Published online Dec 15, 2023. doi: 10.4251/wjgo.v15.i12.2053
Peer-review started: July 27, 2023
First decision: September 26, 2023
Revised: October 11, 2023
Accepted: November 10, 2023
Article in press: November 10, 2023
Published online: December 15, 2023
Processing time: 139 Days and 15.6 Hours
Gall bladder cancer (GBC) is becoming a very devastating form of hepatobiliary cancer in India. Every year new cases of GBC are quite high in India. Despite recent advanced multimodality treatment options, the survival of GBC patients is very low. If the disease is diagnosed at the advanced stage (with local nodal metastasis or distant metastasis) or surgical resection is inoperable, the prognosis of those patients is very poor. So, perspectives of targeted therapy are being taken. Targeted therapy includes hormone therapy, proteasome inhibitors, signal transduction and apoptosis inhibitors, angiogenesis inhibitors, and immunotherapeutic agents. One such signal transduction inhibitor is the specific short interfering RNA (siRNA) or short hairpin RNA (shRNA). For developing siRNA-mediated therapy shRNA, although several preclinical studies to evaluate the efficacy of these key molecules have been performed using gall bladder cells, many more clinical trials are required. To date, many such genes have been identified. This review will discuss the recently identified genes associated with GBC and those that have implications in its treatment by siRNA or shRNA.
Core Tip: The frequency of gall bladder cancer (GBC) in India has increased. The survival of GBC patients is also poor. In this context, some genes have been recognized which are involved in the carcinogenesis of GBCs. In this review, we have discussed such genes which could be aimed for the development of targeted therapy for GBC.
- Citation: Ghosh I, Dey Ghosh R, Mukhopadhyay S. Identification of genes associated with gall bladder cell carcinogenesis: Implications in targeted therapy of gall bladder cancer. World J Gastrointest Oncol 2023; 15(12): 2053-2063
- URL: https://www.wjgnet.com/1948-5204/full/v15/i12/2053.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i12.2053
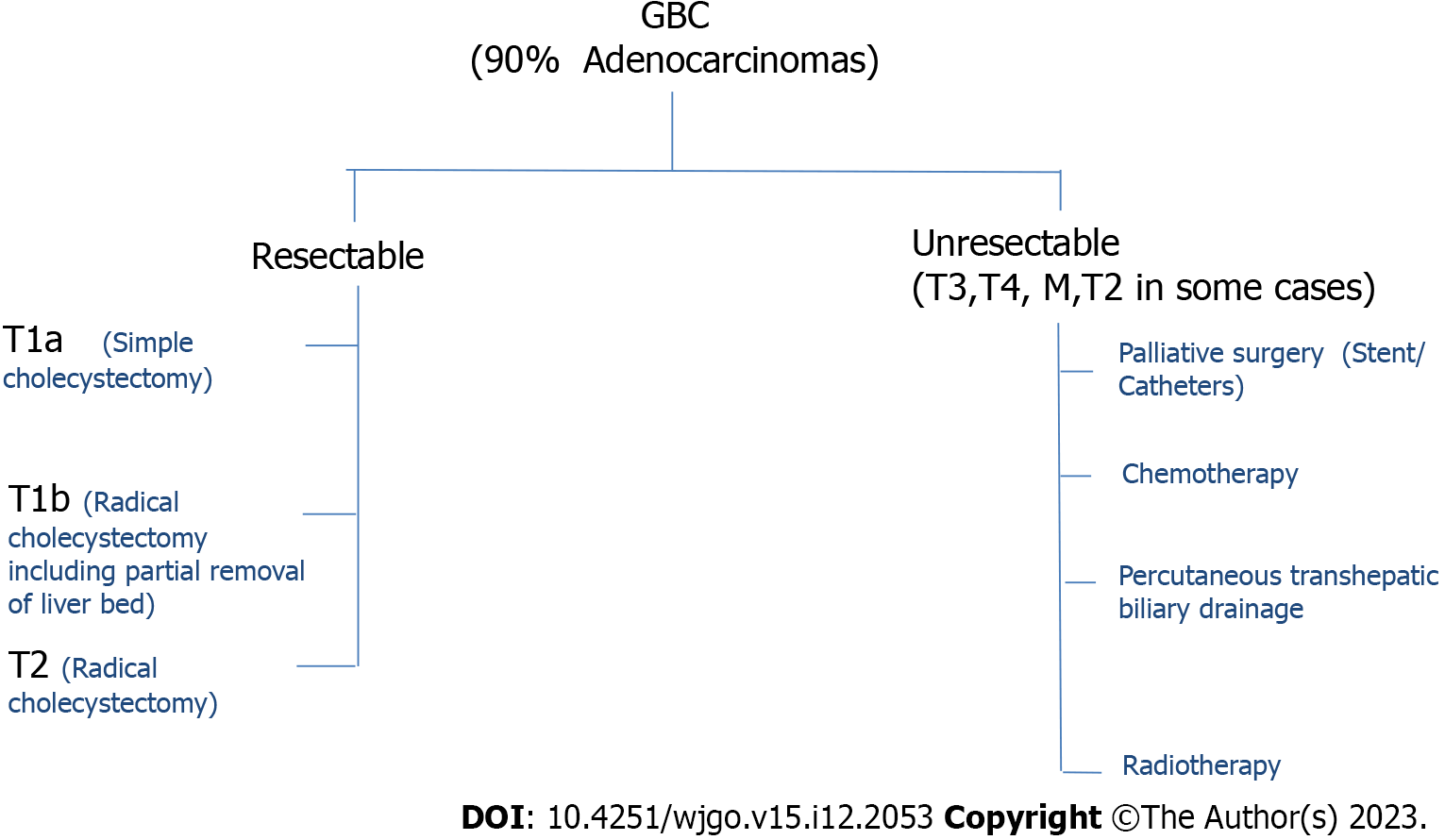
Gall bladder cancer (GBC) is the most common among hepatobiliary cancers. In India every year approximately 19570 new cases are detected and approximately 14736 death occurs due to GBC. According to the Cancer Registry, the rate of GBC is highest in Bolivia (14/100000) and Chile (9.3/100000)[1]. Recently, the occurrence of GBC in India is comparable and close to these countries. In 2001-2004, 6-7 individuals suffered from GBC among 100000 individuals, but in 2012-2014, 10-11 individuals suffered from GBC among 100000 individuals[2]. The total number of GBC cases in India is about 10% of the total GBC cases in the world. The rate of GBC is increasing in India in both males and females. If only the north and northeast are considered, the rates are even higher, which are 11.8/100000 and 17.1/10000 respectively (National Cancer Registry Program, 2012-2014). GBC is found to have a poor prognosis. Among the patients diagnosed with GBC, the average 5-year survival rate is as low as 19%. Unfortunately, most of the GBCs are detected after malignant cells have spread to other organs. In that case, chemotherapy and palliative treatment are the only options (Figure 1). Other approaches of tailored treatment are necessary for the treatment of this disease (Figure 1). Next-generation sequencing, whole exome sequencing, RNA sequencing, single cell isolation, and proteomics are some of the approaches to tailored treatment. This has been applied to specific target treatment, immunotherapy, vaccine therapy, and treatment with nanoparticles, which are already in clinical trials[3]. Therefore, a detailed study of the key molecules involved in the process of carcinogenesis of GBC is necessary for the tailored treatment of this disease.
Short interfering RNA (siRNA) is a small double-stranded RNA interfering molecule whereas, short hairpin RNA (shRNA) is a single-stranded RNA molecule folded into a hairpin or stem-loop-like structure. siRNA can be endogenous, exogenous, or artificial in origin for transient expression. Primarily, siRNA is to provide viral/bacterial defense and genomic stability. It is very similar to microRNA (miRNA), which mainly functions as an endogenous regulator of gene expression through gene silencing. ShRNA is an artificial molecule integrated into genomic DNA for long-term gene silencing of a specific gene expression. The shRNA is processed by DICER to generate siRNA. These siRNAs are then untwisted by helicases into two single strands, one is called the passenger strand and the other is called the guide strand. The guide strand combines with other components to form the RNA-induced silencing complex (RISC). The other main components of RISC are transactivation response RNA binding protein, protein activator of protein kinase R, and Argonaute2 (Ago2). The guide strand binds to its complementary mRNA strand. Ago2 cuts and removes the targeted m-RNA. This mRNA becomes inactive and then cannot be translated and expressed. So, when a specific siRNA is introduced into the cell, it stops the translation of the corresponding mRNA.
In this review, we will describe some siRNA-based gene therapeutic techniques that have yielded positive results for the implication of treatment of GBC. In the present review, we have identified some genes that are responsible for the spread of GBC cells. Approaches for silencing these genes could have the potential for the treatment of this disease. One approach to silencing these genes is by constructing and delivering specific short-interfering RNAs or siRNAs. Technology for the construction of specific siRNA has already been developed and is in practice.
KRAS (Kirsten sarcoma viral oncogene homolog): The gene KRAS encodes a protein which is 21 kDa and occurs in the inner side of the plasma membrane of those cells which could bind GTP and have the ability to convert GTP into GDP. However, when KRAS is attached to GTP, it remains active and plays an important role in the cell signaling pathways[4]. When GTP is converted to GDP, KRAS becomes inactive. Mutations in the KRAS gene result in the loss of the ability of the KRAS protein to return to its inactive form. This affects cell signaling pathways[5]. It was evident from a set of experiments that mutation of KRAS causes the development of gall bladder adenoma[6]. The possible mechanism is that KRAS activates the NOTCH signaling pathway and leads to tumor development.
PIK3CA (Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha): The gene PIK3CA encodes a protein (p110), which is the stimulating subunit of the enzyme PI3K. It is well known now that PI3K participates in many events of cell signaling and is well-linked to cell growth and proliferation[7]. In a set of experiments examining the role of PI3K-AKT in gall bladder tumorigenesis, it was observed that the PI3K-AKT pathway is disturbed when PIK3CA is mutated. The gall bladder epithelial cells are directed towards mesenchymal transition and lead to tumor formation[8].
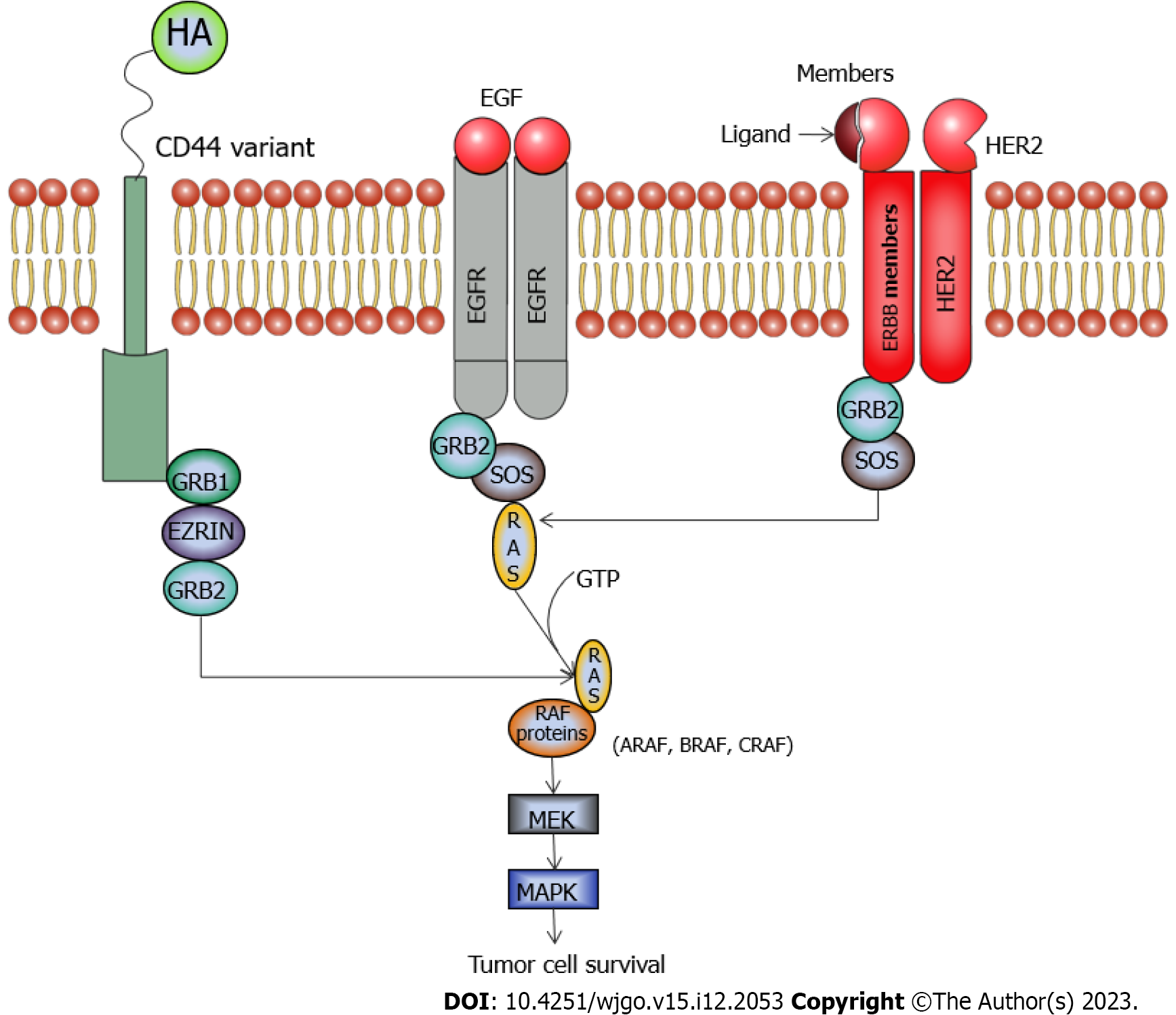
CD44 (Cluster of differentiation 44): CD44 is a glycoprotein which is found in the lipid bilayer. It is encoded by the gene CD44 present on chromosome 11[9]. This glycoprotein is required in the interplay between the cells, mainly in cell attachment and migration. It is found to have a function in the activation of lymphocytes, formation of blood cell components, cell proliferation, cell differentiation, and angiogenesis. Though, CD44 has a few other ligands, mainly hyaluronan binds to CD44 and activates it for the regulation of many pathways like the RAS, MAPK, and PI3K pathways which are associated with cell proliferation, cell differentiation, and angiogenesis[10,11]. Figure 2 shows how CD44 is linked to the MAPK pathway.
It is overexpressed in many cancers including those of the liver and gall bladder. The movement and occupation of the stroma by the GBC cells are also decreased on CD44 inactivation. CD44/CD133 positive cells also behave as cancer stem cells. CD44, in union with NANOG (another cancer stem cell marker), is found to have stimulated some multidrug-resistant genes and thus tumor development[12]. When tested for cancer stem cells, they were found to be reduced in activation of CD44 by CD44-siRNA. The total life span of patients in whom CD44 was overexpressed was much less than those who did not express CD44[13].
In a set of experiments by He et al[13], CD44-siRNA constructs were prepared by Sangon Biotech. A GBC cell line (GBC-SD) was transfected with CD44-siRNA. The expression of CD44 decreased significantly in the CD44-siRNA transfected cells as revealed by immunohistochemical staining, Western blot, and real-time PCR. The apoptosis of these cells increased considerably, which was detected by flow cytometry, while the proliferation capacity of these cells was considerably decreased as observed by cell counting kit-8 (CCK-8) assay. In this study involving CD44-mRNA it was found that CD44 is unhindered in hepatobiliary tumors and CD44 protein is expressed much more in gall bladder carcinoma tissues than non-malignant tissues. The overall survival of these patients is also poor. Silencing of the CD44 gene by delivering the corresponding siRNA in the cells has led to decreased expression of CD44.
EGFR (Epidermal growth factor receptor): EGFR/ErBB1 is a member of the receptor tyrosine kinase family. It is a transmembrane protein that is turned by the binding of its ligand (epidermal growth factor or transforming growth
In a set of experiments by Iyer et al[16], exome analysis and sequencing were performed to detect tumor-specific mutations in GBC tissues. EGFR and ERBB2 were turned off using EGFR-specific shRNA and ERBB2-specific shRNA. Cell proliferation of the transfected cells was assessed by MTT colorimetric assay. Expression of various tumor markers in the transfected cells including EGFR was estimated by Western blot assay and Immunohistochemistry. Migration assays of the transfected cells revealed that there was a considerable decrease in migration and invasive abilities of the transfected cells.
ERBB2 (erythroblastic oncogene B 2): It is a tyrosine kinase, also known as HER2/neu or receptor tyrosine–protein kinase ERBB-2 or CD340, belonging to the Erb family of receptor tyrosine kinases. In humans this Erb family consists of Her1 (EGFR/ErbB1), Her2 (Neu/ErbB2), Her3 (ErbB3) and Her4 (ErbB4)[17,18]. HER2/neu is a protein encoded by the gene ERBB2, present in the long arm of chromosome 17. When a ligand binds to this receptor, it undergoes dimerization activates many signaling pathways, and finally leads to cell proliferation and growth[19]. It has been found that HER2/neu could initiate the PI3K/Akt[20,21] and also the MAP kinase pathway[22]. Figure 2 shows how HER2/neu could activate the MAP kinase pathway. In an experimental setup by Kiguchi et al[23], HER2 was overexpressed in Gall bladder adenocarcinoma of mice. Silencing of ERBB2 was found to curb the penetrating, relocating, and docking free growth properties of human GBC-SDs, provided that KRAS (G12V) is not mutated in these GBC-SDs[16], as visualized by proliferation and migration assays. It was also visualized that due to the silencing of ERBB2, it was unable to signal target proteins of the MAPK pathway.
ARAF: It is a serine-threonine kinase of the Raf-kinase family. There are three members of the Raf (rapidly accelerated fibrosarcoma) kinase family - ARAF, BRAF, and CRAF. These three enzymes have roles in the MAP kinase pathway (Figure 2). In humans, ARAF is encoded by the ARAF gene, located on the X chromosome (xp11.3)[24]. ARAF activates MEK proteins, which in turn activates extracellular regulated kinase (ERK) which is known to activate many transcription factors and drive the MAPK pathway toward cell proliferation[25,26]. In a set of experiments by Lin et al[27], the ARAF gene was found to be elevated in GBC cells. Silencing of ARAF by ARAF-siRNA hinders the proliferation of GBC cells in a GBC-SD, as studied by the CCK-8 assay. The drifting and intruding nature of GBC cells declined, as estimated from transwell and wound healing assays. When the GBC-SD was used for tumor formation in athymic nude mice, ARAF-siRNA was injected. On regular monitoring of tumor size and volume, it was found that ARAF-siRNA was able to reduce the tumor volume remarkably.
BMI1 (B-cell specific Moloney murine leukemia virus integration site 1): BMI1 is a member of the polycomb group family proteins which suppresses transcription. To date, many such polycomb proteins are known[28,29]. Some of the common ones are PRC1, PRC2, PHC1, PHC2, HP1, BMI1, PCGF1, PCGF2, RYBP, RING1, SUV39H1, L3MBTl2, EZH2, EE2, SUZ12, JARID2, REST, RNF2, CBF-β and YY1. Basically, they organize the chromatin of the specific gene in such a way that they are suppressed for a long period of time and during cell division. The human BMI1 gene is placed at the short arm of chromosome 10[30].
BMI1 gene has been found to interact with p16 and p19[31], which are inhibitors of cyclin-dependent kinases (CDK4 and CDK6). P16 is a protein that disallows the cell from the G1 phase to the S-phase. P19 is a protein that has been found to direct p53 to stop the cell cycle at G1 and apoptosis in fibroblast cells[32]. BMI1 has been found to downregulate these inhibitors[33] and thus plays an important role in the progression of the cell cycle and sustaining cell division[34]. In a set of experiments by Jiao et al[35], BMI1-siRNA constructs were prepared by Shanghai Biotech. A GBC-SD was transfected with BMI1-siRNA. Initially, BMI1 was found to be expressed in a majority (84%) of the GBC patients as compared to 40% of normal patients who were taken up for the study. The expression of BMI1 decreased significantly in the BMI1-siRNA transfected cells as revealed by immunohistochemical staining, Western blot, and real-time PCR. The apoptosis of these cells increased considerably, which was detected by flow cytometry, while the proliferation capacity of these cells was considerably decreased as observed by the CCK-8 assay. Silencing of BMI1 by specific siRNA could induce apoptosis of the GBC cells and decrease the proliferation of GBC cells, which could hinder the growth of GBC cells.
BCL2 (B-cell lymphoma 2): BCL2 belongs to the Bcl-2 family of proteins. It is present in the outer mitochondrial membrane, embedded in it, and plays an important role in the suppression of the pro-apoptotic proteins BAX and BAK[36,37]. Thus, it plays a role in cell growth and proliferation. Overexpression of this gene with the upregulation of the proto-oncogene MYC has been found to be associated with some cancers[38]. In a set of experiments, GBC-SD were transfected with BCl2-siRNA according to the standard procedure by Lipofectamine[39]. The cells were cultured in media. The growth and the morphology of the resultant cells were observed. After a certain period of culture, expression of BCL2 was measured in the resulting cells by quantitative real time PCR (qRT-PCR) and Western blot. Expression of the BCL2 gene was found to be significantly lower in the BCL2-siRNA transfected group. On silencing of this gene, the GBC cells were found to be more responsive to chemotherapy drugs. In the nude mouse model experiments, the tumor volume also significantly decreased in the experimental group where this gene was silenced.
JAB1 (c-JUN activation domain binding protein 1): The gene JAB1 is positioned on chromosome 8. JAB1 is a co-activator of c-JUN, which is a well-known oncogene[40,41]. JAB1 is a member of a complex called constitutive photomorphogenic-9 signalosome (CSN) complex, which positively controls cell proliferation and transcription of various genes[42]. It is essential in the advancement of the cell cycle from the G1 phase to the S-phase, as c-JUN controls the level of Cyclin D1. If c-JUN is not activated, the expression of p53 and p21 could be upregulated to prevent the cell cycle from advancing farther[43,44]. Activating factor AP1 binds to the promoter region of JUN and transcription of JUN begins[45]. AP1 is a transcription factor consisting of mainly four groups of proteins i.e. Jun (C-Jun, Jun-B, and Jun-D), Fos (FosC, FosB, Fra1, and Fra2), ATF/cyclic AMP responsive element, and Maf family proteins. Thus, c-JUN transcription is also controlled by c-JUN itself. JUN transcription could also be started with active ERK[46]. In a set of experiments by Pandey et al[47], increased levels of JAB1 were found in GBC tissues. JAB1-siRNA constructs were prepared and used to transfect GBC cells. JAB1 expression, cell growth, and apoptosis of these GBC cells were studied by qRT-PCR, Western blot, MTT, reactive oxygen species, Hoechst, and FITC/Annexin-V staining. In the cells in which JAB1 is expressed highly, proliferation is turned on in these cells. GBC cells when transfected with JAB1-SiRNA decreased this proliferation considerably and programmed cell death was initiated. The cell cycle was found to halt at the G1 phase. Expression of the apoptotic gene CASP 3 and apoptotic regulatory genes p27, P53, and BAX were evident.
MIF (Macrophage migration inhibitory factor): MIF is a protein that plays a role in inflammatory responses. It has been found that though inflammation is a mechanism of the body’s defense if it occurs for a prolonged period, which occurs in the case of chronic inflammation could lead to cancer in some cases. One way, that chronic inflammation could be linked to cancer is thought to be through MIF[48,49]. MIF binds to its receptor CD74 and activates it and it then activates PI3K-AKT, ERK, and NF-kappa B pathways[50,51]. It has been found to subdue the anti-inflammatory cytokines and enhance the other inflammatory cytokines[52]. MIF has been found to be upregulated in many cancers including GBC[53,54]. Inactivation of the MIF gene using MIF-siRNA reduces the proliferation and intruding properties of cancer cells as observed in the colony formation assays.
CD73: CD73 resists the proper functioning of the T-cells by interfering with their clonal expansion, their activation, and their cytolytic activities. It plays an important role in the process due to which tumors can escape the immune system. CD73 is a cell surface enzyme that induces the dephosphorylation of AMP into adenosine[55]. Adenosine activates the G-protein coupled receptors (A2AR and A2BR) and plays a role in the escape of tumor cells by the immune system[56,57]. NOZ cells, a human GBC-SD were transfected with specific CD73-siRNA by Cao et al[58], and the expression of CD73 was found to be reduced in transfected cells as observed by quantitative reverse transcription PCR and Western blot. Adherent cells and spherical aggregates of cancer cells were found to decrease when CD73 was silenced. When CD73 was silenced by CD73-siRNA, epithelial to mesenchymal transition was found to reduce in GBC cells. It could be one of the important reasons for the opposition to drugs by cancer stem cells. The growth of GBC cells in single layers and attached to the surface and also those which can grow without embankment were retarded. The migrating nature of the GBC-NOZ cells was found to be reduced as detected by trans well assays.
PDL1 (Programmed cell death protein 1 ligand): PDL1 binds to its receptor programmed cell death protein 1 (PD1) which is found on activated T-cells’ and B-cells’ surfaces[59]. The interplay between PD1 and PDL1 hinders the proper functioning of the T-cells[60]. PDL1 is expressed on the surface of many cancer cells[61]. The interaction of PD1 with PDL1 interrupts major histocompatibility complex and thus obstructs the interaction of PDL1 with its receptor PD1, as a result, antigen presentation to cytotoxic T-lymphocytes[3]. PDL1 is overexpressed in the GBC-NOZ cells. When PDL1 was silenced with PDL1-siRNA cell growth and transportability of GBC-NOZ cells were diminished. This was revealed by proliferation as well as wound healing assays[58].
Other genes: miRNA is short (about 20 nucleotides), single-stranded, intrinsic, and non-coding sequences of RNA found in all tissues or blood. They could silence a gene by binding it to the specific RNA, complementary to it. Their mechanism of action is similar to that of siRNA. However, miRNAs are procured from different regions of DNA than siRNA. About more than 2500 miRNAs have been found in humans. In GBC several miRNAs have been found to be uncontrolled.
In a microarray analysis of GBC tissues from patients who survived for a long period after diagnosis and those who survived for a short time[62], changes in the expression of miRNA were identified. It was found that only two miRNAs (hsa-miR-30a-3p and hsa-miR-660-5p) were suppressed in patients who survived long. However, 11 miRNAs were suppressed and 11 were stimulated in patients who did not survive long. This recommended the possible roles of these miRNAs in GBC. In some other microarray analyses, changes in the expression of many other miRNAs have been detected in GBC. The table below (Table 1) lists the important ones and the genes associated with these miRNAs.
| miRNA | Status in gall bladder cancer | Target genes |
| miR-146b-5p | Suppressed | EGFR[80] |
| miR-124 | Suppressed | CDK6, ROCK1[81] |
| miR20a | Stimulated | SMAD7[82] |
| miR155 | Stimulated | [82] |
| miR182 | Stimulated | CADM1[82] |
| miR122 | Stimulated | BMI1[82] |
| miR34a | Suppressed | PNUTS[82] |
| miR335 | Suppressed | [82] |
| miR130a | Suppressed | HOTAIR[82] |
| miR135a5p | Suppressed | VLDLR[82] |
| miR-145-5p | Suppressed | STAT1[83] |
| miR26a | Suppressed | HMGA2[82] |
| miR145 | Suppressed | AXL[82] |
| miR143 | Suppressed | [82] |
| miR2185p | Suppressed | BMI1[84] |
Some of the miRNAs including the miR-55 and miR-20a have been considered as oncogenic miRNA or onco-miR as when they were expression levels were increased, the cell proliferation and intruding capabilities of the Gall bladder cells also increased[63]. The status of the important miRNAs in the GBC tissue of a particular patient could determine his response to a particular kind of therapy and prognosis.
Mutations of KRAS, INK4A (p16), TP53, and HER 2/neu have been commonly noticed in GBC[5,64-66]. Mutations of PIK3CA in GBC are also not rare[65]. BRAF and PI3K mutations also have been detected in GBC but they are not as common as KRAS[8,67,68]. There have been attempts to develop drugs against KRAS but the chemical nature of these drugs was such that they could not be controlled, so they were not approved. Some therapeutic drugs, targeting some genes are under development for the treatment of GBC. They are in different phases of clinical trials (Table 2). Many immune checkpoint inhibitors against PDL1 and CTLA4 for the treatment of GBC are also under various stages of clinical trial[69,70].
| Target genes silenced by siRNA (under investigation) | Target genes for GBC treatment (under clinical trial) |
| BMI1, CD44, CLIC1, JAB1, EGFR | EGFR and Her/2 together (Afatnib-NCT04183712, Apatinib-NCT03702491) |
| HER 2/neu, ARAF | HER/2 (Trastuzumab-NCT00478140) |
| MIF | MEK (Trametinib-NCT02042443) |
| CD73, PDL1 | DNMT (Guadecitabine-NCT03257761) |
The combined chemotherapy regimens have been found to increase the overall survival of patients in clinical trials, but still, they have been found to have toxic effects, and resistance is developed later[3,71,72]. Whenever clinical trials were performed some unforeseen hurdles were found, including the side effects and development of resistance to a particular drug. The cause of this resistance to a particular drug could be diversity within the same tumor and genetic differences among the patients[73].
The siRNAs have been found to be capable of silencing a specific gene in experimental models. However, there are certain constraints for the siRNA-mediated therapy. These are mainly the firmness and delivery of siRNA. Choosing the gene to be silenced is another issue with siRNA-mediated therapy. All these issues have to be answered before starting clinical trials in patients. As the siRNA molecules are negatively charged and have an inflexible structure, their diffusion across the membrane becomes difficult. So, they are taken up by the cell through endocytosis. In this case, there is a huge chance of the collection of many molecules in the endosomal compartments rather than reaching the cytosol where it can form the RISC. In the past few years, scientists have attempted to prepare strategies for the endosomal escape of these molecules, and a few have been reported[74]. Few endosomolytic media have been obtained from natural substances or manufactured and many other agents like polymers, liposomes, nano-particles, and other coupling agents have also been developed for the successful carriage of siRNA. Some recent clinical trials have disclosed the safety of nano-particle-based delivery of siRNA in some other cancers[62]. Still, there have been issues regarding the delivery of shRNA[75,76]. However, various attempts have been made to optimize the delivery of shRNA by viral vectors. Many vectors have been studied for their efficiency in the delivery of shRNAs within the target cell[77,78]. These viral vectors have improved the delivery of shRNAs but the safety of these vectors is in question as they are based on viruses. Consequently, these approaches are not applicable to clinical trials. Selection of the gene to be silenced is another important aspect of these siRNA-based technologies. It is suggested by experts to select such a combination therapy for patients which targets the important molecular pathways governing cancer metastasis and also would have less toxicity. To date, many genes have been identified which are responsible for the cells being able to avoid senescence and those which are responsible for metastasis. Genes that are linked to multiple pathways and have been expressed in many cancers are perhaps suitable for targeting. In the case of GBC, a recent study suggests that there are predominantly mutations of the genes ARID1A, ARID2, ATM, CTNNB1, ERBB2, ERBB3, KMT2C, KMT2D, KRAS, PIK3CA, SMAD4, TERT, TP53, and ZNF521[65]. High expression of the gene GLI2 has also been found to increase the number of GBC cells and their aggressive property[79].
All the genes mentioned in this review are linked to important cell signaling pathways. Among them, CD44, EGFR, and MIF have been found to be linked to multiple pathways. CD44 has been found to activate the MAPK, PI3K-AKT pathways, MIF has been linked with the MAPK, PI3K-AKT, and the NF-κB pathways and EGFR has been found to stimulate the MAPK, PI3K-AKT, and JNK pathways. The genes ERBB2, ERBB3, KRAS, and PIK3CA were already known to have been linked with multiple pathways. So, these genes which could activate multiple pathways could be potential targets for si-RNA/shRNA mediated knockdown. siRNA and shRNA-mediated knockdown of the genes mentioned above has shown to have decreased the invasiveness of the GBC considerably. The question of delivery of specific siRNA/shRNA in patients is expected to be answered. If the question is answered, they seem to have the potential for the tailored treatment of GBC. In that case, the toxicity resulting from the knockdown of the selected gene has also to be tested in preclinical models. Another aspect is the high cost of this siRNA/shRNA-mediated therapy. Therefore, before it comes to regular clinical practice all these issues need to be resolved. All these challenges have made it quite far from commercialization.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Dumitraşcu T, Romania; Yu PF, China S-Editor: Lin C L-Editor: A P-Editor: Zhao S
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55758] [Article Influence: 7965.4] [Reference Citation Analysis (132)] |
| 2. | Phadke PR, Mhatre SS, Budukh AM, Dikshit RP. Trends in gallbladder cancer incidence in the high-and low-risk regions of India. Indian J Med Paediatr Oncol. 2019;40:90-93. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Song X, Hu Y, Li Y, Shao R, Liu F, Liu Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct Target Ther. 2020;5:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 4. | Verma K, Dixit R, Singh J, Tiwary SK, Khanna AK, Narayan G, Kumar P. Molecular genetic changes in gall bladder carcinoma. Int J Mol Immuno Oncol. 2020;5:49-61. [DOI] [Full Text] |
| 5. | Rashid A, Ueki T, Gao YT, Houlihan PS, Wallace C, Wang BS, Shen MC, Deng J, Hsing AW. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002;8:3156-3163. [PubMed] |
| 6. | Chung WC, Wang J, Zhou Y, Xu K. Kras(G12D) upregulates Notch signaling to induce gallbladder tumorigenesis in mice. Oncoscience. 2017;4:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Kuipers H, de Bitter TJJ, de Boer MT, van der Post RS, Nijkamp MW, de Reuver PR, Fehrmann RSN, Hoogwater FJH. Gallbladder Cancer: Current Insights in Genetic Alterations and Their Possible Therapeutic Implications. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 8. | Lunardi A, Webster KA, Papa A, Padmani B, Clohessy JG, Bronson RT, Pandolfi PP. Role of aberrant PI3K pathway activation in gallbladder tumorigenesis. Oncotarget. 2014;5:894-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Spring FA, Dalchau R, Daniels GL, Mallinson G, Judson PA, Parsons SF, Fabre JW, Anstee DJ. The Ina and Inb blood group antigens are located on a glycoprotein of 80,000 MW (the CDw44 glycoprotein) whose expression is influenced by the In(Lu) gene. Immunology. 1988;64:37-43. [PubMed] |
| 10. | Herishanu Y, Gibellini F, Njuguna N, Hazan-Halevy I, Farooqui M, Bern S, Keyvanfar K, Lee E, Wilson W, Wiestner A. Activation of CD44, a receptor for extracellular matrix components, protects chronic lymphocytic leukemia cells from spontaneous and drug induced apoptosis through MCL-1. Leuk Lymphoma. 2011;52:1758-1769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006;20:1715-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Bourguignon LY, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703-39712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 178] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | He Y, Xue C, Yu Y, Chen J, Chen X, Ren F, Ren Z, Cui G, Sun R. CD44 is overexpressed and correlated with tumor progression in gallbladder cancer. Cancer Manag Res. 2018;10:3857-3865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 1239] [Article Influence: 154.9] [Reference Citation Analysis (0)] |
| 15. | Terakado M, Gon Y, Sekiyama A, Takeshita I, Kozu Y, Matsumoto K, Takahashi N, Hashimoto S. The Rac1/JNK pathway is critical for EGFR-dependent barrier formation in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L56-L63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Iyer P, Shrikhande SV, Ranjan M, Joshi A, Gardi N, Prasad R, Dharavath B, Thorat R, Salunkhe S, Sahoo B, Chandrani P, Kore H, Mohanty B, Chaudhari V, Choughule A, Kawle D, Chaudhari P, Ingle A, Banavali S, Gera P, Ramadwar MR, Prabhash K, Barreto SG, Dutt S, Dutt A. ERBB2 and KRAS alterations mediate response to EGFR inhibitors in early stage gallbladder cancer. Int J Cancer. 2019;144:2008-2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Vermeulen Z, Segers VF, De Keulenaer GW. ErbB2 signaling at the crossing between heart failure and cancer. Basic Res Cardiol. 2016;111:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Riese DJ 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Iqbal N, Iqbal N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol Biol Int. 2014;2014:852748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 642] [Cited by in RCA: 815] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 20. | Mishra R, Patel H, Alanazi S, Yuan L, Garrett JT. HER3 signaling and targeted therapy in cancer. Oncol Rev. 2018;12:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Ortega-Cava CF, Raja SM, Laiq Z, Bailey TA, Luan H, Mohapatra B, Williams SH, Ericsson AC, Goswami R, Dimri M, Duan L, Band V, Naramura M, Band H. Continuous requirement of ErbB2 kinase activity for loss of cell polarity and lumen formation in a novel ErbB2/Neu-driven murine cell line model of metastatic breast cancer. J Carcinog. 2011;10:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Lenferink AE, Busse D, Flanagan WM, Yakes FM, Arteaga CL. ErbB2/neu kinase modulates cellular p27(Kip1) and cyclin D1 through multiple signaling pathways. Cancer Res. 2001;61:6583-6591. [PubMed] |
| 23. | Kiguchi K, Carbajal S, Chan K, Beltrán L, Ruffino L, Shen J, Matsumoto T, Yoshimi N, DiGiovanni J. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61:6971-6976. [PubMed] |
| 24. | Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1930] [Cited by in RCA: 1923] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 25. | Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 617] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 26. | Rebocho AP, Marais R. ARAF acts as a scaffold to stabilize BRAF:CRAF heterodimers. Oncogene. 2013;32:3207-3212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Lin W, Tong C, Zhang W, Cen W, Wang Y, Li J, Zhu Z, Yu J, Lu B. Silencing ARAF Suppresses the Malignant Phenotypes of Gallbladder Cancer Cells. Biomed Res Int. 2020;2020:3235786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Di Croce L, Helin K. Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol. 2013;20:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 682] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 29. | Chan HL, Morey L. Emerging Roles for Polycomb-Group Proteins in Stem Cells and Cancer. Trends Biochem Sci. 2019;44:688-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 30. | Beà S, Tort F, Pinyol M, Puig X, Hernández L, Hernández S, Fernandez PL, van Lohuizen M, Colomer D, Campo E. BMI-1 gene amplification and overexpression in hematological malignancies occur mainly in mantle cell lymphomas. Cancer Res. 2001;61:2409-2412. [PubMed] |
| 31. | Meng S, Luo M, Sun H, Yu X, Shen M, Zhang Q, Zhou R, Ju X, Tao W, Liu D, Deng H, Lu Z. Identification and characterization of Bmi-1-responding element within the human p16 promoter. J Biol Chem. 2010;285:33219-33229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Radfar A, Unnikrishnan I, Lee HW, DePinho RA, Rosenberg N. p19(Arf) induces p53-dependent apoptosis during abelson virus-mediated pre-B cell transformation. Proc Natl Acad Sci U S A. 1998;95:13194-13199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Abdouh M, Facchino S, Chatoo W, Balasingam V, Ferreira J, Bernier G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J Neurosci. 2009;29:8884-8896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 34. | Fatma H, Akhtar MS, Ali S, Ahmad M, Siddique HR. BMI-1 expression as a marker for gallbladder cancer progression. Journal of Medicine Surgery and Public Health. 2023;1:100002-100009. [DOI] [Full Text] |
| 35. | Jiao K, Jiang W, Zhao C, Su D, Zhang H. Bmi-1 in gallbladder carcinoma: Clinicopathology and mechanism of regulation of human gallbladder carcinoma proliferation. Oncol Lett. 2019;18:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 470] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 37. | Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 647] [Cited by in RCA: 1070] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 38. | Otake Y, Soundararajan S, Sengupta TK, Kio EA, Smith JC, Pineda-Roman M, Stuart RK, Spicer EK, Fernandes DJ. Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA. Blood. 2007;109:3069-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 39. | Geng ZM, Zhang M, Pan XT, Wang L. Bcl-2 gene silencing by RNA interference inhibits the growth of the human gallbladder carcinoma cell line GBC-SD in vitro and in vivo. Oncol Rep. 2013;30:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Chopra S, Fernandez De Mattos S, Lam EW, Mann DJ. Jab1 co-activation of c-Jun is abrogated by the serine 10-phosphorylated form of p27Kip1. J Biol Chem. 2002;277:32413-32416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Pandey P, Khan F, Mishra R, Singh R, Upadhyay TK, Singh SK. Jab1 (COPS5) as an Emerging Prognostic, Diagnostic and Therapeutic Biomarker for Human Cancer. Biointerface Research in Applied Chemistry. 2022;12:7001-7011. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Shackleford TJ, Claret FX. JAB1/CSN5: a new player in cell cycle control and cancer. Cell Div. 2010;5:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Oh W, Lee EW, Sung YH, Yang MR, Ghim J, Lee HW, Song J. Jab1 induces the cytoplasmic localization and degradation of p53 in coordination with Hdm2. J Biol Chem. 2006;281:17457-17465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Berg JP, Zhou Q, Breuhahn K, Schirmacher P, Patil MA, Chen X, Schäfer N, Höller TT, Fischer HP, Büttner R, Gütgemann I. Inverse expression of Jun activation domain binding protein 1 and cell cycle inhibitor p27Kip1: influence on proliferation in hepatocellular carcinoma. Hum Pathol. 2007;38:1621-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Claret FX, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 360] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 46. | Gazon H, Barbeau B, Mesnard JM, Peloponese JM Jr. Hijacking of the AP-1 Signaling Pathway during Development of ATL. Front Microbiol. 2017;8: 2686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 47. | Pandey P, Siddiqui MH, Behari A, Kapoor VK, Mishra K, Sayyed U, Tiwari RK, Shekh R, Bajpai P. Jab1-siRNA Induces Cell Growth Inhibition and Cell Cycle Arrest in Gall Bladder Cancer Cells via Targeting Jab1 Signalosome. Anticancer Agents Med Chem. 2019;19:2019-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 493] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 49. | Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1183] [Cited by in RCA: 1380] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 50. | Farr L, Ghosh S, Moonah S. Role of MIF Cytokine/CD74 Receptor Pathway in Protecting Against Injury and Promoting Repair. Front Immunol. 2020;11: 1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 51. | Wen Y, Cai W, Yang J, Fu X, Putha L, Xia Q, Windsor JA, Phillips AR, Tyndall JDA, Du D, Liu T, Huang W. Targeting Macrophage Migration Inhibitory Factor in Acute Pancreatitis and Pancreatic Cancer. Front Pharmacol. 2021;12:638950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Harris J, VanPatten S, Deen NS, Al-Abed Y, Morand EF. Rediscovering MIF: New Tricks for an Old Cytokine. Trends Immunol. 2019;40:447-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 53. | Kindt N, Laurent G, Nonclercq D, Journé F, Ghanem G, Duvillier H, Gabius HJ, Lechien J, Saussez S. Pharmacological inhibition of macrophage migration inhibitory factor interferes with the proliferation and invasiveness of squamous carcinoma cells. Int J Oncol. 2013;43:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Subbannayya T, Leal-Rojas P, Barbhuiya MA, Raja R, Renuse S, Sathe G, Pinto SM, Syed N, Nanjappa V, Patil AH, Garcia P, Sahasrabuddhe NA, Nair B, Guerrero-Preston R, Navani S, Tiwari PK, Santosh V, Sidransky D, Prasad TS, Gowda H, Roa JC, Pandey A, Chatterjee A. Macrophage migration inhibitory factor - a therapeutic target in gallbladder cancer. BMC Cancer. 2015;15:843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Regateiro FS, Cobbold SP, Waldmann H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol. 2013;171:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 56. | Sun C, Wang B, Hao S. Adenosine-A2A Receptor Pathway in Cancer Immunotherapy. Front Immunol. 2022;13:837230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 57. | Zhang B. CD73: a novel target for cancer immunotherapy. Cancer Res. 2010;70:6407-6411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 58. | Cao L, Bridle KR, Shrestha R, Prithviraj P, Crawford DHG, Jayachandran A. CD73 and PD-L1 as Potential Therapeutic Targets in Gallbladder Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1374] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 60. | Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727-742. [PubMed] |
| 61. | Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 616] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 62. | Wang Y, Xie Y, Kilchrist KV, Li J, Duvall CL, Oupický D. Endosomolytic and Tumor-Penetrating Mesoporous Silica Nanoparticles for siRNA/miRNA Combination Cancer Therapy. ACS Appl Mater Interfaces. 2020;12:4308-4322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 63. | Li Z, Yu X, Shen J, Law PT, Chan MT, Wu WK. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget. 2015;6:13914-13921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Ueki T, Hsing AW, Gao YT, Wang BS, Shen MC, Cheng J, Deng J, Fraumeni JF Jr, Rashid A. Alterations of p16 and prognosis in biliary tract cancers from a population-based study in China. Clin Cancer Res. 2004;10:1717-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | D'Afonseca V, Arencibia AD, Echeverría-Vega A, Cerpa L, Cayún JP, Varela NM, Salazar M, Quiñones LA. Identification of Altered Genes in Gallbladder Cancer as Potential Driver Mutations for Diagnostic and Prognostic Purposes: A Computational Approach. Cancer Inform. 2020;19:1176935120922154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Kawamoto T, Krishnamurthy S, Tarco E, Trivedi S, Wistuba II, Li D, Roa I, Roa JC, Thomas MB. HER Receptor Family: Novel Candidate for Targeted Therapy for Gallbladder and Extrahepatic Bile Duct Cancer. Gastrointest Cancer Res. 2007;1:221-227. [PubMed] |
| 67. | Rizzo A, Federico AD, Ricci AD, Frega G, Palloni A, Pagani R, Tavolari S, Marco MD, Brandi G. Targeting BRAF-Mutant Biliary Tract Cancer: Recent Advances and Future Challenges. Cancer Control. 2020;27:1073274820983013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 68. | Saetta AA, Papanastasiou P, Michalopoulos NV, Gigelou F, Korkolopoulou P, Bei T, Patsouris E. Mutational analysis of BRAF in gallbladder carcinomas in association with K-ras and p53 mutations and microsatellite instability. Virchows Arch. 2004;445:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Kim JH, Kim K, Kim M, Kim YM, Suh JH, Cha HJ, Choi HJ. Programmed death-ligand 1 expression and its correlation with clinicopathological parameters in gallbladder cancer. J Pathol Transl Med. 2020;54:154-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Xie C, Duffy AG, Mabry-Hrones D, Wood B, Levy E, Krishnasamy V, Khan J, Wei JS, Agdashian D, Tyagi M, Gangalapudi V, Fioravanti S, Walker M, Anderson V, Venzon D, Figg WD, Sandhu M, Kleiner DE, Morelli MP, Floudas CS, Brar G, Steinberg SM, Korangy F, Greten TF. Tremelimumab in Combination With Microwave Ablation in Patients With Refractory Biliary Tract Cancer. Hepatology. 2019;69:2048-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 71. | Azizi AA, Lamarca A, McNamara MG, Valle JW. Chemotherapy for advanced gallbladder cancer (GBC): A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;163:103328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Zaidi A, Chandna N, Narasimhan G, Moser M, Haider K, Chalchal H, Shaw J, Ahmed S. Second-line Chemotherapy Prolongs Survival in Real World Patients With Advanced Biliary Tract and Gallbladder Cancers: A Multicenter Retrospective Population-based Cohort Study. Am J Clin Oncol. 2021;44:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Zhou Y, Yuan K, Yang Y, Ji Z, Zhou D, Ouyang J, Wang Z, Wang F, Liu C, Li Q, Zhang Q, Shan X, Zhou J. Gallbladder cancer: current and future treatment options. Front Pharmacol. 2023;14:1183619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 74. | Du Rietz H, Hedlund H, Wilhelmson S, Nordenfelt P, Wittrup A. Imaging small molecule-induced endosomal escape of siRNA. Nat Commun. 2020;11:1809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 75. | Silva JM, Li MZ, Chang K, Ge W, Golding MC, Rickles RJ, Siolas D, Hu G, Paddison PJ, Schlabach MR, Sheth N, Bradshaw J, Burchard J, Kulkarni A, Cavet G, Sachidanandam R, McCombie WR, Cleary MA, Elledge SJ, Hannon GJ. Second-generation shRNA libraries covering the mouse and human genomes. Nat Genet. 2005;37:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 481] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 76. | Li L, Lin X, Khvorova A, Fesik SW, Shen Y. Defining the optimal parameters for hairpin-based knockdown constructs. RNA. 2007;13:1765-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Sliva K, Schnierle BS. Selective gene silencing by viral delivery of short hairpin RNA. Virol J. 2010;7:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Varas-Godoy M, Lladser A, Farfan N, Villota C, Villegas J, Tapia JC, Burzio LO, Burzio VA, Valenzuela PDT. In vivo knockdown of antisense non-coding mitochondrial RNAs by a lentiviral-encoded shRNA inhibits melanoma tumor growth and lung colonization. Pigment Cell Melanoma Res. 2018;31:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Ichimiya S, Onishi H, Nagao S, Koga S, Sakihama K, Nakayama K, Fujimura A, Oyama Y, Imaizumi A, Oda Y, Nakamura M. GLI2 but not GLI1/GLI3 plays a central role in the induction of malignant phenotype of gallbladder cancer. Oncol Rep. 2021;45:997-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 80. | Cai J, Xu L, Cai Z, Wang J, Zhou B, Hu H. MicroRNA-146b-5p inhibits the growth of gallbladder carcinoma by targeting epidermal growth factor receptor. Mol Med Rep. 2015;12:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Kai D, Yannian L, Yitian C, Dinghao G, Xin Z, Wu J. Circular RNA HIPK3 promotes gallbladder cancer cell growth by sponging microRNA-124. Biochem Biophys Res Commun. 2018;503:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 82. | Yang G, Zhang L, Li R, Wang L. The role of microRNAs in gallbladder cancer. Mol Clin Oncol. 2016;5:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Goeppert B, Truckenmueller F, Ori A, Fritz V, Albrecht T, Fraas A, Scherer D, Silos RG, Sticht C, Gretz N, Mehrabi A, Bewerunge-Hudler M, Pusch S, Bermejo JL, Dietrich P, Schirmacher P, Renner M, Roessler S. Profiling of gallbladder carcinoma reveals distinct miRNA profiles and activation of STAT1 by the tumor suppressive miRNA-145-5p. Sci Rep. 2019;9:4796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Chandra V, Kim JJ, Mittal B, Rai R. MicroRNA aberrations: An emerging field for gallbladder cancer management. World J Gastroenterol. 2016;22:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |