through the development of a predictive model for gastric cancer in South Korea. Cancer Nurs. 2009;32:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Pei B, Wen Z, Yang Q, Wang J, Cao Q, Dai L, Li X. Risk Factors Analysis and Prediction Model Establishment of Intestinal Metaplasia or Dysplasia in Patients With Chronic Atrophic Gastritis: A Multi-Center Retrospective Study. Front Med (Lausanne). 2022;9:912331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 96. | Gu J, Chen R, Wang SM, Li M, Fan Z, Li X, Zhou J, Sun K, Wei W. Prediction Models for Gastric Cancer Risk in the General Population: A Systematic Review. Cancer Prev Res (Phila). 2022;15:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | He S, Sun D, Li H, Cao M, Yu X, Lei L, Peng J, Li J, Li N, Chen W. Real-World Practice of Gastric Cancer Prevention and Screening Calls for Practical Prediction Models. Clin Transl Gastroenterol. 2023;14:e00546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 98. | Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 749] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 99. | Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J Nutr. 2020;150:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 535] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 100. | Park JY, Herrero R. Recent progress in gastric cancer prevention. Best Pract Res Clin Gastroenterol. 2021;50-51:101733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 101. | Rugge M, de Boni M, Pennelli G, de Bona M, Giacomelli L, Fassan M, Basso D, Plebani M, Graham DY. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 102. | Correa P, Piazuelo MB, Camargo MC. The future of gastric cancer prevention. Gastric Cancer. 2004;7:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 103. | Mn W, L J, Jw H, Ks R, J T, At H, Cf W. Use of SNP chips to detect rare pathogenic variants: retrospective, population based diagnostic evaluation. BMJ. 2021;372:n214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 104. | Chen B, Wang J, Chen Y, Gu X, Feng X. The MDM2 rs937283 A > G variant significantly increases the risk of lung and gastric cancer in Chinese population. Int J Clin Oncol. 2018;23:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 105. | Lee JK, Merchant SA, Schneider JL, Jensen CD, Fireman BH, Quesenberry CP, Corley DA. Proton Pump Inhibitor Use and Risk of Gastric, Colorectal, Liver, and Pancreatic Cancers in a Community-Based Population. Am J Gastroenterol. 2020;115:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 106. | Bidel S, Hu G, Jousilahti P, Pukkala E, Hakulinen T, Tuomilehto J. Coffee consumption and risk of gastric and pancreatic cancer--a prospective cohort study. Int J Cancer. 2013;132:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | Ibrahim A, Morais S, Ferro A, Lunet N, Peleteiro B. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: Systematic review and meta-analysis of 244 studies. Dig Liver Dis. 2017;49:742-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 108. | Song HR, Shin MH, Kim HN, Piao JM, Choi JS, Hwang JE, Park YK, Ryang DW, Cho D, Kweon SS. Sex-specific differences in the association between ABO genotype and gastric cancer risk in a Korean population. Gastric Cancer. 2013;16:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Verma HK, Bhaskar L. Gender differences in the relationship between alcohol consumption and gastric cancer risk are uncertain and not well-delineated. World J Gastrointest Oncol. 2021;13:2216-2218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (0)] |
| 110. | Wang Z, Butler LM, Wu AH, Koh WP, Jin A, Wang R, Yuan JM. Reproductive factors, hormone use and gastric cancer risk: The Singapore Chinese Health Study. Int J Cancer. 2016;138:2837-2845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 111. | Li H, Wei Z, Wang C, Chen W, He Y, Zhang C. Gender Differences in Gastric Cancer Survival: 99,922 Cases Based on the SEER Database. J Gastrointest Surg. 2020;24:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 112. | Luan X, Niu P, Wang W, Zhao L, Zhang X, Zhao D, Chen Y. Sex Disparity in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis. J Oncol. 2022;2022:1269435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |