Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1725
Peer-review started: December 18, 2023
First decision: January 10, 2024
Revised: January 23, 2024
Accepted: March 25, 2024
Article in press: March 25, 2024
Published online: May 15, 2024
Processing time: 143 Days and 0.9 Hours
Gastric organoids are models created in the laboratory using stem cells and sophisticated three-dimensional cell culture techniques. These models have shown great promise in providing valuable insights into gastric physiology and advanced disease research. This review comprehensively summarizes and analyzes the research advances in culture methods and techniques for adult stem cells and induced pluripotent stem cell-derived organoids, and patient-derived organoids. The potential value of gastric organoids in studying the pathogenesis of stomach-related diseases and facilitating drug screening is initially discussed. The construction of gastric organoids involves several key steps, including cell extraction and culture, three-dimensional structure formation, and functional expression. Simulating the structure and function of the human stomach by disease modeling with gastric organoids provides a platform to study the mechanism of gastric cancer induction by Helicobacter pylori. In addition, in drug screening and development, gastric organoids can be used as a key tool to evaluate drug efficacy and toxicity in preclinical trials. They can also be used for precision medicine according to the specific conditions of patients with gastric cancer, to assess drug resistance, and to predict the possibility of adverse reactions. However, despite the impressive progress in the field of gastric organoids, there are still many unknowns that need to be addressed, especially in the field of regenerative medicine. Meanwhile, the reproducibility and consistency of organoid cultures are major challenges that must be overcome. These challenges have had a significant impact on the development of gastric organoids. Nonetheless, as technology continues to advance, we can foresee more comprehensive research in the construction of gastric organoids. Such research will provide better solutions for the treatment of stomach-related diseases and personalized medicine.
Core Tip: This paper provides a comprehensive summary and analysis of the advances in culture methods and techniques for adult stem cells, induced pluripotent stem cell-derived organoids and patient tumor-derived organoids. The potential value of gastric organoids in the study of the pathogenesis of gastric-related diseases, drug screening, and individualized treatment of gastric cancer patients is preliminarily discussed. Such research will provide better solutions for the treatment of stomach-related diseases and personalized medicine.
- Citation: Liu YY, Wu DK, Chen JB, Tang YM, Jiang F. Advances in the study of gastric organoids as disease models. World J Gastrointest Oncol 2024; 16(5): 1725-1736
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1725.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1725
Gastric organoids are a novel cell culture technique that utilizes stem cells for cultivation in a three-dimensional environment. Unlike traditional two-dimensional cell culture, gastric organoids mimic the growth state of human cells in their natural three-dimensional environment, providing a microenvironment more suitable for cell growth. As a result, organoids are closer to the true physiological state of cells, allowing for more accurate observation and study of stomach disease models. Gastric organoids can be constructed using adult stem cells (AdSCs), induced pluripotent stem cells (iPSCs), and embryonic stem cells (ESCs)[1]. However, there are ethical constraints on the study of ESCs; therefore, this aspect will not be discussed further in this review. Current research still relies heavily on animal models or in vitro cultured cells; however, they cannot fully simulate the complex physiological environment and immune reactions in the human body. Therefore, we need to develop more accurate and closer-to-human physiological model systems. Gastric organoids are a highly complex and multifunctional model system that holds great potential for advancing our understanding of various aspects of basic science and translational research[2]. They serve as powerful tools to study the intricate processes involved in the development and function of different organs. By replicating the complex microenvironment of the stomach, these organoids provide valuable opportunities to acquire an in-depth understanding of cellular and molecular mechanisms, enabling researchers to interpret factors influencing normal and abnormal organ growth[3,4]. Additionally, in the context of Helicobacter pylori (H. pylori) infection, organoids have been proven to be a valuable resource. Using organoids allows the investigation of host-pathogen interactions and the complex molecular signaling pathways involved in H. pylori infection[5,6]. Patient-derived organoids (PDO) as potential predictive biomarkers in the treatment of cancer, have been shown to maintain a high degree of consistency with the pathohistological features, molecular profiles, and drug sensitivities of patients' tumors. This not only contributes to our understanding of disease progression, but also aids in the development of more effective treatment strategies to combat this disease. In-depth characterization and analysis of these samples provides a better understanding of the mechanisms, pathological features, and treatment strategies of gastric cancer. This biobank will serve as a crucial tool for research on gastric cancer, providing scientists with abundant resources to conduct relevant studies and offering clinicians more accurate means of diagnosis, treatment, and prognosis assessment[7].
AdSC-derived and iPSC-derived gastric organoids are the two main modes of culture for gastric organoids. iPSC-derived gastric organoids comprise epithelial and mesenchymal cells with the intrinsic ability to differentiate into any type of cell. Therefore, iPSC-derived gastric organoids require a gradual differentiation process that directs the pluripotent stem cells to adopt the desired tissue characteristics. In contrast, AdSC-derived gastric organoids contain only epithelial cells and can be established from the outset using a single growth factor medium[8].
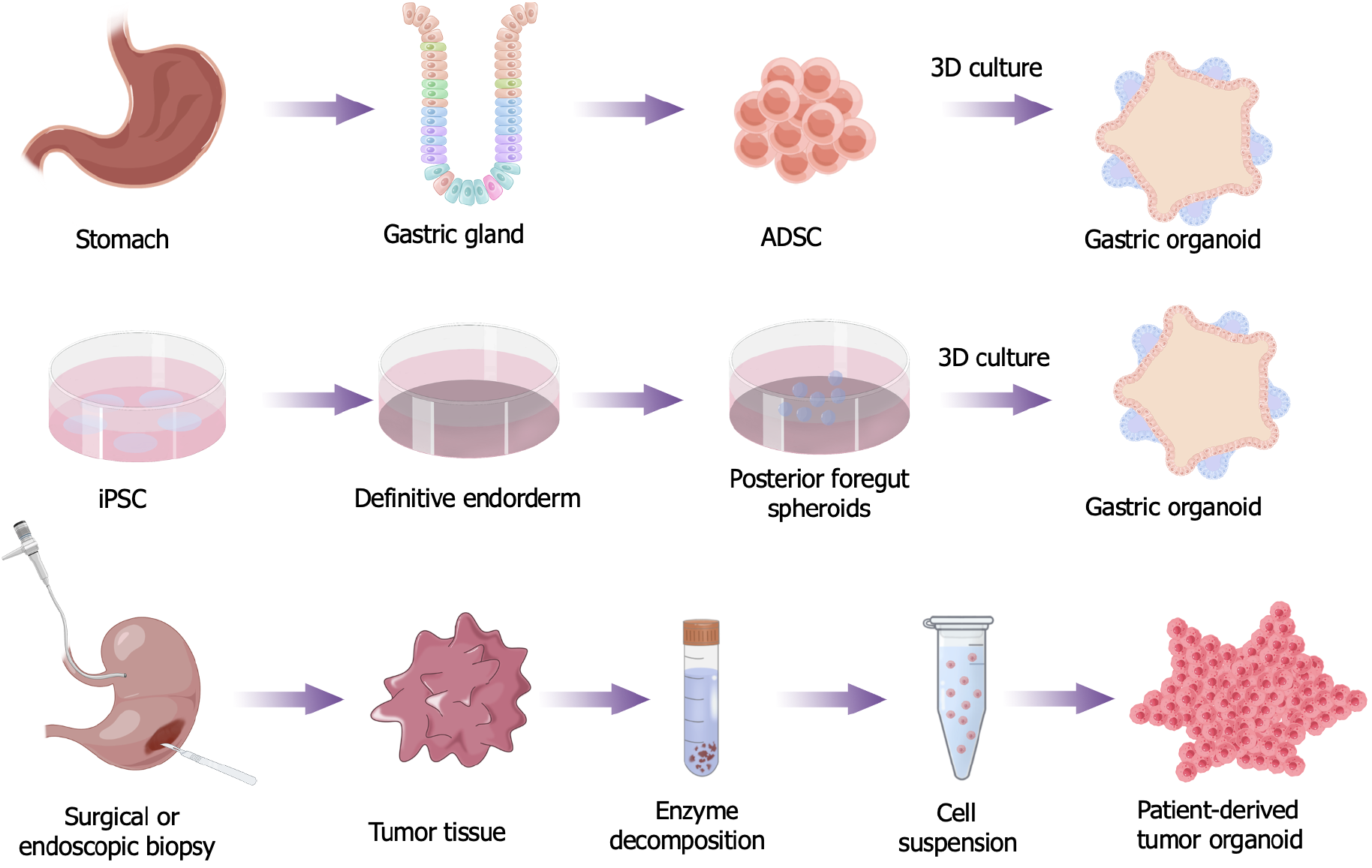
Gastric organoids from stem cells: Gastric organoids are structures derived from AdSCs found in the gastric glands. These organoids rely on growth factors that imitate the natural process of tissue regeneration. Researchers have utilized both organoids and animal models to study gastric homeostasis. The Wnt3/β-catenin signaling pathway, enhanced by R-spondin, plays a crucial role in promoting the growth and division of gastric stem cells, according to recent studies[9]. Additionally, the Notch signaling pathway plays a role in cell differentiation[10]. The presence of epidermal growth factor (EGF), along with other factors, such as bone morphogenic factor and Noggin signals, creates various gradients that are responsible for directing cells towards specific cell types, such as foveolar, chief, and acid-producing parietal cells[11]. These gradients also contribute to the overall formation of either a gland-type or a pit-type phenotype. Wölffling et al[12] unveiled the major impact of EGF on the gastric gland. R-spondin-1 is a cytokine that functions by inhibiting the ring finger protein 43 (RNf43)/zinc and ring finger 3 (ZnRF3) signaling pathway. The RNf43/ZnRF3 signaling pathway is a key factor in the negative regulation of the Wnt signaling pathway[13,14]. R-spondin-1 also interacts with leucine rich repeat containing G proteincoupled receptor 5 (Lgr5), further enhancing the continuous activity of the Wnt signaling pathway[15]. This sustained Wnt signaling is crucial to maintain the activity of stem cells. Lgr5 is widely recognized as a crucial marker to identify stem cells[16]. Thanks to these advances, well-established protocols to cultivate gastric organoids and specific commercially available media for organoid cultures have become available[17](Figure 1).
McCracken et al[18] devised a systematic method to generate human gastric organoids, starting with PSCs and proceeding through several differentiation steps. They first differentiated the PSCs into a definitive endoderm by introducing activin A. This endoderm was then patterned into both anterior and foregut regions by inhibiting bone morphogenic protein (BMP) signaling using Noggin. They then directed the foregut spheroids to differentiate into the posterior foregut through the activation of the retinoic acid signaling pathway. To generate human fundic gastric organoids, researchers activated the Wnt pathway[19]. By contrast, the differentiation of acid-secreting parietal cells, which respond to histamine, was achieved by inhibiting MAPK/ERK Kinase (MEK) and simultaneously activating the BMP signaling pathway[20]. These human gastric organoids are considered groundbreaking because they fully mimic the antrum of the human stomach in an in vitro setting and successfully reproduce key aspects of stomach physiology. Furthermore, these PSC-derived gastric organoids have proven to be valuable in understanding the signaling mechanisms that govern stomach development and physiology[18,21] (Figure 1).
Organoids from gastric tumors: Patient-derived tumor organoids are also referred to as PDOs. Patient tissue sources are the most common method of acquisition. These tissues, obtained by surgical or endoscopic biopsy, are broken down by relevant enzymes to extract tumor cells for in vitro culture. Tumor tissue is mechanically disrupted and enzymatically digested to obtain a cell suspension. This cell suspension is then placed in a Matrigel matrix, allowing the cells to grow and form three-dimensional structures[22]. To enhance the formation of PDOs, a growth factor mixture is added to the Matrigel. These growth factors include various cell proliferation factors, cell differentiation factors, and cell survival factors, which stimulate and promote cell proliferation, differentiation, and survival. These factors have an important role in the formation of PDOs, and can help cells self-organize and form structures similar to real tissues (Figure 1).
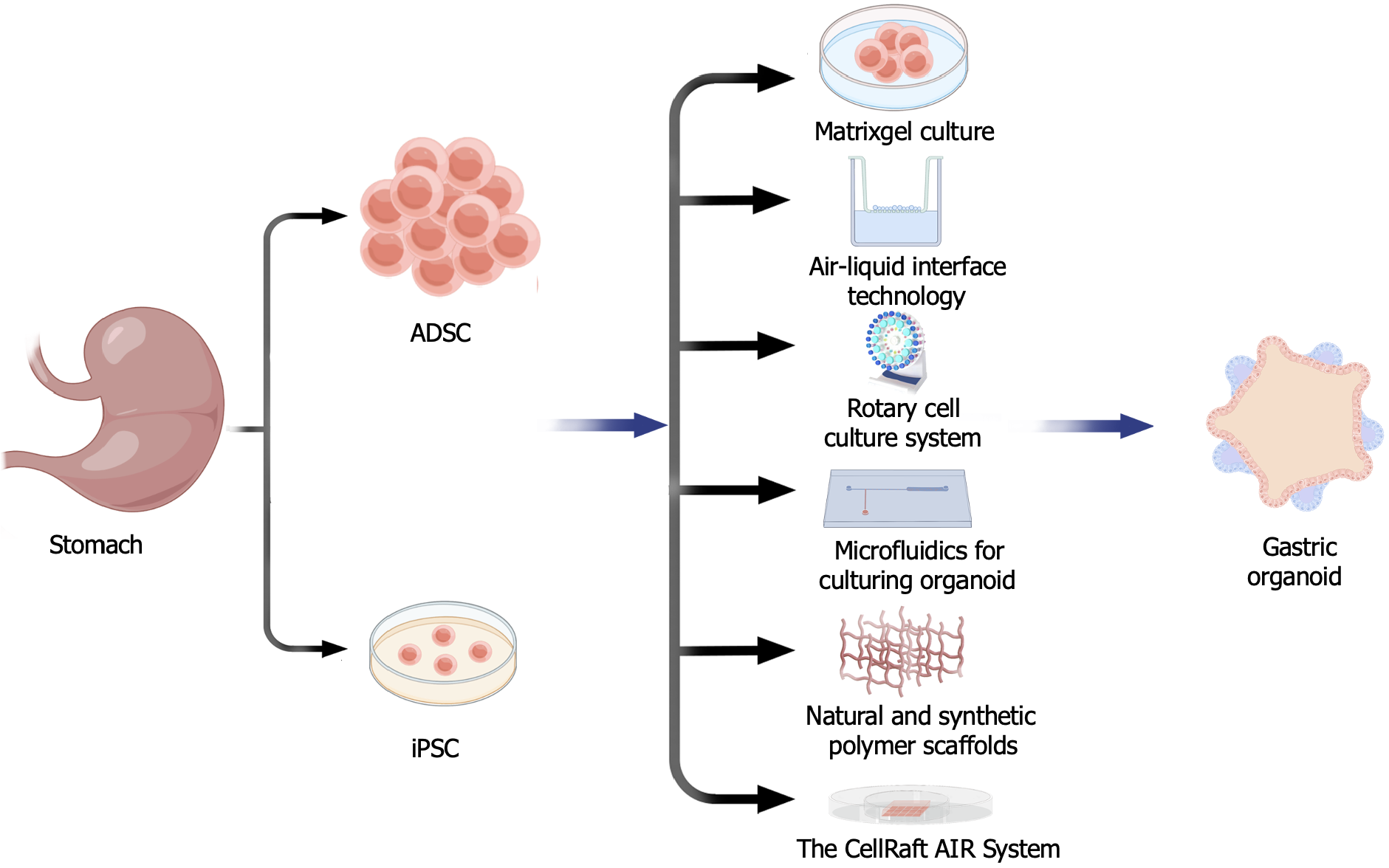
Matrigel culture: With the development of cell culture technology, gastric organoids can be cultured in a variety of culture modes. Among them, matrix gel culture is a commonly used method that simulates the real cell tissue by providing an environment similar to the extracellular matrix around the cells[23]. Matrigel is widely used for three-dimensional organoid cultures but has limitations related to its composition control and batch-to-batch variability, which affects the stability of organoid cultures. Moreover, its slow gelation rate, complex preparation process, and long incubation times hinder its use in high-throughput screening and rapid production.
Air-liquid interface organoid culture: Air-liquid interface organoid culture mimics the growth environment of tumor cells in real organisms by adding an appropriate amount of liquid to the bottom of the culture medium to form a liquid film on the surface of the culture. Through this method, we are able to study the mechanisms of tumorigenesis and the interactions between cells and the microenvironment more accurately, providing a more reliable experimental basis for tumor therapy and drug development[24-26].
Rotary cell culture system: Scientists have managed to create a promising in vitro three-dimensional model using a rotary cell culture system. The rotary cell culture system creates a more realistic environment for cells to receive accurate signals and stimuli, mimicking the in vivo situation. By controlling the speed of rotation, the system can simulate various directions and intensities of stimuli, allowing cells to better detect real signals. Controlling the speed of rotation also allows the system to simulate the effects of gravity and microgravity to study issues related to cell growth, differentiation, and functional expression. The culture system is also very controllable, allowing scientists to control the temperature and other parameters to simulate different physiological states and thus study different disease mechanisms[27-29].
Microfluidics for culturing organoids: Microfluidics is an emerging technology that offers researchers a new possibility to simulate physiological environments in experimental equipment by controlling the flow of fluids. Through microfluidics, the supply of nutrients can be precisely controlled, allowing it to mimic the supply of nutrients required by organs in a real physiological environment. Microfluidics also allows for the integration of external stimuli, which enables researchers to gain a more comprehensive understanding of how organs respond to and function in different environments. Based on the advantages of microfluidics, researchers are beginning to apply this technique to the study of organoids. Consequently, researchers can more accurately simulate and study the developmental process of human organs, disease mechanisms, and the efficacy of drugs. The introduction of such human models provides researchers with a convenient and powerful tool to better understand and solve problems in related fields[30-32].
Natural and synthetic polymer scaffolds: Natural scaffolds have a similar matrix structure and biocompatibility to Matrigel, These alternative scaffolds include natural collagen and fibrin scaffolds, as well as synthetic polymer gel and nanofiber scaffolds. Their composition or preparation method can be altered for better control and customization. Synthetic scaffolds have better control and customizability, allowing precise adjustment of their physical and chemical properties to suit the physiological requirements of different organs[33,34]; however, their cost is relatively high.
The CellRaft AIR® System: Researchers have developed improved methods to address the limitations of conventional organoid culture, such as low throughput and the inability to meet specific imaging, assessment, and isolation needs. By adapting tissue culture consumables and instruments, they have automated the process of imaging, identifying, and isolating individual organoids. Organoids grown on the 3D CellRaft AIRSystem can be reliably time-tracked, imaged, and phenotyped using bright-field and fluorescence microscopy, and then released and transferred intact for use in downstream applications. Stern et al[35] validated the ability of the CellRaft AIR System to overcome several key issues in organoid research, such as time-course imaging and phenotypic assessment, creation of single cell-derived organoids, and the isolation and retrieval of single organoids for downstream applications, thus facilitating efficient, user-friendly, and automated workflows in the field of organoid research[35].
These six organoid culture models offer a variety of choices, allowing researchers to better simulate and study the physiological and pathological conditions of human organs (Figure 2).
H. pylori is a bacterium that can grow in the stomach and cause inflammation. It comes into contact with the stomach wall through the gastric mucosa, leading to increased stomach acid secretion and damage to the gastric mucosa[36]. H. pylori can adhere to the surface of gastric pits and tightly bind to gastric mucosa, thereby invading gastric glands. Research has found that H. pylori can infiltrate the glands and interact with stem cells located at the base. This interaction might involve H. pylori releasing chemical signals to guide stem cell migration, as well as directly interacting with stem cells to influence their differentiation and proliferation[37]. Long-term infection and inflammation can eventually lead to the development of gastric ulcers or stomach cancer[38,39]. According to statistics, more than 10% of the global population is infected with H. pylori and thus faces the risk of developing these stomach diseases[40]. For a long time, the study of gastric epithelial biology has been hindered by the lack of accurate models, because gastric tissue has historically been difficult to culture, and there are significant physiological differences between mice and human stomachs. These difficulties present challenges for scientists to gain a deeper understanding of the key mechanisms in gastric epithelial biology[41]. Current research primarily relies on transformed cell lines as an in vitro model to study H. pylori infections, and most of these cell lines are derived from gastric adenocarcinoma. These tumor cell lines already represent the end point of the Correa cascade and serve as the final product of the required research processes; therefore, they are not suitable to study the early effects of H. pylori on host epithelial cells. Hence, there is an urgent need to develop primary cell systems to evaluate the early impact of H. pylori infection on healthy epithelial cells[5].
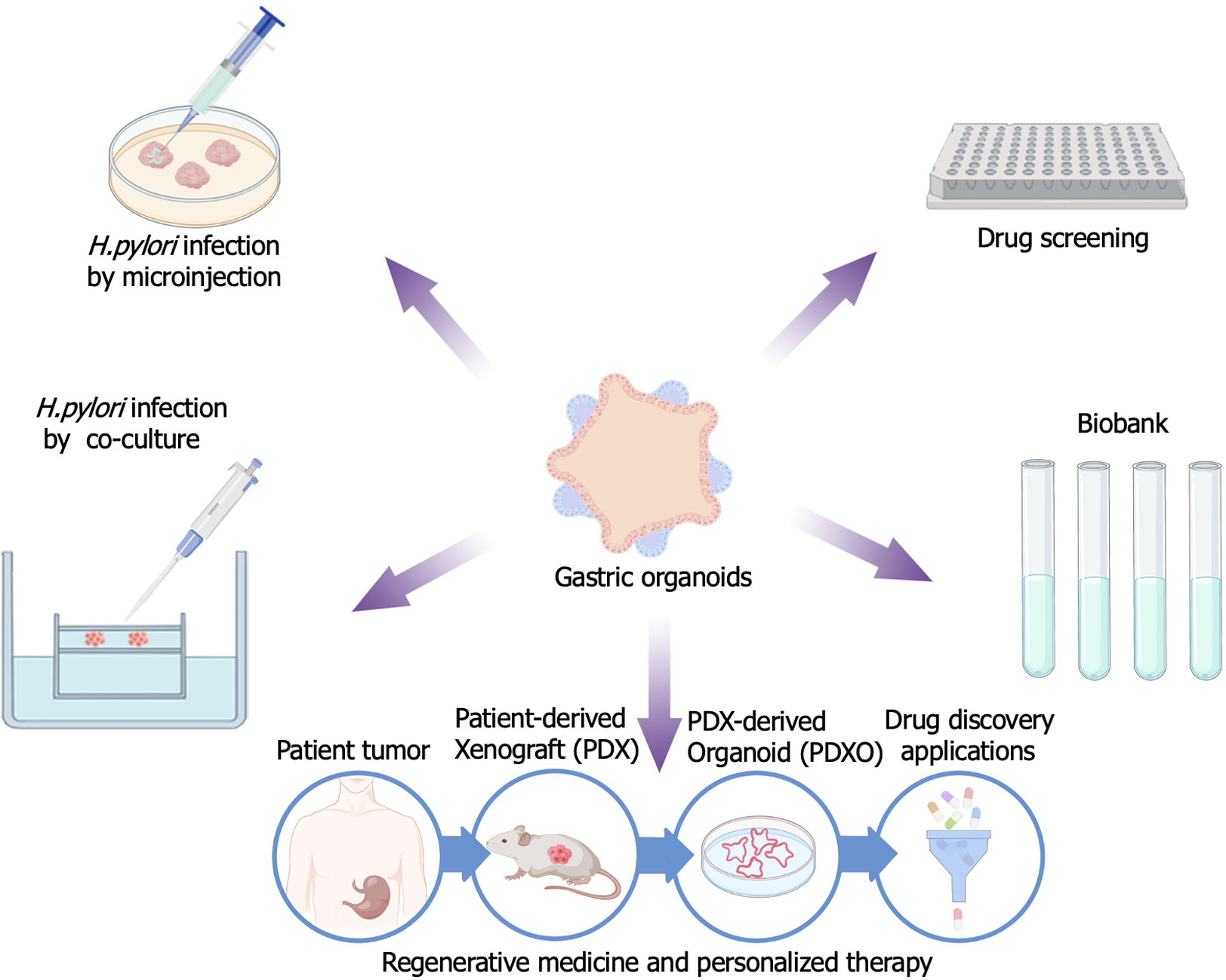
In the three-dimensional organoid model, the lumen is wrapped into a spherical shape. Researchers used a microinjection method to inject H. pylori directly into the gastric epithelial organoid sphere, creating an infection within the enclosed space[18]. H. pylori can infect not only the top cells of the gastric epithelium, but also the bottom cells. The researchers observed that the gastric fundus fossa responded very mildly to bacterial infection, producing almost no inflammatory response. By contrast, the gastric glands showed a strong inflammatory response, which might indicate that they are more sensitive to H. pylori infection[42,43]. This finding might provide insights into the pathogenesis of H. pylori-induced gastritis and thus promote further research into the treatment and prevention of the disease (Figure 3).
By co-culturing gastric organ tissues of individuals infected with H. pylori with host immune cells, researchers could observe in more detail the interactions between gastric epithelial cells and T lymphocytes during the infection process. They found that H. pylori can interfere with the normal role of the immune system in gastric epithelial cells. During the infection process, H. pylori inhibits the activation and function of T cells by regulating the function of immune checkpoints, such as the programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) signaling pathway, resulting in ineffective clearance of the bacteria by T cells. Additionally, H. pylori infection suppresses the immune response of gastric epithelial cells, thereby altering the homeostasis of the gastric microenvironment. These infected organ models provide researchers with an opportunity to better understand the complex interactions between H. pylori and gastric epithelial cells or the microenvironment[44]. Cheng et al[45] successfully demonstrated that the effects of chronic infection with H. pylori can lead to malignant transformation of gastric mucosal epithelial cells. They identified a stimulatory factor called Cag (cytotoxin-associated gene) A + H, which plays an important role in the malignant transformation of gastric mucosal epithelial cells. Further studies revealed that the key role of the FTO gene (encoding fat mass and obesity-associated protein) in this process. FTO can promote the malignant transformation of gastric mucosal cells by modifying the mRNA of the CD44 gene, and this process is associated with the methylation of gastric mucosal cells. These findings suggested that FTO expression plays an important role in the malignant transformation of gastric mucosal cells and provides new potential molecular therapeutic targets for a better understanding of gastric cancer development[45]. PD-L1 expression in gastric adenocarcinoma patients is mediated by the hedgehog (Hh) signaling pathway, which is induced by H. pylori. The phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin kinase (mTOR) signaling pathway is activated in gastric cancer and might have immunomodulatory potential. Immunosuppressive myeloid-derived suppressor cells expressing arginase 1 (Arg1), CEA cell adhesion molecule 8 (CEACAM8 also known as CD66b), V-domain Ig suppressor of T cell activation (VISTA), and indoleamine 2,3-dioxygenase 1 (IDO1) were identified in gastric cancer tissues using organoid technology. The organoids did not respond to nivolumab immunotherapy, but after removal of myeloid-derived suppressor cells, the organoids became sensitive to PD-1/PD-L1-induced cancer cell death. Rapamycin decreased the expression of ribosomal protein S6 kinase B1 (S6K), GLI family zinc finger 2 (Gli2), and PD-L1. Blockade of transcriptional regulator 1 and rapamycin receptor 2 inhibited PD-L1 expression. The results suggested that the mTOR signaling pathway mediates GLI-induced PD-L1 expression[46].
In the process of drug screening and development, drug testing is necessary, including preclinical experiments and clinical trials.
Effectiveness, toxicity: The results obtained throughout the entire process need to be consistent to evaluate the effectiveness and toxicity of the drugs[47]. In pre-clinical drug screening, 2D cell lines or patient-derived xenografts (PDXs) models are widely used[48,49]. Gastric cancer is a highly complex disease with various histological features and molecular subtypes. To better simulate and study the occurrence and development of gastric cancer, and to explore the mechanisms underlying the disease, researchers need to establish experimental models with the corresponding characteristics. In 2018, Nanki et al[50] successfully established gastric cancer organoids with multiple mutations using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) technology. They also established a large-scale library of gastric cancer organoid samples from 37 patients. Through this sample library, they were able to study the relationship between genetic changes and histopathological alterations, providing an important platform and resource to model gastric cancer, drug screening, and personalized treatment. In 2019, Seidlitz et al[51] made a significant breakthrough in the field of cancer research by successfully establishing human gastric cancer organoids. These organoids, grown from patient-derived cells, proved to be an excellent model to study gastric cancer. They accurately mimicked the typical characteristics and pathway variations observed in human gastric cancer, providing researchers with a powerful tool to explore the mechanisms underlying this deadly disease. In addition to studying molecular mutation patterns, the use of gastric cancer organoids also sheds light on drug sensitivity. Yan et al[52] conducted detailed exome and transcriptome analysis, deeply exploring the genomic information of tumors, thus providing a more detailed understanding of the mechanisms underlying tumor occurrence and development. Their research results indicated that there are a series of important gene variations in tumor cells that are closely related to tumor formation and progression. In addition to genomic research, they also carried out large-scale drug screening experiments. They found that gastric cancer organoids are very sensitive to anti-tumor drugs such as napabucasin, abexinostat, and VE-822, an inhibitor of ataxia-telangiectasia mutated and rad3-related protein kinase, which causes uncoordinated capillary vasodilation. These findings provide important clues for the development of new anti-tumor drugs.
Drug resistance: Chemotherapy is still one of the main approaches to treat gastric cancer. However, chemotherapy faces challenges, such as drug resistance and adverse reactions in tumor treatment. Therefore, the development of new targeted therapies through in-depth study of the molecular mechanisms of tumors is expected to improve the therapeutic efficacy and quality of life of patients with gastric cancers[53,54]. In 2021, Yuanhong utilized primary human gastric organoids combined with gene editing technology to establish the first genetic model of ARID1A (encoding AT-rich interaction domain 1A) mutation, revealing many characteristic phenotypes of ARID1A-mutated gastric cancer through multi-omics analysis. In addition to drug screening, gastric cancer organoids can also be combined with techniques such as CRISPR)/Cas9 gene editing to explore the phenotypic and functional characteristics of gastric cancer with different mutation types[55]. Ouyang et al[56] made significant progress in developing a potent and highly selective inhibitor called W1131, which specifically targets signal transducer and activator of transcription 3 (STAT3). Experiments showed that W1131 exhibits strong anti-tumor effects in various gastric cancer models. Specifically, promising results were observed in tests on xenograft, organoid, and PDX models of gastric cancer. What makes W1131 even more promising is its ability to restore the sensitivity of gastric cancer to chemotherapy. Chemoresistance is a major challenge in the treatment of advanced gastric cancer, and this resistance can be overcome by targeting the STAT3-iron death pathway. By inhibiting STAT3 and promoting iron death, W1131 enhances the effectiveness of chemotherapeutic drugs, thereby providing a new treatment strategy for patients with advanced gastric cancer.
An established organoid biobank refers to a specialized repository used for the preservation and study of various artificially synthesized organoids. The establishment of such a biobank is crucial for drug development research because it can provide a large amount of drug sensitivity data, assisting scientists to evaluate the effectiveness and safety of drugs. This, in turn, provides a basis for individualized drug development and research in regenerative medicine. Additionally, this organoid biobank could provide significant value for the study of the dynamic occurrence, development, and treatment of living tumors[57].
Patient-derived xenografts organoids (PDXOs) offer new possibilities for individualized therapy by culturing patient-derived tumor xenograft models (PDTXs) in a way that preserves tumor properties and stem cell properties, representing a new tool for personalized therapy and regenerative medicine research. PDTX[58] models have become valuable and powerful tools in translational research, aiming to develop a model that closely resembles human tumors[59]. The formation of PDTXs involves directly implanting patient-derived tumor tissues into immunodeficient mice, and allowing the tumor to grow and develop while faithfully reproducing the unique characteristics and heterogeneity observed in human tumors. Compared with traditional cell lines or genetically engineered mouse models, this method provides a more accurate representation of the complexity and dynamics of human tumors[60]. These models are highly valuable in preclinical research because of their ability to accurately reproduce the characteristics of patient tumors and predict the efficacy of anticancer drugs[61]. It is widely believed that cancer stem cells exist in PDTXs tumors, which is what makes them similar to patients' tumors. However, PDTXs have practical limitations, such as low throughput, high cost, and long duration[62]. To address these limitations, Xu et al[63] proposed a new method of creating PDXOs. By culturing PDX tumor cells in a way that preserves the tumor characteristics and stemness properties, researchers can generate PDXOs paired with corresponding PDX models[64]. This makes in vivo and in vitro pharmacological research more efficient and cost-effective.
Meanwhile, the complexity of tumor genomes means that there are multiple targets that affect drug sensitivity, and a single target cannot accurately assess a patient's response to drugs. Therefore, PDXOs are considered one of the best current tools for simulating the behavior and response of tumors in patients.
Matrigel is a matrix gel prepared from the secretions of Engelbreth-Holm-Swarm mouse sarcoma cells. Many different types of organoids have been studied in culture in Matrigel. Matrigel for organoid culture contain matrix proteins such as laminin, collagen type IV, and nidogen, which provide scaffolding structures for cell adhesion and proliferation. In addition, the gels contain a variety of growth factors, including transforming growth factor beta (TGF-β), EGF, and insulin-like growth factor, which encourage cell proliferation and differentiation, thereby promoting organoid growth and development. The complexity of Matrigel leads to a lack of clarity and certainty in defining its composition and structure. This has made it difficult for researchers to accurately identify the specific factors in Matrigel that affect organ development. Although Matrigel provides a three-dimensional support structure that helps to mimic a realistic tissue environment, the components are numerous, complex and not easily measured. Researchers need to explore and understand the regulatory role of Matrigel on organ development by analyzing the various molecular components in Matrigel and the mechanisms by which they interact with cells[65].
Currently, the reproducibility and consistency of organoids cultures are the primary issues that need to be addressed. These issues have affected the development of organoids. The establishment of an organoid involves many factors, such as the source of cells used by different researchers, differences in growth environments, the preparation of culture media, and the use of culture tools, all of which have an impact on the formation and function of organoids tissues. In addition, there is a lack of standardized processes for the collection, culture, cryopreservation and recovery of organ tissues, and there are no established industry standards for reference. The collection process of organ tissues may be disrupted, resulting in cell death or loss of function. In the process of culturing organ tissues, because of the lack of standardized culture conditions, the growth and development of cells might be disturbed by different factors, resulting in differences in the structure and function of organ tissues. Meanwhile, cryopreservation and resuscitation of organ tissues is also a challenge. There is no uniform method or standard for the cryopreservation and regeneration of organ tissues. This brings difficulties to the preservation and transportation of organ tissues, and makes it difficult to ensure that organ tissues can restore their original structure and function after recovery. These problems have meant that organoid technology has not been widely used in drug development or diagnosis. Although organoid technology has made some breakthroughs in laboratory research, it needs to solve the above problems and establish unified standards and processes if it is to be usefully applied in the field. In this way, the reproducibility and consistency of organoids can be ensured, making it a reliable and effective tool to promote the development of drug discovery and diagnosis[66].
The structure of the stomach is complex and has diverse functions. There are still many unknowns in the field of regenerative medicine for stomach organs. In recent years, scientists have successfully developed tissue engineering techniques for the stomach through animal experiments[67]. This technique can reconstruct an artificial stomach organ with a complete mucosa and smooth muscle layers. However, this technology is still in the experimental stage and further research is needed before it can be applied in the human body. Therefore, to promote the development of gastric regenerative medicine, new technologies should be introduced. These technologies will help us better understand the process of gastric regeneration and the treatment methods for gastric-related diseases.
While PDXOs enable researchers to perform drug screening in vitro and have potential applications in functional precision oncology, it is important to recognize the limitations associated with using organoids as tools. In comparison to PDX models, PDXOs offer the advantage of allowing large-scale drug screenings to be conducted within a short timeframe. However, one significant limitation is that PDXOs lack other cell types that are typically present in the tumor microenvironment or the immune system. Therefore, they are not suitable for evaluating the effects of drugs on these crucial components of cancer biology. Moreover, the drug library that can be used for PDXOs drug testing is restricted to drugs that do not require metabolism by the liver. This limitation narrows the scope of drugs that can be tested using PDXOs. Additionally, at the moment, PDXOs cannot be used to assess the effects of immunotherapies. Lastly, if real-time co-clinical drug testing is required, where results are needed shortly after obtaining patient tissue, PDXOs might not be the most ideal tool. The inability to conduct immediate testing using PDXOs hinders their ability to provide timely results in such scenarios[68].
Compared with traditional 2D monolayer cell cultures, three-dimensional organoid disease models offer a more accurate representation of human physiological conditions. This is because organoids can better mimic the complex structure and function of human organs, allowing for a more realistic study of disease mechanisms. These organoid models serve as valuable tools for researchers to compare and reference various disease models, leading to a deeper understanding of their underlying mechanisms.
Personalized organoid culture technology provides a new breakthrough for gastric cancer research, and provides an important basis for clinicians to develop individualized treatment strategies. At the same time, establishing a living biobank containing specific cancer entities provides valuable resources for drug development and repurposing. However, to better utilize this technology, researchers need to continuously improve and optimize culture methods with the aim of enhancing the accuracy and reliability of gastricorganoid models.
The heterogeneity of stomach cancer means that a universal drug that effectively treats all patients has not yet been discovered. Therefore, to advance cancer treatment outcomes, personalized medicine has become an important approach. Tumor-derived organoids have emerged as a crucial tool in achieving the goals of personalized medicine. These organoids, generated from patient tumor samples, closely resemble the characteristics and behavior of the original tumor and can be used to test the efficacy of different therapies tailored to each individual's unique cancer profile. Utilizing tumor-derived organoids, clinicians can identify the most effective treatment options for specific patients, thereby increasing the chances of successful cancer treatment while minimizing unnecessary side effects. This personalized approach is revolutionizing cancer treatment and offering hope for more targeted and successful therapies in the future.
The use of organoid platforms for drug screening has proven to be feasible and highly effective. The similarity between organoids and the human body makes them an ideal platform for drug research. By testing the efficacy and safety of drugs in organoids, researchers can obtain more reliable and accurate evaluations of their effects. This provides crucial references for drug development, enabling the development of safer and more effective treatments. In particular, the use of PDXOs allows for personalized drug screening, thus tailoring treatment options to the specific needs of individual patients. As a result, organoid technology has the potential to drive the future development of precision medicine. The regenerative and proliferative abilities of organoids also make them highly promising in the field of regenerative medicine. Through the cultivation and repair of organoids, researchers can facilitate the regeneration of human tissues and organs. This has significant implications for treating a wide range of diseases and injuries. However, to fully realize the potential of organoid technology in regenerative medicine, further advances are needed to better simulate the structure and function of human organs. With continued progress, organoid technology has the potential to greatly impact the fields of disease modeling, drug screening, and regenerative medicine.
Although the current organoid systems have some limitations, such as the need for further optimization in disease modeling and personalized medicine, these systems provide important new opportunities to the field of regenerative medicine. By combining additional biotechnological methods, these systems will continue to develop into valuable tools for preclinical and clinical research. The development of these organoid technologies holds tremendous potential for disease treatment and personalized healthcare, providing clinicians with more accurate disease models and patients with personalized treatment plans. Although current organoid systems still face some technical challenges, it is believed that with ongoing research and improvements, they will be able to better simulate and rebuild human organs, resulting in breakthroughs in medical research and clinical applications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Toyoshima O, Japan S-Editor: Yan JP L-Editor: A P-Editor: Zhang XD
| 1. | Rauth S, Karmakar S, Batra SK, Ponnusamy MP. Recent advances in organoid development and applications in disease modeling. Biochim Biophys Acta Rev Cancer. 2021;1875:188527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Nguyen R, Bae SDW, Zhou G, Read SA, Ahlenstiel G, George J, Qiao L. Application of organoids in translational research of human diseases with a particular focus on gastrointestinal cancers. Biochim Biophys Acta Rev Cancer. 2020;1873:188350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Li F, Zhang P, Wu S, Yuan L, Liu Z. Advance in Human Epithelial-Derived Organoids Research. Mol Pharm. 2021;18:3931-3950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Zeng Y, Jin RU. Molecular pathogenesis, targeted therapies, and future perspectives for gastric cancer. Semin Cancer Biol. 2022;86:566-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 5. | Di Giorgio C, Roselli R, Biagioli M, Marchianò S, Distrutti E, Bordoni M, Donini A, Fiorucci S. Organoids as ex vivo culture system to investigate infection-host interaction in gastric pre-carcinogenesis. Recent Adv Inflamm Allergy Drug Discov. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Chakrabarti J, Zavros Y. Generation and use of gastric organoids for the study of Helicobacter pylori pathogenesis. Methods Cell Biol. 2020;159:23-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Perrone F, Zilbauer M. Biobanking of human gut organoids for translational research. Exp Mol Med. 2021;53:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Azar J, Bahmad HF, Daher D, Moubarak MM, Hadadeh O, Monzer A, Al Bitar S, Jamal M, Al-Sayegh M, Abou-Kheir W. The Use of Stem Cell-Derived Organoids in Disease Modeling: An Update. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Malagola E, Hayakawa Y, Wang TC. R-spondin signaling in the stomach: isthmal Lgr4 rules. EMBO J. 2022;41:e111696. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Wu A, Yu B, Zhang K, Xu Z, Wu D, He J, Luo J, Luo Y, Yu J, Zheng P, Che L, Mao X, Huang Z, Wang L, Zhao J, Chen D. Transmissible gastroenteritis virus targets Paneth cells to inhibit the self-renewal and differentiation of Lgr5 intestinal stem cells via Notch signaling. Cell Death Dis. 2020;11:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Hanyu H, Sugimoto S, Sato T. Visualization of Differentiated Cells in 3D and 2D Intestinal Organoid Cultures. Methods Mol Biol. 2023;2650:141-153. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Wölffling S, Daddi AA, Imai-Matsushima A, Fritsche K, Goosmann C, Traulsen J, Lisle R, Schmid M, Reines-Benassar MDM, Pfannkuch L, Brinkmann V, Bornschein J, Malfertheiner P, Ordemann J, Link A, Meyer TF, Boccellato F. EGF and BMPs Govern Differentiation and Patterning in Human Gastric Glands. Gastroenterology. 2021;161:623-636.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Colozza G, Koo BK. Ub and Dub of RNF43/ZNRF3 in the WNT signalling pathway. EMBO Rep. 2021;22:e52970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Toh Y, Wu L, Park S, Wang A, Tu J, Yu W, Zuo M, Carmon KS, Liu QJ. LGR4 and LGR5 form distinct homodimers that only LGR4 complexes with RNF43/ZNRF3 to provide high affinity binding of R-spondin ligands. Sci Rep. 2023;13:10796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Wu N, Sun H, Zhao X, Zhang Y, Tan J, Qi Y, Wang Q, Ng M, Liu Z, He L, Niu X, Chen L, Li HB, Zeng YA, Roulis M, Liu D, Cheng J, Zhou B, Ng LG, Zou D, Ye Y, Flavell RA, Ginhoux F, Su B. MAP3K2-regulated intestinal stromal cells define a distinct stem cell niche. Nature. 2021;592:606-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 16. | Xiao S, Zhou L. Gastric Stem Cells: Physiological and Pathological Perspectives. Front Cell Dev Biol. 2020;8:571536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Poudel H, Sanford K, Szwedo PK, Pathak R, Ghosh A. Synthetic Matrices for Intestinal Organoid Culture: Implications for Better Performance. ACS Omega. 2022;7:38-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y, Wells JM. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 717] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 19. | Hirshorn ST, Steele N, Zavros Y. Modeling pancreatic pathophysiology using genome editing of adult stem cell-derived and induced pluripotent stem cell (iPSC)-derived organoids. Am J Physiol Gastrointest Liver Physiol. 2021;320:G1142-G1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Broda TR, McCracken KW, Wells JM. Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat Protoc. 2019;14:28-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Yasui R, Sekine K, Yamaguchi K, Furukawa Y, Taniguchi H. Robust parameter design of human induced pluripotent stem cell differentiation protocols defines lineage-specific induction of anterior-posterior gut tube endodermal cells. Stem Cells. 2021;39:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Jensen LH, Rogatto SR, Lindebjerg J, Havelund B, Abildgaard C, do Canto LM, Vagn-Hansen C, Dam C, Rafaelsen S, Hansen TF. Precision medicine applied to metastatic colorectal cancer using tumor-derived organoids and in-vitro sensitivity testing: a phase 2, single-center, open-label, and non-comparative study. J Exp Clin Cancer Res. 2023;42:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 23. | Guan S, Wang Y, Xie F, Wang S, Xu W, Xu J, Sun C. Carboxymethyl Chitosan and Gelatin Hydrogel Scaffolds Incorporated with Conductive PEDOT Nanoparticles for Improved Neural Stem Cell Proliferation and Neuronal Differentiation. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Wakamatsu T, Ogawa H, Yoshida K, Matsuoka Y, Shizuma K, Imura Y, Tamiya H, Nakai S, Yagi T, Nagata S, Yui Y, Sasagawa S, Takenaka S. Establishment of Organoids From Human Epithelioid Sarcoma With the Air-Liquid Interface Organoid Cultures. Front Oncol. 2022;12:893592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, Sutcliffe M, Boulanger J, Tripodi M, Derivery E, Paulsen O, Lakatos A, Lancaster MA. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci. 2019;22:669-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 26. | Chandrasekaran A, Kouthouridis S, Lee W, Lin N, Ma Z, Turner MJ, Hanrahan JW, Moraes C. Magnetic microboats for floating, stiffness tunable, air-liquid interface epithelial cultures. Lab Chip. 2019;19:2786-2798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Kumar S, Jach D, Macfarlane W, Crnogorac-Jurcevic T. A 3-Dimensional Coculture Model to Visualize and Monitor Interaction Between Pancreatic Cancer and Islet β Cells. Pancreas. 2021;50:982-989. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Singh R, Singh RP. Study of Rotary Cell Culture System-Induced Microgravity Effects on Cancer Biomarkers. Methods Mol Biol. 2022;2413:77-96. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Rembiałkowska N, Baczyńska D, Dubińska-Magiera M, Choromańska A, Bieżuńska-Kusiak K, Gajewska-Naryniecka A, Novickij V, Saczko J, Przystupski D, Kulbacka J. RCCS Bioreactor-Based Modeled Microgravity Affects Gastric Cancer Cells and Improves the Chemotherapeutic Effect. Membranes (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Zheng F, Xiao Y, Liu H, Fan Y, Dao M. Patient-Specific Organoid and Organ-on-a-Chip: 3D Cell-Culture Meets 3D Printing and Numerical Simulation. Adv Biol (Weinh). 2021;5:e2000024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Saorin G, Caligiuri I, Rizzolio F. Microfluidic organoids-on-a-chip: The future of human models. Semin Cell Dev Biol. 2023;144:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 32. | Huang J, Xu Z, Jiao J, Li Z, Li S, Liu Y, Qu G, Wu J, Zhao Y, Chen K, Li J, Pan Y, Wu X, Ren J. Microfluidic intestinal organoid-on-a-chip uncovers therapeutic targets by recapitulating oxygen dynamics of intestinal IR injury. Bioact Mater. 2023;30:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Kaur S, Kaur I, Rawal P, Tripathi DM, Vasudevan A. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021;504:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 34. | Kim S, Min S, Choi YS, Jo SH, Jung JH, Han K, Kim J, An S, Ji YW, Kim YG, Cho SW. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat Commun. 2022;13:1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 187] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 35. | Stern A, Thompson B, Williams K, McClellan R, Gebhart S, Hartman J. The CellRaft AIR(®) system: A novel system enabling organoid imaging, identification, and isolation. SLAS Discov. 2022;27:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Seeger AY, Ringling MD, Zohair H, Blanke SR. Risk factors associated with gastric malignancy during chronic Helicobacter pylori Infection. Med Res Arch. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Ku CC, Wuputra K, Pan JB, Li CP, Liu CJ, Liu YC, Saito S, Chan TF, Lin CS, Wu DC, Yokoyama KK. Generation of Human Stomach Cancer iPSC-Derived Organoids Induced by Helicobacter pylori Infection and Their Application to Gastric Cancer Research. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | El Khadir M, Boukhris Alaoui S, Benajah DA, Ibrahimi SA, Chbani L, El Abkari M, Bennani B. VacA genotypes and cagA-EPIYA-C motifs of Helicobacter pylori and gastric histopathological lesions. Int J Cancer. 2020;147:3206-3214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Laucirica I, García Iglesias P, Calvet X. Peptic ulcer. Med Clin (Barc). 2023;161:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 40. | Eslami O, Nakhaie M, Rezaei Zadeh Rukerd M, Azimi M, Shahabi E, Honarmand A, Khazaneha M. Global Trend on Machine Learning in Helicobacter within One Decade: A Scientometric Study. Glob Health Epidemiol Genom. 2023;2023:8856736. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Won Y, Choi E. Mouse models of Kras activation in gastric cancer. Exp Mol Med. 2022;54:1793-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Kapalczynska M, Lin M, Maertzdorf J, Heuberger J, Muellerke S, Zuo X, Vidal R, Shureiqi I, Fischer AS, Sauer S, Berger H, Kidess E, Mollenkopf HJ, Tacke F, Meyer TF, Sigal M. BMP feed-forward loop promotes terminal differentiation in gastric glands and is interrupted by H. pylori-driven inflammation. Nat Commun. 2022;13:1577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Boccellato F, Woelffling S, Imai-Matsushima A, Sanchez G, Goosmann C, Schmid M, Berger H, Morey P, Denecke C, Ordemann J, Meyer TF. Polarised epithelial monolayers of the gastric mucosa reveal insights into mucosal homeostasis and defence against infection. Gut. 2019;68:400-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Holokai L, Chakrabarti J, Broda T, Chang J, Hawkins JA, Sundaram N, Wroblewski LE, Peek RM Jr, Wang J, Helmrath M, Wells JM, Zavros Y. Increased Programmed Death-Ligand 1 is an Early Epithelial Cell Response to Helicobacter pylori Infection. PLoS Pathog. 2019;15:e1007468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 45. | Cheng S, Li H, Chi J, Zhao W, Lin J, Liu X, Xu C. FTO-mediated m(6)A modification promotes malignant transformation of gastric mucosal epithelial cells in chronic Cag A(+) Helicobacter pylori infection. J Cancer Res Clin Oncol. 2023;149:7327-7340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 46. | Koh V, Chakrabarti J, Torvund M, Steele N, Hawkins JA, Ito Y, Wang J, Helmrath MA, Merchant JL, Ahmed SA, Shabbir A, Yan So JB, Yong WP, Zavros Y. Hedgehog transcriptional effector GLI mediates mTOR-Induced PD-L1 expression in gastric cancer organoids. Cancer Lett. 2021;518:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 47. | Ren X, Chen W, Yang Q, Li X, Xu L. Patient-derived cancer organoids for drug screening: Basic technology and clinical application. J Gastroenterol Hepatol. 2022;37:1446-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 48. | Yu X, Chen Y, Lu J, He K, Ding Y, Jin K, Wang H, Zhang H, Teng L. Patient-derived xenograft models for gastrointestinal tumors: A single-center retrospective study. Front Oncol. 2022;12:985154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Alzeeb G, Arzur D, Trichet V, Talagas M, Corcos L, Le Jossic-Corcos C. Gastric cancer cell death analyzed by live cell imaging of spheroids. Sci Rep. 2022;12:1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Nanki K, Toshimitsu K, Takano A, Fujii M, Shimokawa M, Ohta Y, Matano M, Seino T, Nishikori S, Ishikawa K, Kawasaki K, Togasaki K, Takahashi S, Sukawa Y, Ishida H, Sugimoto S, Kawakubo H, Kim J, Kitagawa Y, Sekine S, Koo BK, Kanai T, Sato T. Divergent Routes toward Wnt and R-spondin Niche Independency during Human Gastric Carcinogenesis. Cell. 2018;174:856-869.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 51. | Seidlitz T, Merker SR, Rothe A, Zakrzewski F, von Neubeck C, Grützmann K, Sommer U, Schweitzer C, Schölch S, Uhlemann H, Gaebler AM, Werner K, Krause M, Baretton GB, Welsch T, Koo BK, Aust DE, Klink B, Weitz J, Stange DE. Human gastric cancer modelling using organoids. Gut. 2019;68:207-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 52. | Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, Chan D, Chan AS, Ma S, Lam KO, Bartfeld S, Man AHY, Lee BCH, Chan ASY, Wong JWH, Cheng PSW, Chan AKW, Zhang J, Shi J, Fan X, Kwong DLW, Mak TW, Yuen ST, Clevers H, Leung SY. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell. 2018;23:882-897.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 484] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 53. | Putker M, Millen R, Overmeer R, Driehuis E, Zandvliet MMJM, Clevers H, Boj SF, Li QX. Medium-Throughput Drug- and Radiotherapy Screening Assay using Patient-Derived Organoids. J Vis Exp. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Driehuis E, Kretzschmar K, Clevers H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat Protoc. 2020;15:3380-3409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 395] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 55. | Lo YH, Kolahi KS, Du Y, Chang CY, Krokhotin A, Nair A, Sobba WD, Karlsson K, Jones SJ, Longacre TA, Mah AT, Tercan B, Sockell A, Xu H, Seoane JA, Chen J, Shmulevich I, Weissman JS, Curtis C, Califano A, Fu H, Crabtree GR, Kuo CJ. A CRISPR/Cas9-Engineered ARID1A-Deficient Human Gastric Cancer Organoid Model Reveals Essential and Nonessential Modes of Oncogenic Transformation. Cancer Discov. 2021;11:1562-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 56. | Ouyang S, Li H, Lou L, Huang Q, Zhang Z, Mo J, Li M, Lu J, Zhu K, Chu Y, Ding W, Zhu J, Lin Z, Zhong L, Wang J, Yue P, Turkson J, Liu P, Wang Y, Zhang X. Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol. 2022;52:102317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 277] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 57. | Xie X, Li X, Song W. Tumor organoid biobank-new platform for medical research. Sci Rep. 2023;13:1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 58. | Cacciapuoti MT, Cappelli LV, Fiore D, Toruno P, Kayembe C, Tam W, Inghirami G. In Vivo and Ex Vivo Patient-Derived Tumor Xenograft Models of Lymphoma for Drug Discovery. Curr Protoc. 2021;1:e96. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 59. | Jung J, Seol HS, Chang S. The Generation and Application of Patient-Derived Xenograft Model for Cancer Research. Cancer Res Treat. 2018;50:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 60. | Yoshida GJ. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020;13:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 313] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 61. | Zanella ER, Grassi E, Trusolino L. Towards precision oncology with patient-derived xenografts. Nat Rev Clin Oncol. 2022;19:719-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 86] [Reference Citation Analysis (0)] |
| 62. | Abdolahi S, Ghazvinian Z, Muhammadnejad S, Saleh M, Asadzadeh Aghdaei H, Baghaei K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med. 2022;20:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 174] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 63. | Xu X, Shang L, Wang P, Zhou J, Ouyang X, Zheng M, Mao B, Zhang L, Chen B, Wang J, Chen J, Qian W, Guo S, Huang Y, Li QX. Creating Matched In vivo/In vitro Patient-Derived Model Pairs of PDX and PDX-Derived Organoids for Cancer Pharmacology Research. J Vis Exp. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Guillen KP, Fujita M, Butterfield AJ, Scherer SD, Bailey MH, Chu Z, DeRose YS, Zhao L, Cortes-Sanchez E, Yang CH, Toner J, Wang G, Qiao Y, Huang X, Greenland JA, Vahrenkamp JM, Lum DH, Factor RE, Nelson EW, Matsen CB, Poretta JM, Rosenthal R, Beck AC, Buys SS, Vaklavas C, Ward JH, Jensen RL, Jones KB, Li Z, Oesterreich S, Dobrolecki LE, Pathi SS, Woo XY, Berrett KC, Wadsworth ME, Chuang JH, Lewis MT, Marth GT, Gertz J, Varley KE, Welm BE, Welm AL. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer. 2022;3:232-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 233] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 65. | Kozlowski MT, Crook CJ, Ku HT. Towards organoid culture without Matrigel. Commun Biol. 2021;4:1387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 66. | LeSavage BL, Suhar RA, Broguiere N, Lutolf MP, Heilshorn SC. Next-generation cancer organoids. Nat Mater. 2022;21:143-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 249] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 67. | Vogt CD, Panoskaltsis-Mortari A. Tissue engineering of the gastroesophageal junction. J Tissue Eng Regen Med. 2020;14:855-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Scherer SD, Zhao L, Butterfield AJ, Yang CH, Cortes-Sanchez E, Guillen KP, Welm BE, Welm AL. Breast cancer PDxO cultures for drug discovery and functional precision oncology. STAR Protoc. 2023;4:102402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |