Published online Nov 15, 2024. doi: 10.4251/wjgo.v16.i11.4456
Revised: September 11, 2024
Accepted: September 29, 2024
Published online: November 15, 2024
Processing time: 85 Days and 2.3 Hours
Colorectal cancer (CRC) is a considerable global health issue. Dioscin, a com
To find the relationship between CRC cells (HCT116) and diosgenin and clarified their mechanisms of action.
CRC cell line HCT116 was cultured by dividing cells into control and dioscin groups (dioscin + Jagged 1 group; Jagged 1 group, 5 μg/mL; and dioscin group, 2.5 μg/mL). The dioscin groups were given different concentrations of dioscin. Cell Counting Kit-8 was chosen for testing cell viability in different groups. Flow cytometry was established to undiscover the apoptosis rate of human liver cancer cell line 11. Real-time PCR as well as Western blot analyses were applied to reveal the expression levels of caspase-3, Notch, and other proteins. Transwell and scr
This study indicated that dioscin restricted the growth of HCT116 cells, boosted cell apoptosis, and rose the Bax/Bcl-2 ratio as well as the expression of Caspase-3. Dioscin also inhibited physiological activities, for instance cell migration, and sig
Dioscin exerts a certain inhibitory effect on HCT116, and its mechanism of action may be linked, with the inhibition of the Notch1 signaling pathway.
Core Tip: This study investigates the anticancer effects of dioscin on colorectal cancer (CRC) cells (HCT116). Our findings reveal that dioscin inhibits cell viability, promotes apoptosis, and suppresses migration and invasion by modulating the Notch1 signaling pathway. These results highlight the potential of dioscin as a therapeutic agent against CRC.
- Citation: Cai XX, Huang ZF, Tu FY, Yu J. Impact and mechanism study of dioscin on biological characteristics of colorectal cancer cells. World J Gastrointest Oncol 2024; 16(11): 4456-4467
- URL: https://www.wjgnet.com/1948-5204/full/v16/i11/4456.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i11.4456
Analysis of malignant tumors can confirm if they are cancer, and colorectal cancer (CRC) is responsible for the deaths of approximately 600000 individuals globally each year[1]. In developing countries, new cases of CRC will reach 2.5 million by 2035[2]. In China, the incidence and mortality rates of CRC increase every year, and the affected population is young individuals because of rapid economic development, aging population, and changes in residents' lifestyles and dietary patterns[3]. CRC originates from the epithelium and glands of the colorectal mucosa. Abnormal expression or structural changes in core regulatory pathway molecules within cells lead to disruptions in cell growth and metabolic functions. This process prompts the transformation of normal colonic mucosa into colorectal adenomas or polyps, which, with the gradual accumulation of abnormal molecules, can further develop into CRC[4]. Chemotherapy is the most widely used clinical treatment for CRC, but it can cause severe toxic side effects and complications in the body, for instance nausea, vomiting, bone marrow suppression, and liver and kidney toxicity[5]. Therefore, effective treatments should be de
The Notch1 signaling pathway plays a crucial role in the advancement as well as progression of CRC. Notch1 is a transmembrane receptor that interacts with ligands for instance Jagged1 and Delta-like proteins. When the Notch1 signaling pathway is activated, it triggers the cleavage of the Notch intracellular domain (NICD). The NICD subsequently moves into the nucleus, where it initiates the expression of downstream target genes such as Hes1 as well as Hey1. These target genes regulate cell proliferation, differentiation, as well as apoptosis. Dysregulation of the Notch1 pathway has been implicated in the promotion of CRC cell growth and survival.
Traditional Chinese medicine has unique advantages in natural resources, and its pharmacological effects are very extensive. Various Chinese medicines exert anti-tumor activity and have significant advantages in clinical treatment. Dioscin is widely present in medicinal plants of the Dioscoreaceae, Liliaceae, Caryophyllaceae, and Rosaceae families, espe
A few studies investigated the ability of dioscin to suppress CRC. Limited data are available on the inhibitory effects of Dioscoreaceae medicinal plants on CRC cells in vitro. The systematic effects of dioscin on CRC and its underlying me
Dioscin (cat. no. JOT-10207) was purchased from Chengdu Pufei De Biotech Co., Ltd. Notch1 pathway activator Jagged 1 (cat. no. HY-P1846A) was acquired from MedChemExpress. Cell counting kit-8 (CCK-8) staining solution (cat. no. C0037) was obtained from Beyotime Biotechnology. qRT-PCR amplification (cat. no. RR820Q) and reverse transcription reagent kit (cat. no. RR047A) as well as annexin V-FITC/PI cell apoptosis detection kit (cat. no. 630109) were provided by Takara. Primers for Bax, Bcl-2, as well as caspase-3 were synthesized by Shanghai Jima Technology Co., Ltd. Rabbit anti-Notch1 (cat. no. ab52627), Jagged1 (cat. no. ab109536), Hes1 (cat. no. ab108937), and GAPDH (cat. no. ab8245) antibodies and goat anti-rabbit secondary antibody coupled with horseradish peroxidase (HRP) (cat. no. ab181662) were supplied by Abcam (Shanghai, China).
CRC cells HCT116 were sourced from the Shanghai Cell Bank of the Chinese Academy of Sciences. These cells were cultured at 37 °C in an atmosphere containing 5% CO2, using RPMI-1640 medium enriched with 10% fetal bovine serum. Upon reaching 80% cell confluence, the culture medium was disposed. The cells were washed carefully with phosphate buffered saline (PBS) and added with 1 mL of 0.25% trypsin for digestion. Digestion was stopped by adding culture medium when the cell gaps widened and some adherent cells detached. The adherent cells were detached by gentle pi
HCT116 cells were seeded at a density of 1 × 105 cells per milliliter in a 96-well plate and allowed to grow for a duration of 24 hours. Each well was added with culture media containing different agents (1.25, 2.5, 5 μg/mL dioscin, 5 μg/mL Jagged 1, 2.5 μg/mL dioscin + 5 μg/mL Jagged 1). An equivalent volume of drug-free growth media was given to the control group. The cells were cultivated in a carbon dioxide incubator at 37 °C for 48 hours. The cells were then added with 10 μL of CCK-8 solution per well and cultured again for 60 minutes. Absorbance was detected at 450 nm. Cell viability was calculated by the formula: OD value of dioscin-treated group/OD value of control group × 100.
The cells (2 × 104 cells/mL) were cultivated in a 24-well microplate for 24 hours Each well was added with culture media containing different concentrations of dioscin (1.25,2.5,5 μg/mL), 5 μg/mL Jagged 1, and a mixture of 2.5 μg/mL dioscin and 5 μg/mL Jagged 1. The control group received the same volume of culture medium without drugs. The cells were incubated at 37 °C in a 5% CO2 cell culture incubator for 48 hours. After the cells were broken down by 0.25% trypsin, they were centrifuged at 1500 rpm for 3 minutes and then revived with 300 μL of binding buffer. The cell suspension (100 μL) was incubated at room temperature with 1 μL of PI and 5 μL of annexin V for 15 minutes.
The lower chamber of the 24-well plate was added with 600 μL of standard culture media with serum. HCT116 cells were seeded at a density of 2 × 104/mL in serum-free medium at the top chamber of the Transwell. The upper chamber was added with different serum-free media containing dioscin (1.25, 2.5, 5 μg/mL), 5 μg/mL Jagged 1, and a mixture of 2.5 μg/mL dioscin and 5 μg/mL Jagged 1. An equivalent amount of serum-free medium was administered to the blank control group. After 24 hours in a cell culture incubator, the upper chamber was removed. The cells were washed with PBS, fixed with methanol pre-cooled in the bottom chamber for 30 minutes, stained with crystal violet for 10 minutes, and observed under an inverted microscope to check for cell invasion.
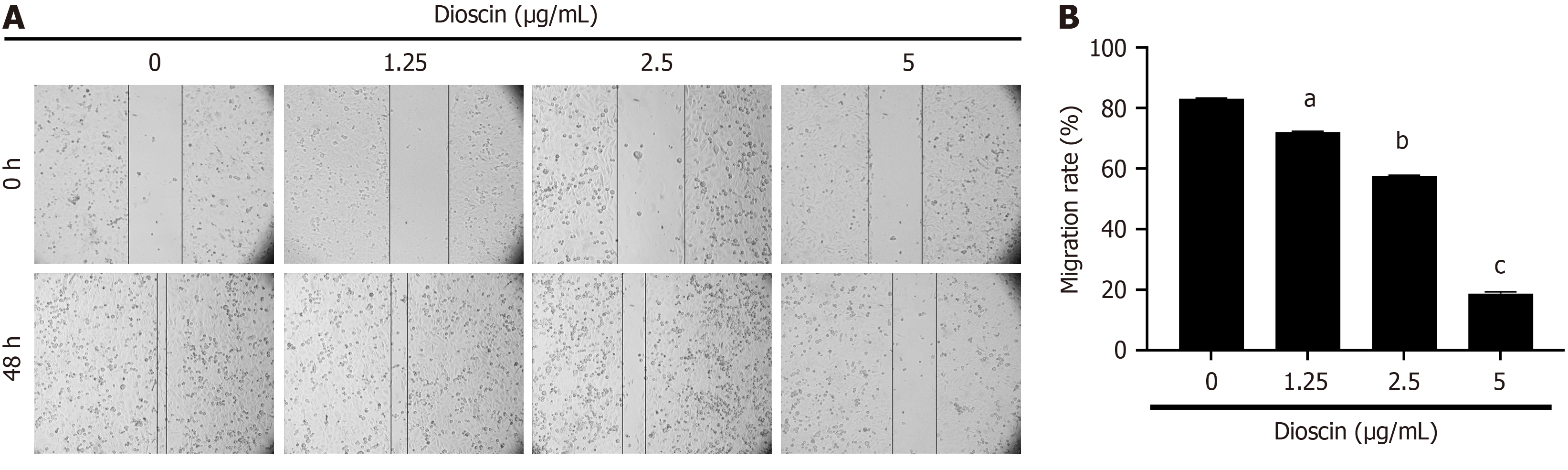
In a 12-well plate, cells (3 × 105 cells/mL) were cultivated until a monolayer formed. A straight incision was made at the center of the cell pore by using the tip of a sterile micropipette. After 48 hours, the cells were viewed under a microscope to determine the healing status of the wound. The rate of cell migration was determined using the formula: Migration rate = (Area at 0 hours - Area at 48 hours) / Area at 0 hours.
Trizol method was used to extract total cellular RNA, and cDNA was synthesized according to the instructions of the reagent kit. The overall reaction volume was 20 μL, and the reaction conditions were set at 25 °C for 30 min, 42 °C for 30 minutes, and 85 °C for 5 minutes. About 2 μL of cDNA was taken as the amplification template, with an overall reaction volume of 20 μL. Amplification was conducted with the following steps: Pre-denaturation at 95 °C for 5 minutes, denaturation at 94 °C for 30 seconds, annealing at 55 °C for 30 seconds, extension at 72 °C for 60 seconds, for a total of 30 cycles, and a final extension at 72 °C for 1 minute. Relative gene expression changes were calculated using 2−△△CT method. The primers used are shown in Table 1.
| Gene | Forward primer | Reverse primer |
| Bax | ATGGAGCTGCAGAGGATGATT | TGATGGTTCTGATCAGCTCGG |
| Bcl-2 | GCGTCAACAGGGAGATGTCA | TTCCACAAAGGCATCCCAGC |
| Caspase-3 | TCTACCGCACCCGGTTACTA | TCAAATTCCGTGGCCACCTT |
| GAPDH | GAAGGTGAAGGTCGGAGTCA | GAAGATGGTGATGGGATTTC |
After extracting all of the cellular proteins from each group, the BCA assay kit was used to quantify the proteins. The samples were concentrated with loading buffer as well as heated at 100 °C for 10 minutes to denature the proteins after their concentrations were adjusted to the same amount. SDS-PAGE was conducted at 100 V for 50 minutes, followed by wet transfer at 320 mA for 90 minutes. The membrane was placed in a room-temperature environment and sealed using 5% skim milk. The membrane was shaken for 60 minutes and washed using TBST. The membrane was placed in the antibody diluted by 1000 times and then placed in a humidification chamber for 12 hours. The membrane was removed, washed using TBST, placed in the second diluted antibody, and shaken for 120 minutes. Signal was detected using the ECL chemiluminescence detection kit. A gel imaging system was used to detect and analyze the image. The optical density ratio of the internal reference standard and the target strip was calculated and statistically analyzed.
After completing data collection, statistical analysis was conducted using SPSS 18.0 software. Measurement data were described by mean ± SD. Normality of continuous variables was tested using the Shapiro-Wilk test. Different groups of data were compared using one-way ANOVA. Values with a P value of less than 0.05 were deemed to be statistically significant.
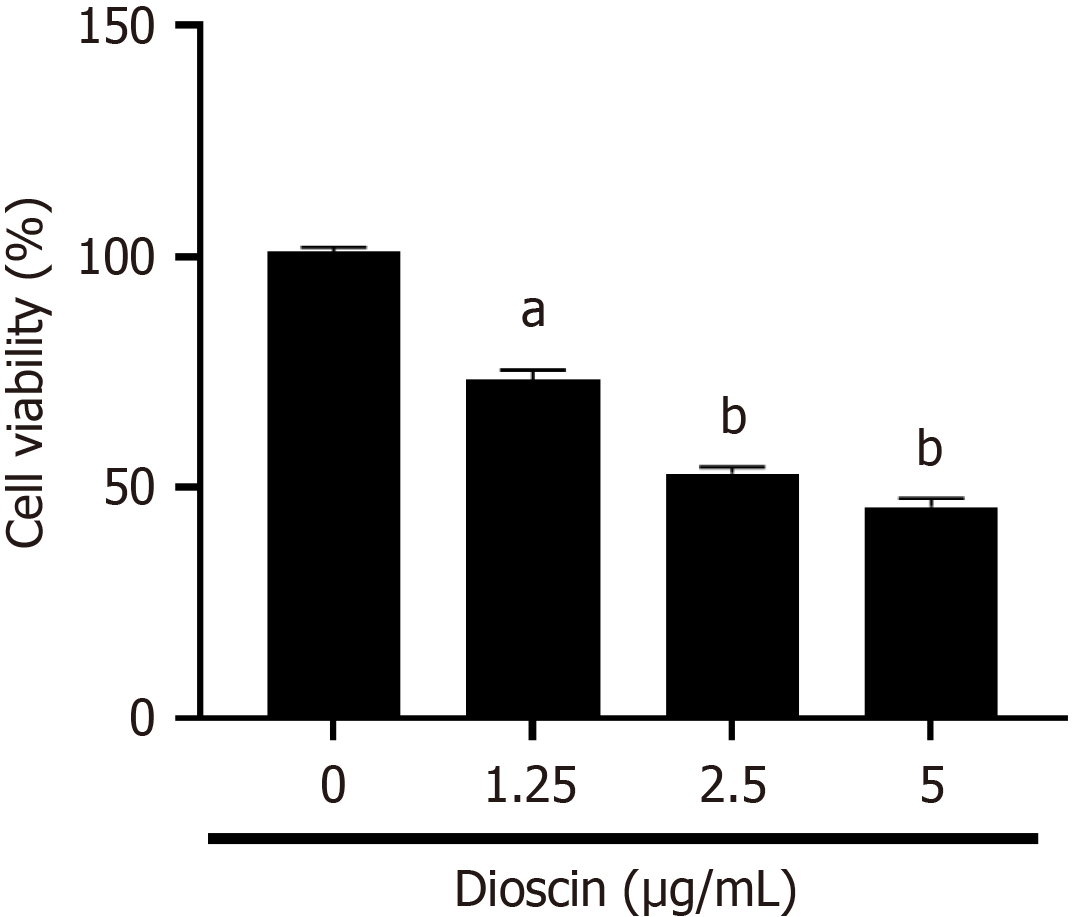
To explore the response of dioscin on the viability of HCT116 cells, we conducted a series of experiments. As shown in Figure 1, different concentrations of dioscin inhibited the viability of HCT116 cells. With increasing concentrations of dioscin, the cell viability of each group gradually reduced (0 μg/mL: 100.90% ± 1.18%, 1.25 μg/mL: 73.34% ± 2.09%, 2.5 μg/mL: 53.00% ± 1.49%, 5 μg/mL: 45.76% ± 1.89%), Cell viability was statistically significantly different between the control group and the dioscin groups (P < 0.05).
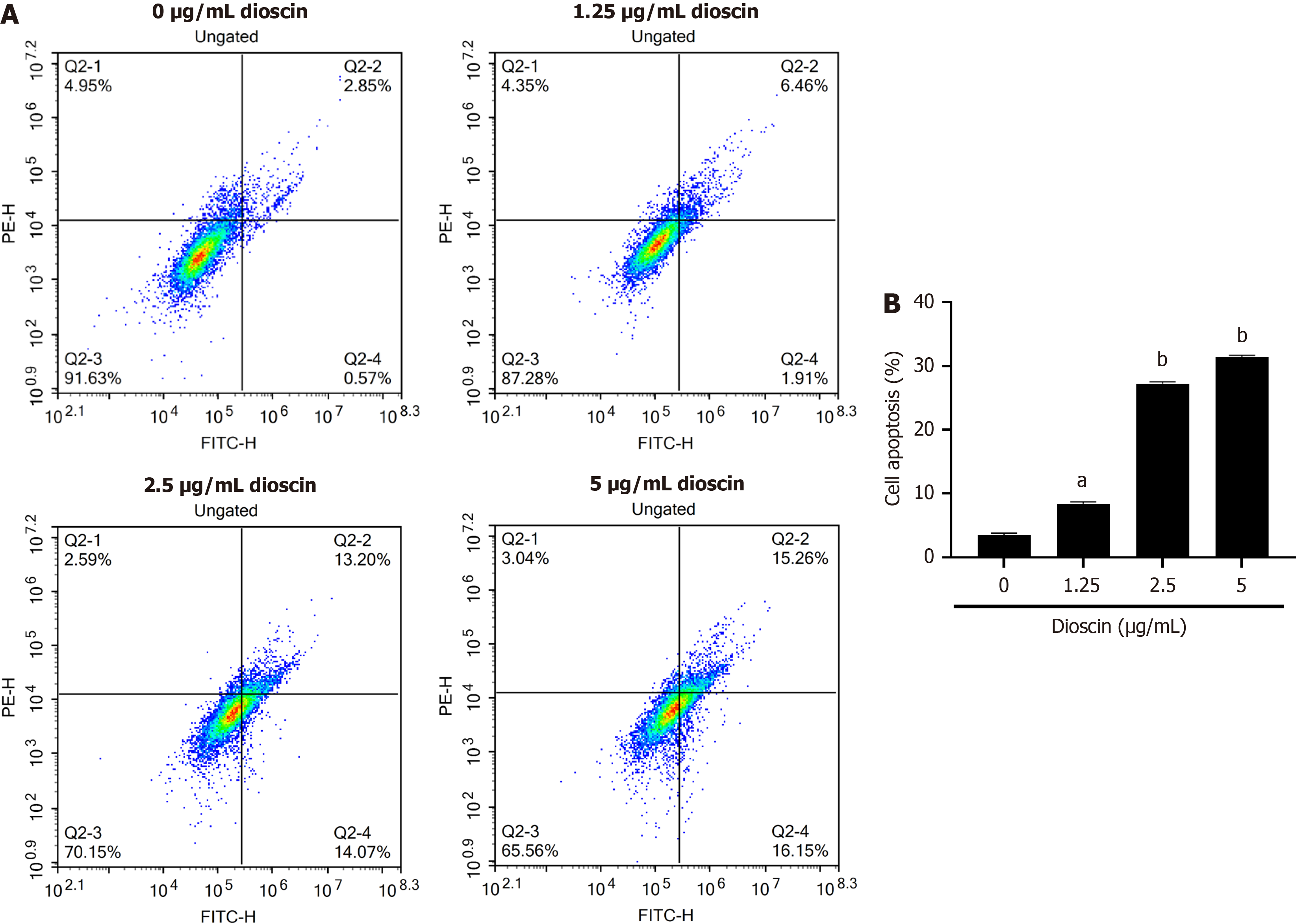
To elucidate the impact of dioscin on apoptosis in HCT116 cells, we applied flow cytometry analysis. As shown in Figure 2, various dioscin doses increased the rate of cell apoptosis relative to the control group (P < 0.05). With increasing dioscin concentration, the degree of apoptosis in each group gradually increased (0 μg/mL: 100.90 ± 1.18, 1.25 μg/mL: 73.34 ± 2.09, 2.5 μg/mL: 53.00 ± 1.49, 5 μg/mL: 45.76 ± 1.89). This finding indicates that dioscin promotes apoptosis in HCT116 cells.
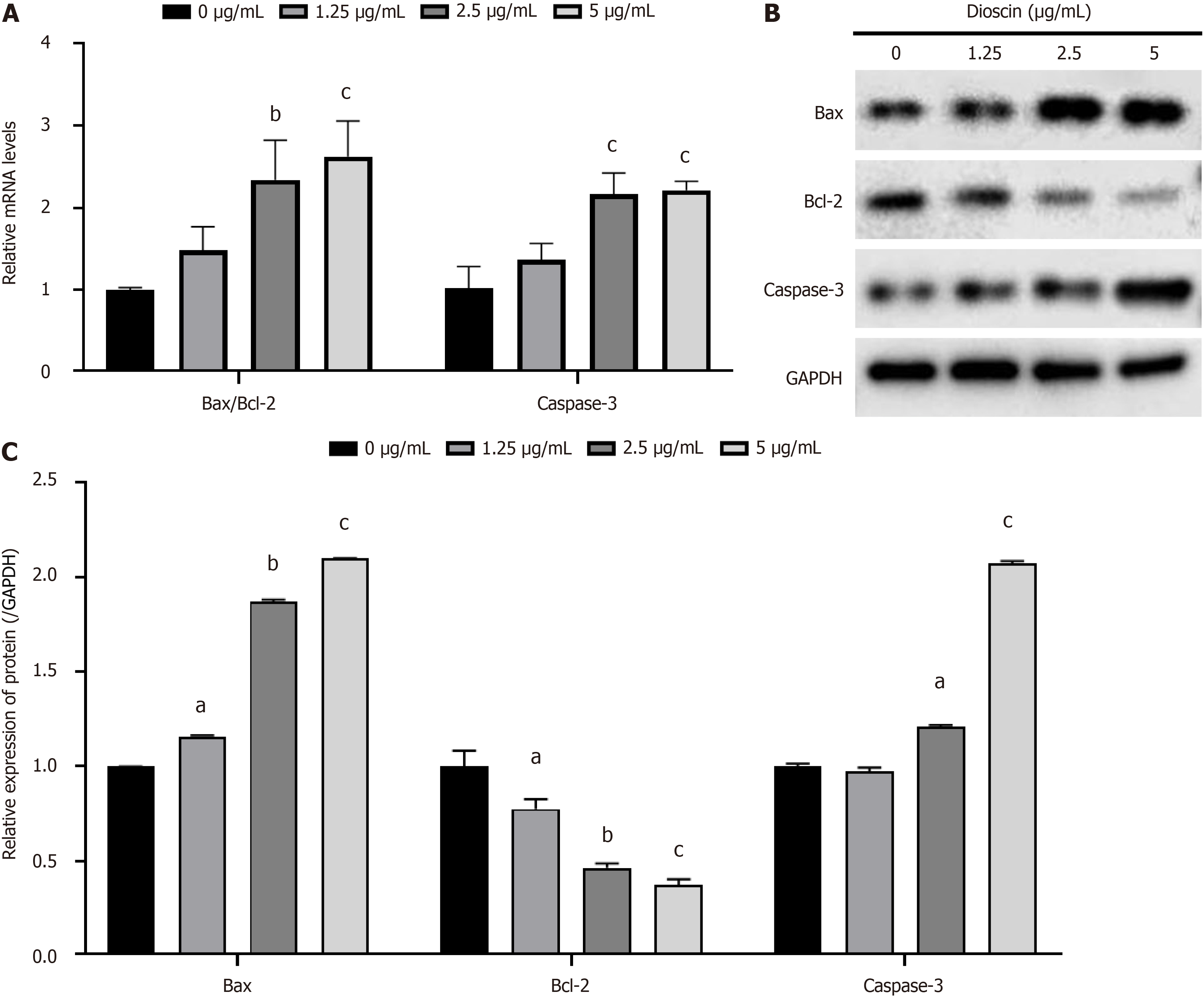
As shown in Figure 3A, with increasing dioscin concentration, the Bax/Bcl-2 ratio (0 μg/mL: 1.00 ± 0.03, 1.25 μg/mL: 1.48 ± 0.29, 2.5 μg/mL: 2.34 ± 0.48, 5 μg/mL: 2.62 ± 0.44) and Caspase-3 mRNA expression (0 μg/mL: 1.62 ± 0.26, 1.25 μg/mL: 1.37 ± 0.20, 2.5 μg/mL: 2.16 ± 0.26, 5 μg/mL: 2.21 ± 0.11) significantly increased (P < 0.01). This finding indicates that dioscin promotes the expression of apoptotic genes in HCT116 cells.
As shown in Figure 3B, compared with the control group, the expression of the Bax protein in the dioscin group (0 μg/mL: 1.00 ± 0.00, 1.25 μg/mL: 1.16 ± 0.01, 2.5 μg/mL: 1.87 ± 0.01, 5 μg/mL: 2.10 ± 0.00) significantly increased (P < 0.05), while the expression of the Bcl-2 protein (0 μg/mL: 1.00 ± 0.08, 1.25 μg/mL: 0.78 ± 0.05, 2.5 μg/mL: 0.47 ± 0.02, 5 μg/mL: 0.38 ± 0.03) significantly reduced (P < 0.05). The expression of the Caspase-3 protein (0 μg/mL: 1.00 ± 0.01, 1.25 μg/mL: 0.97 ± 0.02, 2.5 μg/mL: 1.21 ± 0.01, 5 μg/mL: 2.07 ± 0.01) significantly increased, except that at 1.25 μg/mL (P < 0.05). This finding indicates that dioscin promotes has the expression of apoptotic proteins.
As shown in Figure 3C, the protein expression levels of Bax (0 μg/mL: 1.00 ± 0.00, 1.25 μg/mL: 1.16 ± 0.01, 2.5 μg/mL: 1.87 ± 0.01, 5 μg/mL: 2.10 ± 0.00) and Caspase-3 (0 μg/mL: 1.00 ± 0.01, 1.25 μg/mL: 0.97 ± 0.02, 2.5 μg/mL: 1.21 ± 0.01, 5 μg/mL: 2.07 ± 0.01) significantly increased, whereas Bcl-2 (0 μg/mL: 1.00 ± 0.08, 1.25 μg/mL: 0.78 ± 0.05, 2.5 μg/mL: 0.47 ± 0.02, 5 μg/mL: 0.38 ± 0.03) significantly decreased with increasing dioscin concentration (P < 0.05). These results indicate that dioscin promotes the expression of pro-apoptotic proteins.
To investigate the effect of dioscin on the invasion and migration of HCT116 cells, we performed Transwell assays and wound healing assays. As shown in Figure 4, dioscin markedly reduced the rate of tumor cell invasion in comparison with the control group (0 μg/mL: 100.00% ± 5.43%, 1.25 μg/mL: 97.57% ± 28.72%, 2.5 μg/mL: 73.53% ± 25.89%, 5 μg/mL: 71.40% ± 25.81%; P < 0.05). This finding suggests that dioscin inhibits the invasive ability of HCT116 cells.
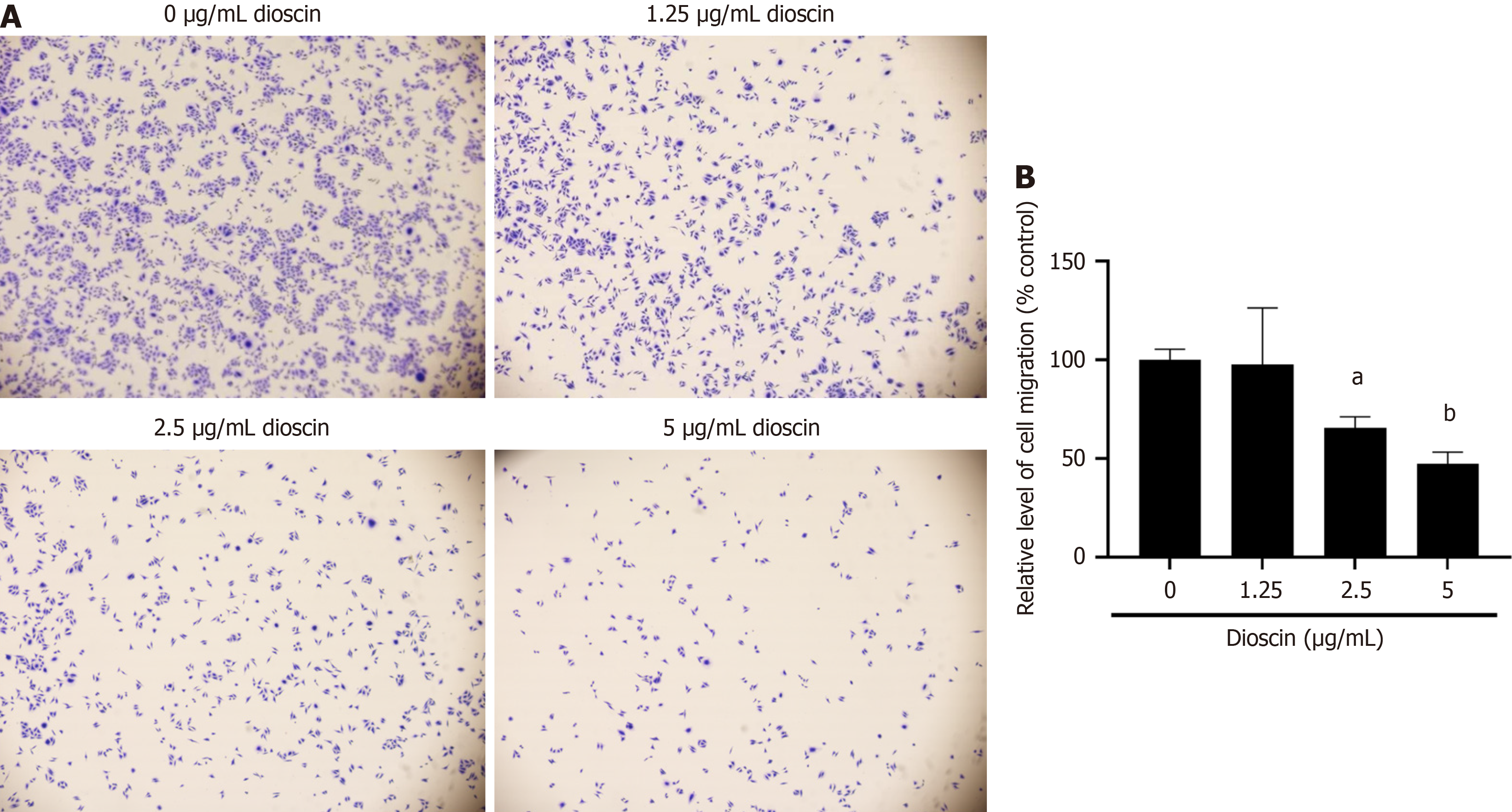
As shown in Figure 5, after 48 hours, diosgenin significantly inhibited the migration of HCT116 cells (0 μg/mL: 86.23% ± 0.26%, 1.25 μg/mL: 72.01% ± 0.46%, 2.5 μg/mL: 57.66% ± 0.28%, 5 μg/mL: 18.89% ± 0.55%; P < 0.001).
To extra understand the molecular mechanisms underlying the inhibitory effects of dioscin on HCT116 cells, we exa
Basing on preliminary experiments, we believe that dioscin has a certain inhibitory effect on HCT116 cells and it could be related to the regulation of the Notch 1 signaling pathway. Therefore, we treated cells with the Notch 1 activator Jagged 1 to further explore the relationship between dioscin and Notch 1. In the preliminary experiments, the difference in cell viability and apoptosis level was relatively small when dioscin was increased from 2.5 μg/mL to 5 μg/mL. Therefore, we chose a concentration of 2.5 μg/mL dioscin for subsequent experiments.
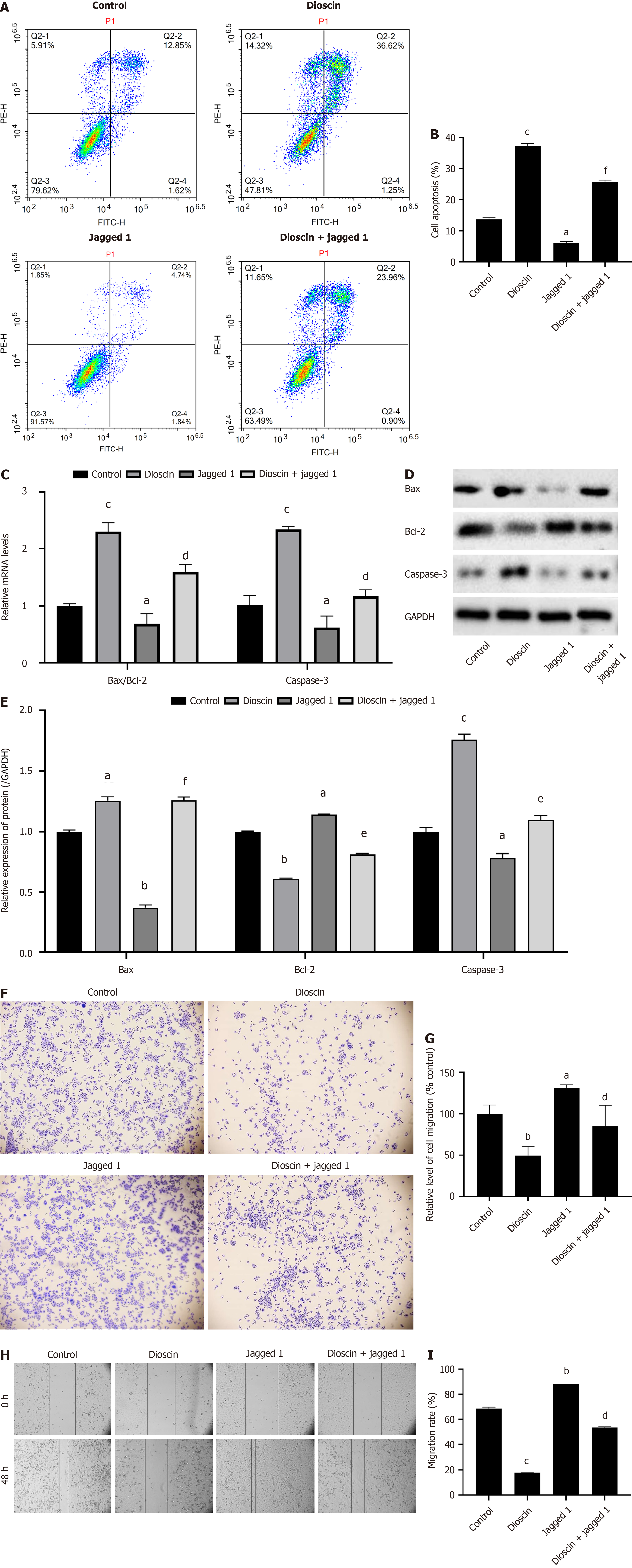
As shown in Figure 7A-C, the apoptosis ratio of cells in the Jagged 1 group dramatically reduced (P < 0.05) compared with the control group (Jagged 1 group: 6.11% ± 0.44%, control group: 13.64% ± 0.72%). The Bax/Bcl-2 ratio (control group: 1.00 ± 0.04, Jagged 1 group: 0.69 ± 0.18) and caspase-3 gene expression (control group: 1.01 ± 0.17, Jagged 1 group: 0.62 ± 0.20) significantly reduced (P < 0.05). The protein expression levels of Bax (control group: 1.00 ± 0.01, Jagged 1 group: 0.37 ± 0.02) and caspase-3 (control group: 1.00 ± 0.04, Jagged 1 group: 0.78 ± 0.04) significantly reduced (P < 0.05), while that of Bcl-2 (control group: 1.00 ± 0.01, Jagged 1 group: 1.14 ± 0.011) significantly increased (P < 0.01). Compared with the Jagged 1 group, the dioscin + Jagged 1 group (Jagged 1 group: 6.11% ± 0.44%, dioscin + Jagged 1 group: 25.60% ± 0.68%) showed a significant increase in the apoptosis ratio (P < 0.001). The Bax/Bcl-2 ratio (Jagged 1 group: 0.69 ± 0.18, dioscin + Jagged 1 group: 1.60 ± 0.13) and caspase-3 gene expression (Jagged 1 group: 0.62 ± 0.20, dioscin + Jagged 1 group: 1.17 ± 0.11) significantly increased (P < 0.05). This finding indicates that dioscin reversed the inhibitory effect of Jagged 1 on cell apoptosis and suppressed the expression of pro-apoptotic genes and proteins.
As shown in Figure 7D and E, the protein expression levels of Bax (control group: 1.00 ± 0.00, Jagged 1 group: 0.37 ± 0.02, dioscin + Jagged 1 group: 1.87 ± 0.01) and Caspase-3 (control group: 1.00 ± 0.04, Jagged 1 group: 0.78 ± 0.04, dioscin + Jagged 1 group: 2.07 ± 0.01) significantly increased, whereas Bcl-2 (control group: 1.00 ± 0.01, Jagged 1 group: 1.14 ± 0.011, dioscin + Jagged 1 group: 0.47 ± 0.02) significantly decreased with increasing dioscin concentration (P < 0.05).
As shown in Figure 7F and G, the rates of cell invasion (control group: 100% ± 10.60%, Jagged 1 group: 131.32% ± 3.56%, dioscin + Jagged 1 group: 84.86% ± 25.36%) and cell migration (control group: 68.79% ± 0.83%, Jagged 1 group: 88.27% ± 0.02%, dioscin + Jagged 1 group: 53.52% ± 0.66%) significantly increased in the Jagged 1 group compared with those in the control group (P < 0.05). The rates of cell invasion and cell migration significantly reduced in the dioscin + Jagged 1 group compared with those in the Jagged 1 group (P < 0.05). Hence, dioscin can reverse the stimulating impact of Jagged 1 on tumor cell invasion and migration.
As shown in Figure 7H and I, the inhibition of HCT116 cell migration and invasion was quantified using the wound healing and Transwell assays, respectively. The dioscin + Jagged 1 group showed a significant reduction in migration and invasion compared to the Jagged 1 group (P < 0.05).
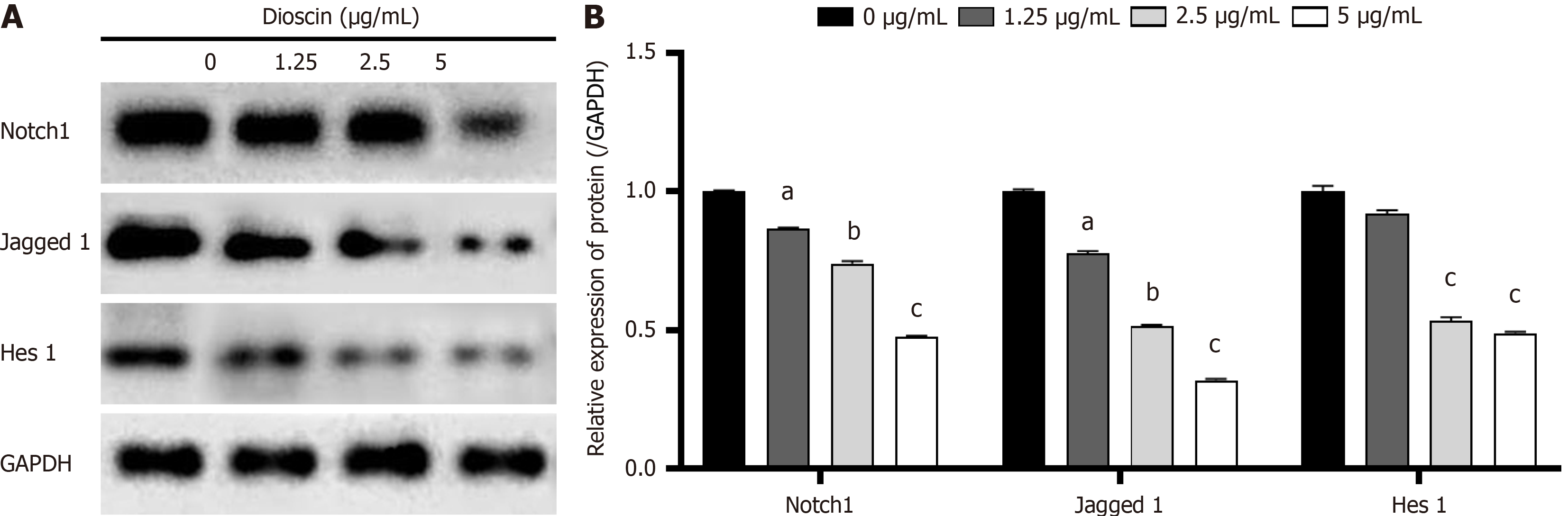
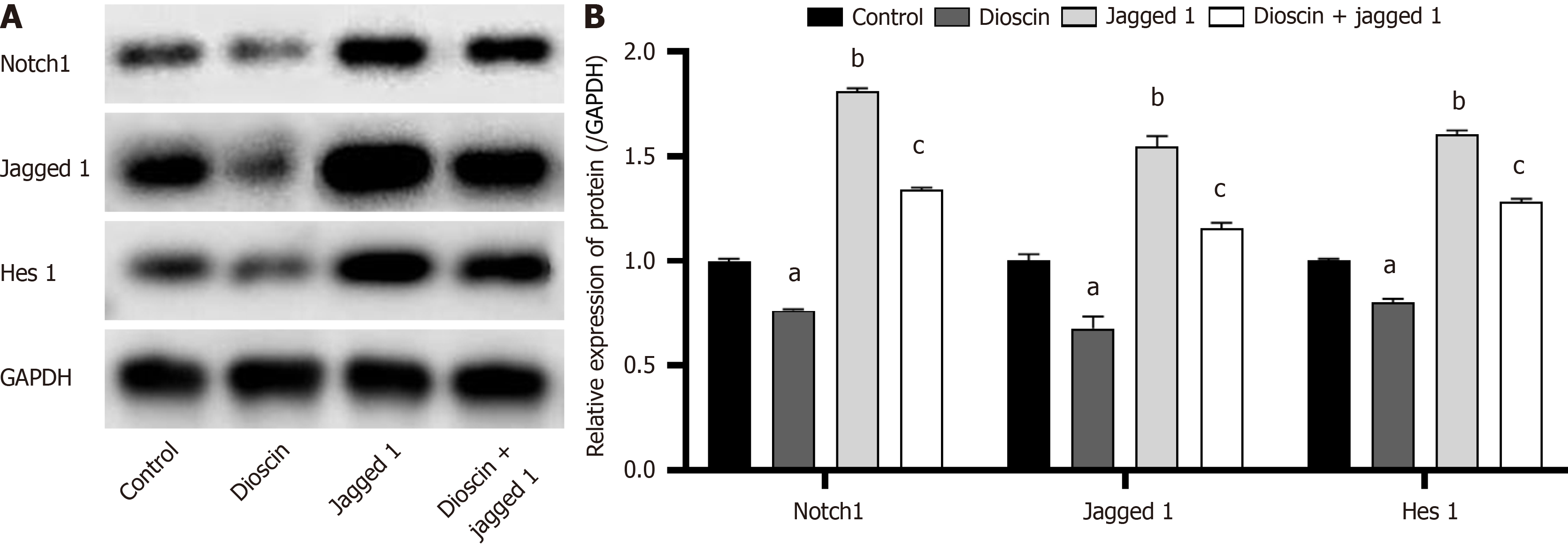
To examine the involvement of Notch 1 in the Notch 1/Hes 1 signaling pathway, we analyzed the protein expression levels of Notch 1, Jagged 1, and Hes 1 using Western blot analysis. As demonstrated in Figure 8, the combination of dioscin and Jagged 1 group resulted in lower (P < 0.05) protein expression of Notch 1, Jagged 1, as well as Hes 1 than the Jagged 1 group. This finding suggests that dioscin can reverse the activation of the Notch 1/Hes 1 signaling pathway by Jagged 1.
CRC is as one of the prevalent malignant neoplasms that affect the digestive system and presents a notable risk to humans. A noteworthy increase in the incidence and mortality rates of CRC has been reported in China; such increase could have been propelled the ongoing enhancement of socio-economic conditions and shifts in lifestyle and dietary patterns. This surge affects individuals in younger age groups[14]. In China, a common approach to the treatment of CRC involves the integration of traditional Chinese medicine. Traditional Chinese medicine has been essential in oncology, significantly aiding in the management of tumors. It has proven effective in alleviating clinical symptoms, boosting immune response, overcoming chemotherapy resistance, and reducing the likelihood of tumor recurrence and metastasis[15]. Dioscin, an active compound extracted from traditional Chinese herbs, exhibits anticancer activity against multi
The occurrence and development of tumors are primarily associated with abnormal cell proliferation, low apoptosis rates, and strong invasiveness. Dioscin significantly inhibits the viability of colorectal HCT116 cancer cells, and this inhibition is concentration-dependent, consistent with previous findings. Li et al[18] inquisited the sequels of dioscin on the proliferation of human CRC cells SW480, SW620, RKO, and Caco-2 and reported its dose-dependent inhibitory effect.
CRC has diverse etiological factors that involve numerous cellular pathways, for instance Notch signaling pathway. This pathway plays a great role in the initiation as well as progression of CRC[19,20]. The combination of different li
In the cascade of apoptosis, caspases assume a pivotal function, with caspase-3 situated downstream in this protein family. Once activated, it promotes cell apoptosis[24]. Bax is an important regulatory factor in the activation of caspase-3, and its pro-apoptotic effect may be achieved by enhancing the activity of caspase-3[25]. Bcl-2 can cause glutathione to accumulate in the cell nucleus, leading to an imbalance in nuclear redox status, thereby reducing caspase activity and protecting the cell. Bcl-2 is a substrate for the activated caspase-3[26]. Bcl-2 can bind to Bax, thereby diminishing the pro-apoptotic outcome of Bax and restraining the progression of apoptosis. The equilibrium in the Bcl-2 to Bax ratio functions as a regulatory factor in the initiation of apoptosis[27]. Dioscin can impede the proliferation of HepG2 Liver cancer cells and induce apoptosis by downregulating Bcl-2 expression and concurrently increasing Bax expression[28]. This study found that dioscin intervention significantly increased the Bax/Bcl-2 ratio, promoted caspase-3 gene expression at the gene level, and increased the expression of Bax and Caspase-3 proteins while downregulating Bcl-2 protein expression. Hence, dioscin affects the mitochondrial apoptosis pathway and regulates the expression of Bcl-2 as well as Bax, thereby inducing apoptosis in cells and the development of CRC. The Notch 1 pathway can be regulated by miRNAs, which participate in tumor progression. miRNA-449a has a negative regulatory effect on Notch1. If miRNA-449a is overexpressed, then it will have a significant effect on Bcl-2, causing a significant reduce in its expression level and a significant increase in Bax expression level[29]. The present work shows that Jagged1 can activate signaling pathways. After intervention with dioscin, it can have various effects on cell HCT116, significantly reducing the expression levels of genes for instance caspase-3 and related proteins. Hence, diosgenin can inhibit the activeness of signaling pathways and induce the apoptosis of HCT116 cells.
Invasion and migration are two different but related processes by which cancer cells invade local tissues and spread to distant sites. Dioscin can hinder the movement and infiltration of A549 Lung cancer cells by suppressing TGF-β1-induced epithelial-mesenchymal transition (EMT)[30]. Dioscin can also inhibit the invasion and EMT process in lung adenocarcinoma by modulating the AKT/GSK3β/mTOR signaling pathway[31]. In the present experiment, dioscin inhibited the invasion as well as migration of CRC cells HCT116. However, the addition of Jagged1, an activator of the Notch signaling pathway, significantly attenuated the inhibitory effect of dioscin. Hence, dioscin can inhibit cell invasion and migration by suppressing the activity of the Notch 1 signaling pathway.
The loss of Jagged-1 is an important factor that promotes the development of CRC by inhibiting tumor cell differentiation and inducing angiogenesis[32]. Hes1 is a downstream factor of Notch-1 and can inhibit Kruppel-like factor 4 (KLF4). High expression of KLF4 has an inhibitory effect on CRC[33]. Previous studies indicated that the Notch1 protein is overexpressed in CRC tissues[34]. In the present work, CRC cells HCT116 were treated with dioscin, and the protein expression levels of Notch1, Jagged-1, and downstream target gene HES-1 in the Notch1 signaling pathway lessened. The inhibitory effect of dioscin was significantly attenuated when the Notch1 signaling pathway was energized by the addition of Jagged1. Hence, dioscin can inhibit the occurrence and development of CRC by suppressing related signaling pathways.
In summary, dioscin can inhibit the growth, migration, and other activities of HCT116 cells, thereby effectively promoting cell apoptosis, by preventing the activation of relevant signaling pathways.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64583] [Article Influence: 16145.8] [Reference Citation Analysis (176)] |
| 2. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3302] [Article Influence: 412.8] [Reference Citation Analysis (3)] |
| 3. | Zhu J, Tan Z, Hollis-Hansen K, Zhang Y, Yu C, Li Y. Epidemiological Trends in Colorectal Cancer in China: An Ecological Study. Dig Dis Sci. 2017;62:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 4. | Sinha R. Colorectal cancer. Clin Radiol. 2021;76:870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Zhu D, Zhao D, Wang N, Cai F, Jiang M, Zheng Z. [Current status and prospects of GREM1 research in cancer (Review)]. Mol Clin Oncol. 2023;19:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Chen YQ, Cao YJ. [Advances in modern pharmacology of dioscin]. Xiandai Zhongxiyijiehe Zazhi. 2019;28:2613-2617. [DOI] [Full Text] |
| 7. | Tao X, Yin L, Xu L, Peng J. Dioscin: A diverse acting natural compound with therapeutic potential in metabolic diseases, cancer, inflammation and infections. Pharmacol Res. 2018;137:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Xi P, Niu Y, Zhang Y, Li W, Gao F, Gu W, Kui F, Liu Z, Lu L, Du G. The mechanism of dioscin preventing lung cancer based on network pharmacology and experimental validation. J Ethnopharmacol. 2022;292:115138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Gao H, Wang Z, Zhu D, Zhao L, Xiao W. Dioscin: Therapeutic potential for diabetes and complications. Biomed Pharmacother. 2024;170:116051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Li XL, Ma RH, Ni ZJ, Thakur K, Cespedes-Acuña CL, Wang S, Zhang JG, Wei ZJ. Dioscin inhibits human endometrial carcinoma proliferation via G0/G1 cell cycle arrest and mitochondrial-dependent signaling pathway. Food Chem Toxicol. 2021;148:111941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Zhou L, Yu X, Li M, Gong G, Liu W, Li T, Zuo H, Li W, Gao F, Liu H. Cdh1-mediated Skp2 degradation by dioscin reprogrammes aerobic glycolysis and inhibits colorectal cancer cells growth. EBioMedicine. 2020;51:102570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Si L, Zheng L, Xu L, Yin L, Han X, Qi Y, Xu Y, Wang C, Peng J. Dioscin suppresses human laryngeal cancer cells growth via induction of cell-cycle arrest and MAPK-mediated mitochondrial-derived apoptosis and inhibition of tumor invasion. Eur J Pharmacol. 2016;774:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Hsieh MJ, Tsai TL, Hsieh YS, Wang CJ, Chiou HL. Erratum to: Dioscin-induced autophagy mitigates cell apoptosis through modulation of PI3K/Akt and ERK and JNK signaling pathways in human lung cancer cell lines. Arch Toxicol. 2017;91:2495-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Wang XS. [Discussion of the importance of early diagnosis and treatment of colorectal cancer from the epidemiological characteristics of colorectal cancer in China and United States of America]. Zhonghua Jiezhichang Jibing Dianzi Zazhi. 2021;10:26-33. [DOI] [Full Text] |
| 15. | Pei XH, Peng YM. [Seventy years of traditional Chinese medicine in treatment of cancer]. Zhongguo Zhongliu Waike Zazhi. 2019;11:305-308. [DOI] [Full Text] |
| 16. | Gao LL, Li FR, Jiao P, Yao ST, Sang H, Si YH. Apoptosis of Human Ovarian Cancer Cells Induced by Paris Chinensis Dioscin via a Ca2+-Mediated Mitochondrion Pathway. Asian Pac J Cancer Prev. 2011;12:1361-1366. |
| 17. | Wei Y, Xu Y, Han X, Qi Y, Xu L, Xu Y, Yin L, Sun H, Liu K, Peng J. Anti-cancer effects of dioscin on three kinds of human lung cancer cell lines through inducing DNA damage and activating mitochondrial signal pathway. Food Chem Toxicol. 2013;59:118-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Li R, Qin J, Wang Z, Lv F, Guo J, Zhu H, Huang Y. Dioscin reduced chemoresistance for colon cancer and analysis of sensitizing targets. Biochem Biophys Res Commun. 2023;638:94-102. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 20. | Tyagi A, Sharma AK, Damodaran C. A Review on Notch Signaling and Colorectal Cancer. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Li L, Tang P, Li S, Qin X, Yang H, Wu C, Liu Y. Notch signaling pathway networks in cancer metastasis: a new target for cancer therapy. Med Oncol. 2017;34:180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Meurette O, Mehlen P. Notch Signaling in the Tumor Microenvironment. Cancer Cell. 2018;34:536-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 467] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 23. | Wang YH, Zhou SL, Yao W. [Dioscin inhibits the proliferation and invasion of thyroid cancer SW579 cells by down-regulating Notch 1 signaling pathway]. Jiepou Kexue Jinzhan. 2021;27:462-466. [DOI] [Full Text] |
| 24. | Koyun E, Karadag R, Ozkanli S, Oguztuzun S, Kocdogan AK, Ozsoy I. Caspase-3, p53 and Bcl-2 expression in basal cell carcinoma of the eyelid. Postepy Dermatol Alergol. 2020;37:535-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Lin HC, Lin JY. GSF3, a polysaccharide from guava (Psidium guajava L.) seeds, inhibits MCF-7 breast cancer cell growth via increasing Bax/Bcl-2 ratio or Fas mRNA expression levels. Int J Biol Macromol. 2020;161:1261-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Hartman ML, Czyz M. BCL-G: 20 years of research on a non-typical protein from the BCL-2 family. Cell Death Differ. 2023;30:1437-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 27. | Wanderoy S, Hees JT, Klesse R, Edlich F, Harbauer AB. Kill one or kill the many: interplay between mitophagy and apoptosis. Biol Chem. 2020;402:73-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Liang YQ, Huang Q, Chen LK, Su XJ, Sun J, Liu YS. [Inhibitory effect of Dioscin on proliferation of HEPG2 hepatoma cells]. Xiandai Shipin Keji. 2021;37:62-66. [DOI] [Full Text] |
| 29. | Hou Y, Feng F, Yang R. Effect of miR449amediated Notch signaling pathway on the proliferation, apoptosis and invasion of papillary thyroid carcinoma cells. Oncol Rep. 2020;43:471-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Lim WC, Kim H, Kim YJ, Choi KC, Lee IH, Lee KH, Kim MK, Ko H. Dioscin suppresses TGF-β1-induced epithelial-mesenchymal transition and suppresses A549 lung cancer migration and invasion. Bioorg Med Chem Lett. 2017;27:3342-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Mao W, Yin H, Chen W, Zhao T, Wu S, Jin H, Du B, Tan Y, Zhang R, He Y. Network Pharmacology and Experimental Evidence Reveal Dioscin Suppresses Proliferation, Invasion, and EMT via AKT/GSK3b/mTOR Signaling in Lung Adenocarcinoma. Drug Des Devel Ther. 2020;14:2135-2147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Dai Y, Wilson G, Huang B, Peng M, Teng G, Zhang D, Zhang R, Ebert MP, Chen J, Wong BC, Chan KW, George J, Qiao L. Silencing of Jagged1 inhibits cell growth and invasion in colorectal cancer. Cell Death Dis. 2014;5:e1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Ma Y, Wu L, Liu X, Xu Y, Shi W, Liang Y, Yao L, Zheng J, Zhang J. KLF4 inhibits colorectal cancer cell proliferation dependent on NDRG2 signaling. Oncol Rep. 2017;38:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Jin HY, Zhang HY, Wang X, Xu J, Ding Y. Expression and clinical significance of Notch signaling genes in colorectal cancer. Tumour Biol. 2012;33:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |