Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.36
Peer-review started: September 20, 2022
First decision: November 15, 2022
Revised: December 6, 2022
Accepted: December 27, 2022
Article in press: December 27, 2022
Published online: January 15, 2023
Processing time: 111 Days and 23.7 Hours
Gastric cancer (GC) is a common gastrointestinal tumor. Gastric precancerous lesions (GPL) are the last pathological stage before normal gastric mucosa transforms into GC. However, preventing the transformation from GPL to GC remains a challenge. Traditional Chinese medicine (TCM) has been used to treat gastric disease for millennia. A series of TCM formulas and active compounds have shown therapeutic effects in both GC and GPL. This article reviews recent progress on the herbal drugs and pharmacological mechanisms of TCM in preventing the transformation from GPL to GC, especially focusing on anti-inflammatory, anti-angiogenesis, proliferation, and apoptosis. This review may provide a meaningful reference for the prevention of the transformation from GPL to GC using TCM.
Core Tip: Precancerous lesions are precursors of gastric cancer (GC). The molecular mechanism of the transformation of precancerous lesions into GC remains unclear. This article reviews the mechanism of traditional Chinese medicine in the treatment of precancerous lesions and GC, and describes the relationship between the molecular mechanisms of Chinese medicine in treating these two pathological stages, providing a research idea for blocking GC progression through the gastric precancerous lesion stage.
- Citation: Zhong YL, Wang PQ, Hao DL, Sui F, Zhang FB, Li B. Traditional Chinese medicine for transformation of gastric precancerous lesions to gastric cancer: A critical review. World J Gastrointest Oncol 2023; 15(1): 36-54
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/36.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.36
Gastric cancer (GC) is the fifth most commonly diagnosed cancer and the fourth leading cause of cancer-related death worldwide[1]. The direct cause of high mortality in GC is that early GC may occur without any specific symptoms and cannot be treated promptly[2]. Therefore, it is particularly important to prevent GC in early stage[3]. In the 19th century, Virchow proposed “the origin of cancer as a site of chronic inflammation”[4]. Mostly, gastric carcinogenesis is a chronic pathological process, from chronic superficial gastritis, atrophic gastritis, intestinal metaplasia (IM), gastric epithelial dysplasia (GED), to GC, which was demonstrated as the typical process of “inflammation-cancer transformation”[5]. IM and GED are the main pathological stages of gastric precancerous lesions (GPL)[6]. GPL is the last stage before the occurrence of GC. Once this stage is attained, the probability of GC increases by at least 10-fold[7,8]. Therefore, intervention of at the GPL stage and reversal of malignant transformation are of great importance for the prevention of GC. Currently, there is no recommended treatment for GPL in western medicine[9]. The only therapeutic treatment, endoscopic mucosal dissection, is applicable to cases of severe dysplasia and early GC[10]. Helicobacter pylori (H. pylori) eradication and supplementation with vitamins and minerals exert a positive effect on the treatment of GC; however, the current research is not sufficient to support their therapeutic effects on GPL[11].
Inflammation, angiogenesis, and proliferation are the three most important histological features of the transformation from GPL to GC[12-14]. Chronic inflammation is the core driver of GC[15,16]. Inflammation promotes angiogenesis, and its progression depends on the speed of angiogenesis[17,18]. Simultaneously, inflammatory cells are almost inseparable from tumor cells, and the proliferation of tumor cells is directly related to the promotion of inflammation[19]. Moreover, the microenvironment created by tumors can promote further proliferation of inflammatory cells[20]. Angiogenesis can also promote the proliferation of tumor cells[21]. Furthermore, the establishment of a large number of new blood vessels also means that the tumor is about to transition from the dormant to the malignant stage[22]. An important feature is that when the diameter of the tumor tissue is larger than 1-2 mm, it relies heavily on neovascularization to deliver nutrients and clear metabolic wastes in tumor cells[23]. Clinically, tumor angiogenesis is believed to be directly proportional to tumor malignancy[24]. Seeking drugs that regulate the proliferation, inflammation, and angiogenesis of GPL and GC may be a promising avenue for improving clinical efficacy and further drug discovery.
GPL is generally defined as “stomach distension” and “stomach pain”, according to traditional Chinese medicine (TCM) theory, with symptoms including fatigue and weakness, dizziness and wasting, grayish-yellow face, and a pale and dark tongue. TCM has been used for millennia to treat GPL. A series classical formula (fangjis) was documented in Treatise on Febrile Diseases, and Prescriptions of the Pharmacy Bureausuch, and showed curative effects for GPL. For example, the sijunzi decoction, which can be dated back to 1151 AD, was effective in treating both precancerous lesions[25,26] and GC[27,28]. Currently, a variety of traditional Chinese patent medicines, such as the Weiqi decoction[29], WeiFuChun (WFC)[30], and Weipixiao (WPX)[31] have been developed for GPL. Many clinical studies have also suggested that TCM can hinder the transformation process of inflammation and cancer, treat precancerous lesions and early GC, and improve the progression of advanced GC (Table 1). For instance, WFC is a Chinese herbal compound approved by the National Medical Products Administration to treat GPL. Clinical trials have shown that WFC can significantly improve the pathological conditions of patients with GPL compared to vitamin C, especially in the case of atrophy or IM[16]. Moreover, with the advantages of TCM in precancerous lesions and GC, TCM has received increasing attention, and a growing number of clinical studies have been registered at ClinicalTrials.gov, such as the Jianpi Yangzheng Xiaozheng decoction (NCT03823248) and Yiqi Wenyang Jiedu decoction (NCT05229809).
| Pathological stages | Ref. | Clinical drugs | Clinical sample size | Intervention | Control | Treatment duration | Outcome measures |
| GPL | Deng et al[125], 2012 | Weining Granules | 120 | Weining Granules | Weifuchun tablets | 6 mo | Overall response; gastroscopically-determined response; pathologically-confirmed response; eradication of Hp; microvessel density in the gastric mucosa; VEGF; IL-2; IL-6; T lymphocyte subsets; immunoglobulins; symptom scores; QOL; adverse reactions |
| Bian et al[16], 2021 | Weifuchun (WFC) | 120 | WFC tablets | Vitacoenzyme tablets | 6 mo | Histopathology of gastric tissues; intestinal microbiota; sensitivity and specifcity of diferent intestinal microbiota | |
| Li et al[126], 2006 | Weiansan (WAS) | 76 | Weiansan | Weifuchun tablets | 24 wk | Inflammation of gastric mucosa; degree of glandular atrophy; IM and dysplasia; Hp infection | |
| NCT03823248 | MoLuoDan and Sanchi powder | 480 | Moluodan combined with Sanchi powder | Folic acid tablets | 24 wk | The disappearance rate of dysplasia; Histopathological score; endoscopic findings score; main symptom score; the patient-reported outcome scale integrals | |
| GC | Pan et al[128], 2020 | Jianpi Yangzheng Xiaozheng decoction | 210 | Chemotherapy combined with JPYZXZ decoction | Chemotherapy | 24 wk | One-year survival rate; progression-free survival; overall survival; immune related hematology test; objective response rate; tumor makers; TCM syndrome points; fatigue scale; QOL scale |
| Xu et al[129], 2013 | Wei Chang’An | 399 | Chemotherapy combined with Wei Chang’An decoction | Continuously | 3 mo or more | Survival trends; survival time | |
| Shu et al[130], 2019 | Yiqi Huayu Jiedu decoction | 489 | Chemotherapy combined with YHJD | Chemotherapy | 6 mo or more | Disease-free survival rate; 5-yr survival rate; QOL; TCM symptoms | |
| NCT05229809 | Yiqi Wenyang Jiedu prescription | 212 | Yiqi Wenyang Jiedu prescription | Simulation agent of Yiqi Wenyang Jiedu prescription | 24 wk | Two-year disease-free survival rate; disease-free survival; overall survival; cumulative annual recurrence and metastasis rate for 1-3 yr; cumulative annual survival rate for 1-3 yr; Indexes related to fat distribution; visceral adiposity Index; tumor marker; peripheral blood inflammatory index; prognostic nutritional index; QOL of the patient; evaluation of the patient’s symptoms; medication compliance; percentage of participants with adverse events |
Many experimental studies have investigated the efficacy and mechanisms of TCM. For example, WFC can increase the secretion of pepsin by inhibiting the MAPK signaling pathway, thereby regulating the weight of rats with GPL and improving histopathological changes in the gastric mucosa[32]. Another study showed that Ginsenoside Rb1 (GRb1), which is contained in WFC, can prevent the occurrence and progression of GPLs by reducing the protein expression and nuclear translocation of β-catenin, interfering with the interaction of β-catenin/TCF4, and inhibiting the transcriptional activity of downstream genes, including c-myc, Cyclin D1 and Birc5[33]. Therefore, it is important to study the effect of TCM on GPL to improve the clinical efficacy and transformation of drug research and development (R&D). In this study, we summarize the mechanisms and targets of TCM in the treatment of GPL and GC, with a particular focus on their action in the processes of inflammation, angiogenesis, cell proliferation, and apoptosis.
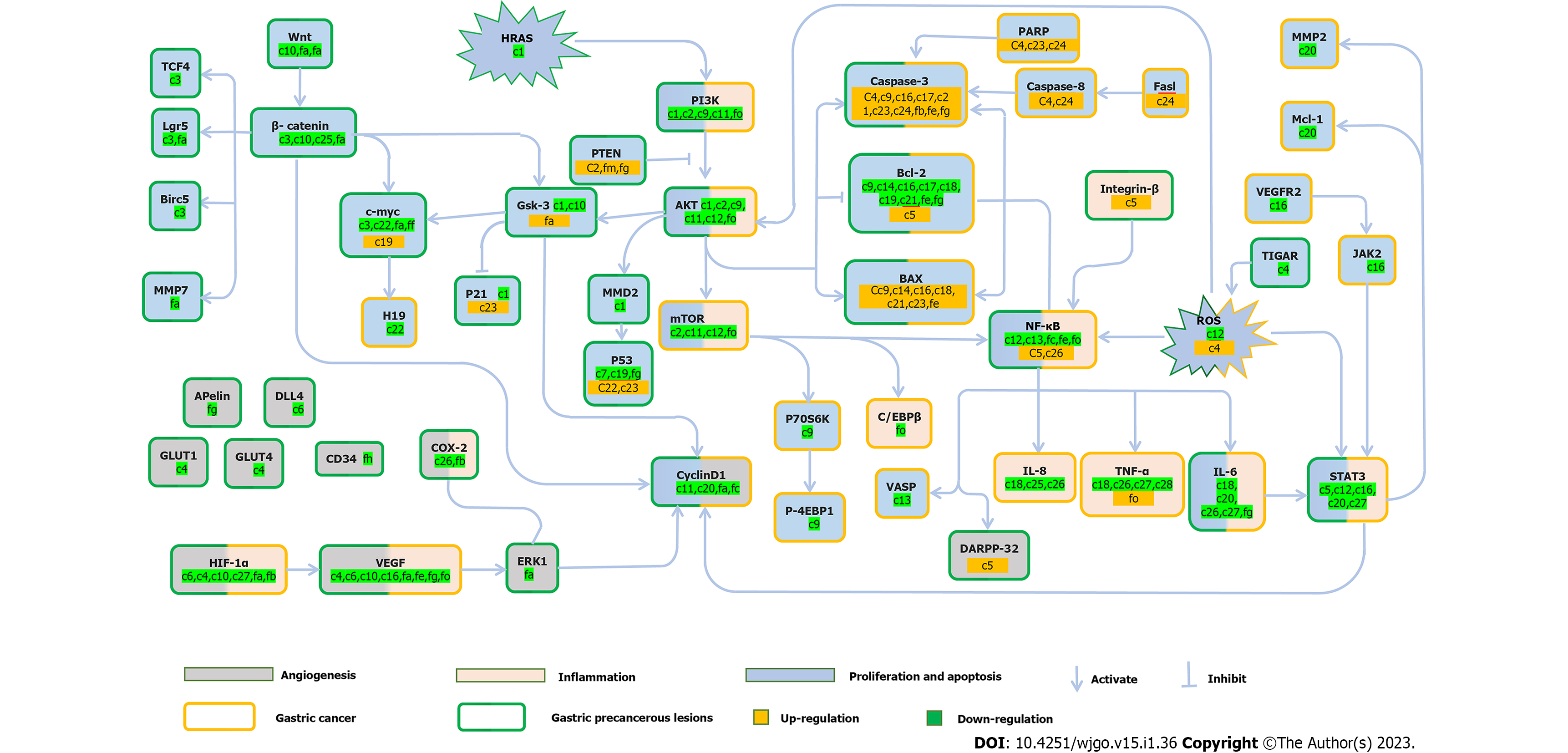
The GPL stage is a critical stage in the development of GC. The intervention goal of GPL is to reverse malignant transformation and block the progression to GC. However, the molecular mechanisms underlying GPL have not yet been fully elucidated. Many TCMs have been shown to be effective in the treatment of GPL, focusing on the regulation of proliferation and apoptosis, anti-inflammation, and inhibition of angiogenesis (Tables 2 and 3, Figure 1).
| Pathological stages | Effect | No. | Ref. | Active component | Animal/cells | Pathways/targets |
| Gastric precancerous lesions (GPL) | Anti-proliferation inducing apoptosis | c1 | Wang et al[37], 2021 | Erianin | GES-1 cell | HRAS-PI3K-Akt↓; p-Gsk3β, MDM2, p21, CyclinD1↓ |
| c2 | Zhu et al[38], 2021 | Epigallocatechin gallate | Male Wistar rats, PLGC model | PI3K/Akt/mTOR↓; PTEN↑; PI3K, Akt, mTOR↓ | ||
| c3 | Zeng et al[33], 2021 | Ginsenoside Rb1 | Sprague-Dawley rats, PLGC model | β-catenin/TCF4↓; c-myc, cyclin, Birc5↓ | ||
| c4 | Lv et al[131], 2022 | Ginsenoside Rg3 | Male Sprague-Dawley rats, PLGC model GPL cell | TIGAR, G6PDH, NADP, GSH↓; ROS↑ | ||
| Anti-inflammatory | c5 | Liu et al[50], 2020 | Calycosin | Male (SD) rats, PLGC model | Integrinβ1/NF-κB/DARPP-32; Integrinβ1, NF-κB, DARPP-32↑; STAT3↓ | |
| Anti-angiogenesis | c5 | Liu et al[50], 2020 | Calycosin | Male (SD) rats, PLGC model | Integrinβ1/NF-κB/DARPP-32; Integrinβ1, NF-κB, DARPP-32↑; STAT3↓ | |
| c6 | Gao et al[58], 2022 | Atractylenolide III | Female SD rats, Gastric Precancerous, Lesions Model | HIF-1α, VEGF-A, DLL4↓ | ||
| c4 | Zeng et al[120], 2022 | Ginsenoside Rg3 | Male Sprague Dawley rats, PLGC modelAGS cell, HGC-27 cell | GLUT1, GLUT4↓ | ||
| Inhibit glycolysis | c4 | Liu et al[63], 2020 | Ginsenoside Rg3 | Male Atp4a/ C57Bl/6 mice, PLGC model | PI3K/Akt/mTOR↓; PI3K/Akt/miRNA-21↓; PI3K, AKT, mTOR, HIF-1α, miRNA-21↓; caspase-3↑ | |
| c7 | Zhang et al[65], 2018 | Astragaloside IV | Male Sprague-Dawley rats, PLGC model | LDHA, MCT1, MCT4, HIF-1α, CD147, TIGAR↓; miRNA-34a, p53↑ | ||
| Improvement of EMT | C8 | Liao et al[68], 2023 | Gallic acid | GES-1 cell, MC cells | Wnt/β-catenin↓ | |
| Induce autophagy | c7 | Cai et al[44], 2018 | Astragaloside IV | Sprague Dawley rats, PLGC model | Bcl-2/Bax, p53, Beclin1, p62, ATG5, ATG12↓; caspase3↑ | |
| GC | Anti-proliferation inducing apoptosis | c9 | Xu et al[74], 2021 | Naringin | SNU-1 cell, GES-1 cell | PI3K/Akt↓; PI3K, Akt, Bcl-2↓; caspase 3, Bax↑ |
| c10 | Yang et al[132], 2016 | Epigallocatechin-3-gallate | SGC-7901 cells, Nude mouse tumour xenograft model | Wnt/β-catenin↓; GSK3b, β-catenin↓ | ||
| c11 | Lee et al[75], 2018 | Pectolinarigenin | AGS cell, MKN28 cell | PI3K/Akt/mTOR↓; PI3K, p-Akt, mTOR, p-p70S6K, p-4EBP1↓ | ||
| c10 | Fu et al[133], 2019 | Epigallocatechin-3-gallate | SGC7901 cell | VEGF, HIF-1α↓ | ||
| c12 | Wang et al[77], 2020 | Aloin | HGC-27 cell, BGC-823 cell | Akt/mTOR, Stat3, NF-κB↓; NOX2, ROS, Akt, mTOR, Stat3, IκBα, p65↓ | ||
| c13 | Chen et al[109], 2020 | Betulinic acid | BGC-823 cells, MNK45 cells | NF-κB, VASP↓ | ||
| c14 | Geng et al[134], 2018 | Usnic acid | BGC823 cell, SGC7901 cell | Bax, LC3-II↑; Bcl-2, p62↓ | ||
| c15 | Xu et al[135], 2020 | T-17 | SGC-7901, AGS cell, MGC-803 cell, BGC-823 cell, NCI-N87 cell,HUVEC cell | JNK, Bcl-2↑ | ||
| c16 | Liu et al[81], 2015 | Ponicidin | MKN28 cell | JAK2/STAT3↓; Bcl-2, VEGF, VEGFR2, JAK2 STAT3↓; Bax, caspase-3↑ | ||
| c17 | Chen et al[136], 2012 | Tanshinone IIA | MKN45 cell, SGC7901 cell | cyto-c, Bax, Caspase-9↑; Bcl-2↓ | ||
| c18 | Yang et al[52], 2020 | Tomentosin | GCCs cell, AGS cell | IL-6, TNF-α, IL-1, IL-8, Bcl-2↓; Bax↑ | ||
| c19 | Sun et al[137], 2007 | Swainsonine | SGC-7901 cell, BALB/c nu/nu mice, GC model | p53, Bcl-2↓; cmyc↑ | ||
| c20 | Tang et al[82], 2019 | Micheliolide | AGS cell, N87 cell | IL-6, STAT3, cyclinD1, Mcl-1, MMP-2↓ | ||
| c21 | Li et al[138], 2013 | Andrographolide | BGC-823 cell | Bax, caspasase-3↑; Bcl-2↓ | ||
| c22 | Liu et al[139], 2016 | Curcumin | SGC7901 cell, GES-1 cell | c-Myc/H19↓; c-Myc, H19↓; p53↑ | ||
| c23 | Lee et al[140], 2016 | Quercetin | NOD/SCID mice, PLGC model SNU719 cell, MKN74 cells | p53, p21, Bax, Puma, caspase-3, caspase-9, PARP↑ | ||
| c4 | Aziz et al[121], 2016 | Ginsenoside Rg3 | SGC-7901 cell | Caspases-3, caspase-8, caspase-9, PARP, SP1↑; HSF1, FUT4↓ | ||
| c24 | Saralamma et al[141], 2015 | Poncirin | AGS cells | Fasl, caspase-8, caspase-3, PARP↑ | ||
| Anti-inflammatory | c18 | Yang et al[52], 2020 | Tomentosin | GCCs cell, AGS cell | IL-6, TNF-α, IL-1, IL-8, Bcl-2↓; Bax↑ | |
| c25 | Tharmalingam et al[115], 2016 | Piperine | AGS cell | β-catenin, IL-8↓ | ||
| c26 | Su et al[53], 2019 | Artemisinin | SGC-7901 cell, GES-1 cells, C57BL/6 J mice | NF-κB↓; IL-8, IL-6, TNF-α, IL-1β, COX-2, p-IκBα↓; IκBα↑ | ||
| c27 | Han et al[54], 2015 | Rosmarinic acid | MKN45 cell | IL-6/STAT3↓; IL-6, IL-1β, TNF-α, TNFsR-1, HIF-1α, miRNA-155-5p↓; IL-10↑ | ||
| c28 | Sun and Meng[55], 2022 | Scutellarin | AGS cell, albino Wistar rats, GC modelr | TNF-α, IL-1β, IL-2↓ | ||
| Anti-angiogenesis | c4 | Li and Qu[89], 2019 | Ginsenoside Rg3 | BGC823 cell | HIF 1α, VEGF↓ | |
| Inhibit glycolysis | C29 | Wang et al[142], 2022 | Licochalcone A | MKN45 cell, SGC7901cell, GES-1 cell | Akt/HK2↓; Akt, HK2↓ | |
| C30 | Chen et al[96], 2015 | Baicalein | AGS cell | PTEN/Akt/HIF-1α↓; HK2, LDH-A, PDK1, Akt, HIF-1α↓; PTEN↑ | ||
| C31 | Wang et al[142], 2022 | Helichrysetin | MGC803 cell, HCT-8 cell | mTOR/p70S6K/c-Myc/PDHK1↓; mTOR/p70S6K, c-Myc, PDHK1↓ | ||
| C27 | Han et al[54], 2015 | Rosmarinic acid | MKN45 cell | IL-6/STAT3↓; IL-6, IL-1β, TNF-α, TNFsR-1, HIF-1α, miRNA-155-5p↓; IL-10↑ | ||
| Improvement of EMT | C32 | Wang et al[99], 2022 | Poria acid | AGS cell, MKN-28 cell | E-cadherin↑; N-cadherin, Vimentin↓ | |
| C33 | Zang et al[143], 2017 | Luteolin | NCI-N87 cell, MKN28 cell, Hs-746T cell | E-cadherin↑; N-cadherin, vimentin, Snail↓ | ||
| c34 | Zhou et al[108], 2019 | Crocin | AGS cell, HGC-27 cell, GES-1 cell | miR-320/KLF5/HIF-1α; KLF5/HIF- | ||
| c7 | Zhu and Wen, 2020[144], 2018 | Astragaloside IV | BGC-823 cell, MKN-74 cell, GES-1 cell | PI3K/Akt/NF-κB↓; TGF-β1↓ | ||
| Regulate immune function | c35 | Zhuang et al[105], 2020 | Sophoridine | MFC cell, RAW264.7 cell | iNOS, IFN-β, IL-12α, Granzyme-B, TNF-α, Perforin↑; Arg-1, CD206, IL-10, PD-1, Tim-3, Lag-3, CCR2↓ | |
| C36 | Lu et al, 2021[104] | Oleanolic acid | MKN-45, Jurkat T cell | IL-1β/ NF-κB /TET3↓; PD-L1↓ | ||
| Induce autophagy | c11 | Lee et al, 2018[75] | Pectolinarigenin | AGS cell, MKN28 cell | PI3K/Akt/mTOR↓; PI3K, p-Akt, mTOR, mTOR, p-p70S6K, p-4EBP1↓ | |
| c14 | Geng et al[134], 2018 | Usnic acid | BGC823 cell, SGC7901 cell | Bax, LC3-II↑; Bcl-2, p62↓ | ||
| c15 | Xu et al[135], 2020 | T-17 | SGC-7901, AGS, MGC-803, BGC-823, NCI-N87, HUVEC cell | JNK, Bcl-2↑ | ||
| Inhibits migration, and invasion | c12 | Wang et al[77], 2020 | Aloin | HGC-27 cell, BGC-823 cell | Akt/mTOR, Stat3, NF-κB↓; NOX2, ROS, Akt, mTOR, Stat3, IκBα, p65↓ | |
| c13 | Chen et al[109] , 2020 | Betulinic acid | BGC-823 cells, MNK45 cells | NF-κB, VASP↓ | ||
| c34 | Zhou et al[108], 2019 | Crocin | AGS cell, HGC-27 cell, GES-1 cell | miR-320/KLF5/HIF-1α; KLF5/HIF-1α; KLF5, HIF-1α↓; miR-320↑ | ||
| c37 | Cai et al[110], 2018 | 18β-glycyrrhetinic acid | SGC-7901 cell | ROS/PKC-α/ERK↓; ROS, PKC-α, ERK↓ | ||
| c30 | Yan et al[111], 2015 | Baicalein | SGC7901 Cell, MGC803 cell | MMP-2, mmp-9, p38↓ | ||
| Anti-Helicobacter pylori | c25 | Tharmalingam et al[115], 2016 | Piperine | AGS cell lines | β-catenin, IL-8↓ | |
| c26 | Su et al[53], 2019 | Artemisinin | SGC-7901 cell, GES-1 cells | NF-κB↓; IL-8, IL-6, TNF-α, IL-1β, COX-2, p-IκBα↓; IκBα↑ | ||
| c28 | Sun and Meng[55], 2022 | Scutellarin | (AGS) cell, albino Wistar rats, GC model | TNF-α, IL-1β, IL-2↓ |
| Pathological stages | Effect | No. | Ref. | Formulas | Main component | Animal/cells | Pathways/targets |
| GPL | Anti-proliferation inducing apoptosis | fa | Zeng et al[31], 2016 | Weipixiao (WPX) | Radix Astragali, Radix Pseudostellariae, Rhizoma Atractylodis Macrocephalae, Radix Salviae Miltiorrhiz, Herba Hedyotis Diffusae | Male SD rats, PLGC model | Wnt/β-catenin↓; Lgr5, MMP-7, Wnt1, β-catenin↓ |
| fa | Zeng et al[145], 2018 | Weipixiao (WPX) | Astragalus Membranaceus, Pseudostellaria Heterophylla, Atractylodis Macrocephalae, Curcuma zedoaria, Salvia Miltiorrhiza and Hedyotis Diffusa Willd | Male SD rats, PLGC model | Wnt/GSK3β; GSK3β↑; C-myc↓ | ||
| fb | Yin et al[29], 2019 | Weiqi decoction (WQD) | Radix Angelicae Sinensis, Radix Astragali, Radix Codonopsis, Rhizoma Curcumae, Fructus Aurantii, Fructus Akebiae and Herba Taraxaci | Male Wistar rats, CAG with Precancerous Lesion Mode | PGE2, caspase-3↑; Ki67, HIF-1, COX-2, VEGF, VEGFR1↓ | ||
| fc | Cai et al[146], 2022 | Sancao Tiaowei Decoction | Pseudostellariae Radix, stirbaked Atractylodis Macrocephalae Rhizoma inbran, Poria, Agrimoniae Herba, Taraxaci Herba, Hedyotis Diffusa Willd, Salviae Miltiorrhizae Radix Etrhizoma, Curcumae Rhizoma and Glycyrrhizae Radix Et Rhizoma | SD male rats, PLGC model | Hh signaling↓; Shh, Gli-1, Smo, cyclinD1, CDKN2A/p16INK4a, NF-κB P65↓; Ptch↑ | ||
| fd | Hao et al[147], 2022 | Huazhuojiedu decoction | Artemisia capillaris Thunb, Scutellaria baicalensis Georgi, Oldenlandia diffusa Roxb, Isatis indigotica Fortune, Lobelia chinensis Lour, Pogostemon cablinBenth, Scutellaria barbata D. Don, Sophora flavescens Aiton, Coptis chinensis Franch, Gynostemma pentaphyllum Makino, and Eupatorium fortunei Turcz | Male SD rats, PLGC model | Lnc 517368↓ | ||
| fe | Xu et al[148], 2018 | Xiao Tan He Wei Decoction | Radix bupleuri, processed rhizomapinelliae, poriacocos, coptischinensis, oldenlandiadiffusa, dandelion,cassia twig, rhubarb, radix paeoniae alba, radix glycyrrhizae preparata | GES-1 cell, Wistar rats, PLGC rat animal models | Bax, caspase-3↑; Bcl-2, NF-κB↓ | ||
| ff | Shen et al[149], 2008 | Jinguo Weikang Capsule (JWC) | Tinospora root, trifoliate-orange Immature fruit, kaempfer dutchmanspipe root | SD rats, PLGC model | H-ras, EGFR, P53, c-myc↓ | ||
| Anti-inflammatory | fb | Yin et al[29], 2019 | Weiqi decoction (WQD) | Radix Angelicae Sinensis, Radix Astragali, Radix Codonopsis, Rhizoma Curcumae, Fructus Aurantii, Fructus Akebiae, and Herba Taraxaci | Male Wistar rats, CAG with Precancerous Lesion Mode | PGE2, caspase-3↑; Ki67, HIF-1, COX-2, VEGF,VEGFR1↓ | |
| fg | Deng et al[125], 2012 | Weining granule | Radix Astragali Mongolici, Herba Hedyotdis, Rhizoma Curcumae Phaeocau, Fructus Lycii | Male Wistar rats, PLGC model | VEGF, IL-6, IgG↓; CD4+, CD4+/CD8+, IL-2↑ | ||
| Anti-angiogenesis | fb | Yin et al[29], 2019 | Weiqi decoction (WQD) | Radix Angelicae Sinensis, Radix Astragali, Radix Codonopsis, Rhizoma Curcumae, Fructus Aurantii, Fructus Akebiae, and Herba Taraxaci | Male Wistar rats, CAG with Precancerous Lesion Mode | PGE2, caspase 3↑; Ki67, HIF-1, COX-2, VEGF,VEGFR1↓ | |
| fa | Zeng et al[59], 2018 | Weipixiao (WPX) | Astragalus Membranaceus, Pseudostellaria Heterophylla, Atractylodis Macrocephalae, Curcuma zedoaria, Salvia Miltiorrhiza and Hedyotis Diffusa Willd | Male SD rats, PLGC model | ERK1/CylinD1; HIF-1α, VEGF, ERK1, CylinD1↓ | ||
| fh | Wang et al[150], 2020 | Jinlongshe (JLS) | Rhizoma Pinelliae, Radix, Rhizome Arisaemat, Glycyrrhizaepreparata, corium stomachiumgalli, etc. | Male SD rats, PLGC model | Apelin, CD34↓ | ||
| Protecting gastric mucosa | fi | Yi et al[151], 2022 | Elian granules | Curcumae Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Angelicae Sinensis, Diels, Coptidis Rhizoma, Hedyotis Diffusa, Codonopsis Radix, Atractylodis Macrocephalae Rhizoma, Glycyrrhizae Radix et Rhizoma, Pinelliae Rhizoma, Citri Reticulatae Pericarpium, Poria | Male SD rats, PLGC model | MAPK; JNK, p38↑ | |
| fj | Wang et al[32], 2020 | WeiFuChun (WFC) | Radix Ginseng Rubra (red ginseng), Rabdosia amethystoides H. Hara, and fried Fructus Aurantii | Male SD rats, PLGC model | MAPK; VEGF, FOXO4, AKT, TP53, FAS, MAPK8, MAPK11, MAPK14↓ | ||
| Inhibit glycolysis | fa | Cai et al[64], 2019 | Weipixiao (WPX) | Astragalus, Radix Pseudostellariae, Atractylodes macrocephala, Salvia miltiorrhiza Bge, Oldenlandia diffusa(Willd.)Roxb | Male SD rats, PLGC model | miRNA-34a/PI3K/AKT/Mtor; LDHA, CD147, MCT4, PI3K, AKT, mTOR, HIF-1α, miRNA-34a↓ | |
| fk | Liu et al[152], 2019 | Weipiling (WPL) | Hedysarum multijugum Maxim, Pseudostellaria heterophylla Pax, Atractylodes macrocephala Koidz, Poria cocos Wolf, Panax notoginseng F.H. Chen, Curcuma zedoaria, Roscoe,Hedyotis diffusa Willd,Hericium erinaceus Pers | Male Atp4a-/-C57Bl/6 mice | mTOR/HIF-1α↓; CDX2, MUC2, ki-67, PTEN and p53, mTOR, HIF-1a, AMPK↓; TSC1, TSC2↑ | ||
| Improvement of EMT | fl | Li et al[69], 2022 | Manpixiao decoction | Heterophylla falsestarwort root, root of red rooted salvia, drug solomonseal, common perilla stem, largehead atractylodes rhizome, corydalis ambigua, japanese apricot fruit, citron, rose, villous amomum fruit, spreading hedyotis herb, liquorice | Wistar male rats, PLGC model | EGFR-PI3K-AKT↓; EGFR, β-catenin, N-cadherin protein↓ | |
| GC | Anti-proliferation inducing apoptosis | fm | He et al[76], 2020 | Weifufang | Astragalus, Codonopsis pilosula, Atractylodes macrocephala, Poria cocos, Nutgrass Galingale Rhizome, Radix Curcumae, Sappan Wood, Rhizoma Curcumae, Zaoxiu Paris Root Rhizoma Paridis, Barbed Skullcap Herb, Ligustrum lucidum, South Dodder Seed, Oldenlandia, Liquorice Root, Chicken’s Gizzard-membrane, fry malt, and fry Rice-grain Sprout | BALB/c-nu nude mice, BGC-823 cell, Nude mice with xenografts | PTEN↑ |
| fn | Fang et al[153], 2021 | Huosu Yangwei (HSYW) | Huoxiang, Zisugeng, Baizhu, Zhike, Doukou, Foshou, Wumei, Shengjiang, Dazao, Gancao, Huangqi, Dihuang, Mudanpi, Tianhuafen, Danggui, Chuanxiong, Ezhu, Gouqizi, Huanglian, Dangsheng, and Pugongying | Male Balb/c mice, PLGC model | DNAJB4, CALD1, AKR1C1, CST1, CASP1, PREX1, SOCS3, PRDM1 | ||
| fo | Yuan et al[154], 2020 | Jianpi Yangzheng Xiaozheng (JPYZXZ) decoction | Radix astragali, Radix codonopsis pilosulae, Rhizoma Sparganiiand Rhizoma Curcumae | HGC-27 cells ,THP-1 cell, MFC cell | PI3Kγ, NF-κB, AKT, p-C/EBPβ, IL-10↓; IL-1β, TNF-α, IL-12p↑ | ||
| fg | Deng et al[92], 2019 | Weining granule | Radix Astragali Mongolici and Herba Hedyotdis Rhizoma Curcumae Phaeocaulis,Fructus Lycii | Male Wistar rats, PLGC model | Bcl-2, VEGF↓; caspase-3, PTEN↑ | ||
| Anti-inflammatory | fp | Li et al[155], 2021 | Guiqi Baizhu prescription | Astragali radix, Atractylodis macrocephalae, Angelicae,Paeoniae radix alba, Pericarpium citri reticulatae, Rhubarb, Glycyrrhizae | MKN-45 cell, SGC-7901 cell, BGC-823 cell, GES-1cell | HER2, PD-L1↑ | |
| Anti-angiogenesis | fg | Deng et al[92], 2019 | Weining granule | Radix Astragali Mongolici and Herba Hedyotdis Rhizoma Curcumae Phaeocaulis, Fructus Lycii | Male Wistar rats, PLGC model | Bcl-2, VEGF↓; caspase-3, PTEN↑ | |
| Improvement of EMT | fq | Liu et al[100], 2020 | Babao Dan | Natural bezoar, snake gall, antelope horn, pearl, musk, and Panax notoginseng | AGS cell, MGC803 cell | TGF-b/Smad↓; TGF-b1, p-Smad2/3↓ | |
| fo | Yuan et al[154], 2020 | Jianpi Yangzheng Xiaozheng (JPYZXZ) decoction | Radix astragali, Radix codonopsis pilosulae, Rhizoma Sparganiiand Rhizoma Curcumae | HGC-27 cells, THP-1 cell, MFC cell | PI3Kγ, NF-κB, AKT, p-C/EBPβ, IL-10↓; IL-1β, TNF-α, IL-12p↑ | ||
| Inhibits migration, and invasion | fr | Chen et al[112], 2018 | Yangzheng Sanjie Decoction (YZSJD) | Astragali Radix, Scutellariae Barbatae, Herba, Arisaematis Rhizoma Preparatum, Citri Sarcodactylis, Fructus, Cremastrae Pseudobulbus and Curcumae Longae, Rhizoma | MKN-45 cell | EGFR, miR-7↑ |
In GPL and GC, the imbalance between proliferation and apoptosis of gastric epithelial cells may be the direct cause of malignant progression[34]. The PI3K/Akt/mTOR signaling pathway can regulate growth in normal cells and in cancers, and the activation of the Akt pathway through PI3K is directly related to tumorigenesis[35]. Pathological changes in the PI3K/Akt/mTOR pathway usually include downregulation of the tumor suppressor gene PTEN, abnormal activation of PI3K, and overexpression/hyperactivation of Akt[36]. Erianin, which is one of the most important natural compounds in Dendrobium, can be directly extracted from Dendrobium. Moreover, dendrobium species are widely used to treat various digestive diseases. Wang et al[37] confirmed that Erianin can significantly reduce Harvey rat sarcoma viral oncogene homolog (HRAS), thereby inhibiting the downstream PI3K/Akt signal pathway; hence, it plays a role in the treatment of precancerous lesions. Green tea polyphenols have been recognized for their anti-GC effects. Epigalocatein gallate is the main component of green tea polyphenols. Similarly, Zhu et al[38] found that epigallocatechin gallate inhibits the downstream PI3K/Akt/mTOR signaling pathway by promoting PTEN expression. This process achieves a balance between cell proliferation and apoptosis. The Wnt/β-catenin signaling pathway is a conserved pathway that plays an important role in maintaining intracellular homeostasis[39]. Inappropriate activation of the Wnt/β-catenin signaling pathway often occurs in GPL gastric epithelial cells, which may be one of the reasons for the transition from GPL to GC[40,41]. Weipixiao is a TCM compound that is found in Radix Astragali, Radix Pseudostellariae, Rhizoma Atractylodis Macrocephalae, Radix Salviae Miltiorrhiz, Herba Hedyotis Diffusae, and other TCMs. It is widely used for the management of GPL in clinical practice. Zeng et al[33] found that Weipixiao can inhibit cell proliferation and induce apoptosis by inhibiting abnormal activation of the Wnt/β-catenin signaling pathway, while GRb1 can inhibit β-catenin protein expression. Downstream targets, such as c-myc, Cyclin D1, Lgr5, MMP-7, and Birc5, in this pathway are inhibited. Autophagy is an intracellular catabolic process[42]. Current research shows that promoting autophagy in the early stages of tumorigenesis has a positive significance in cancer treatment[43]. The Chinese medicine monomer, Astragaloside IV (As-IV), regulates autophagy by mediating the Ambra1/Beclin1 complex[44].
Inflammation is the key to the progression of GPL to GC. Currently, it is generally believed that the noncanonical nuclear factor-kappaB (NF-κB) and STAT3 signaling pathways are the two major signaling pathways that connect inflammation and cancer, and they link inflammation and cancer through synergistic action[45,46]. As one of the most important signaling pathways in the inflammatory response[47], the NF-κB signaling pathway can lead to the transcriptional activation of many pro-inflammatory mediators, including tumour necrosis factor alpha (TNF-α), interleukin-8 (IL-8), and IL-6[48]. Because it also regulates cell proliferation, angiogenesis, metabolism, inflammation, and cell migration and is in the key position of these mechanism-related pathways, it is not difficult to explain why this pathway has become the most important bridge connecting GPL and GC[49]. Calycosin may play an anti-inflammatory role by regulating the integrin β1/NF-κB/DARPP-32 pathway and downregulating STAT3 expression[50]. The upregulated expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1α (HIF-1α) in cells under hypoxic conditions lead to an inflammatory reaction[51]; therefore, WQD can play an anti-inflammatory role by inhibiting the HIF-1 signaling pathway[29].
In addition, TCM monomers and compounds can regulate various inflammatory factors. TCM monomers and compounds can also regulate a variety of inflammatory factors, including IL-6, TNF-α, IL-1, IL-8, COX-2, and IL-2. Among these, TNF-α, IL-6, and IL-8 can be regulated by more than three types of Chinese herbs. For example, TNF-α can be regulated by Tomentosin[52], artemisinin (ART)[53], rosmarinic acid (RA)[54], scutellarin (SC)[55].
HIF-1α binds to the target gene VEGF, and then leads to the transcription of Pro angiogenic protein, which is an important reason for the formation of new blood vessels, or neovasculogenesis[56,57]. Therefore, stabilizing the HIF-1α/VEGF pathway is of great significance for improving angiogenesis under hypoxic conditions. A study showed that early angiogenesis in GPL tissue is accompanied by HIF-1α and VEGF-A activation[58]. Both atractylenolide III (ATIII)[58] and WPX[59] can inhibit the HIF-1α/VEGF signaling pathway, and WPX can also inhibit the downstream targets of the pathway, such as ERK1/Cyclin D1. It then plays a role in angiogenesis inhibition.
Metabolic disorder is a hallmark of GC, the most significant of which are disorders of glucose metabolism[60]. In contrast to normal cells, GC cells preferentially choose glycolysis as the main way to obtain energy, even under conditions of sufficient oxygen[61]. The rapid proliferation of GC cells depends largely on glycolysis[62]. Studies have found that glycolysis also occurs in GPL; therefore, glycolysis is likely to be one of the key points in the transition from GPL to GC[63]. Ginsenoside Rg3 (GRg3) blocks glycolysis by inhibiting the PI3K/AKT pathway and downregulating downstream miRNA-21[63], whereas WPX inhibits the PI3K/AKT/mTOR pathway by upregulating upstream miRNA-34a, and then blocks glycolysis[64]. Interestingly, both WPX and As-IV can regulate LDHA, MCT4, HIF-1α, and CD147 targets inhibits glycolysis[65].
Epithelial-mesenchymal transformation (EMT) refers to the transformation of epithelial cells into mesenchymal cells under specific conditions. EMT is a physiological process that occurs during tissue self-repair[66]. When it is out of control, it may lead to fibrosis, angiogenesis, loss of normal organ function, and cancer, making it one of the key characteristics of GC[67]. Gallic acid (GA) is found in many TCMs. As early as 1552 AD, the Compendium of Materia Medica recorded the method for obtaining GA and its medicinal properties. Liao et al[68] found that GA can inhibit the EMT process by downregulating the Wnt/β-catenin signaling pathway, thereby inhibiting the malignant proliferation of MC cells and finally achieving the goal of treating GPL. The Manpixiao Decoction is a compound used in Chinese medicine. Li et al[69] confirmed that this compound could inhibit the progression of PLGC by reducing the occurrence of systemic inflammatory reactions in the local gastric mucosa and inhibiting the EGFR-PI3K-AKT related EMT pathway.
A variety of TCM formulas and active compounds have been demonstrated to be effective in the treatment of GC, mainly involving the regulation of proliferation, apoptosis, and inflammation (Tables 2 and 3, Figure 1).
The imbalance between cell proliferation and apoptosis destroys tissue homeostasis and promotes tumor occurrence[70]. Therefore, regulating the proliferation and apoptosis of GC cells is an important therapeutic strategy for preventing GC progression[71]. In GC, the PI3K/AKT/mTOR pathway is often activated, and this pathway plays a role in promoting cancer progression[72]. Multiple targets of this pathway have been shown to be mutated or otherwise dysregulated during tumorigenesis[73]. Naringin and pectinarigenin (PEC) can inhibit the PI3K/Akt signaling pathway, and PEC can also inhibit downstream mTOR[74,75]. Weifufang promoted the upregulation of PTEN and inhibited the PI3K/Akt signaling pathway[76]. Aloin (ALO) inhibits the Akt/mTOR signaling pathway mediated by NOX2-ROS[77]. They can both play a role in inhibiting cell formation. Recent studies have shown that the STAT signaling pathway has a strong carcinogenic potential, which can promote proliferation and has an extremely significant anti-apoptotic effect[78,79]. Therefore, dysregulation of the STAT3 signaling pathway is common in GC[80]. Ponicidin can induce apoptosis in MKN28 cells, which may be related to the inhibition of the VEGFR2-mediated JAK2-STAT3 signaling pathway[81]. The study showed that micheliolide inhibited the growth of GC in vitro and in vivo, and this effect was related to downregulation expression of IL-6 and thus inhibition of the STAT3 pathway[82]. ALO can inhibit cell proliferation by inhibiting the NOX2-ROS mediated Stat3 signal pathway[77].
The occurrence of GC is mostly the result of inflammation, and blocking the inflammatory signaling pathway is essential for the treatment of GC[83]. During GC, continuous activation of NF-κB leads to chronic inflammation and further tumorigenesis. ART and its derivatives can be used to treat GC caused by H. pylori infection by downregulating inflammatory factors and inhibiting the NF-κB signaling pathway in vivo. The STAT3 signaling pathway plays an important role in the development of inflammation-related GC, and its activation can induce tumors to promote inflammation[84,85]. RA can downregulate the anti-inflammatory factor IL-10 and upregulate pro-inflammatory cytokines such as TNF-α and IL-1β Expression of. Inhibition of IL-6/STAT3 pathway[54].
Without angiogenesis, it is difficult to achieve large-scale proliferation and invasion of GC cells[86]. Angiogenesis in GC is regulated by a variety of angiogenic or anti-angiogenic factors[87]. For example, VEGF is the most representative promoter of angiogenesis. Currently, it is considered one of the most promising targets for the treatment of GC[88]. Therefore, Weining granules inhibit angiogenesis by downregulating VEGF expression[89]. As an upstream regulator of VEGF, HIF-1α also plays a role in regulating other pro angiogenic factors and anti-angiogenic factors[90,91]. Therefore, the inhibition of HIF-1α and VEGF expression may be the direct reason for GRg3’s excellent inhibition of angiogenesis[92].
Cancer is usually considered a metabolic disease because cancer cells proliferate rapidly by reprogramming their energy metabolism. Glycolysis is the main mechanism by which GC cells obtain energy[93,94]. Current research has shown that many TCMs can inhibit abnormal metabolic processes. Licochalcone A (Lic A) is an important active compound extracted from licorice that has anti-inflammatory, antibacterial, antioxidant, antitumor, and other activities. Wu et al[95] found that Lic A inhibits glycolysis by blocking the Akt/HK2 pathway. In nature, baicalein mainly exists in Scutellaria baicalensis Georgi and has anti-inflammatory, antibacterial, and other effects. Chen et al[96] found that baicalein can inhibit glycolysis by regulating the PTEN/Akt/HIF-1α signaling pathway.
In the malignant progression of GC, tumor cells use the process of EMT to change their cell morphology to improve their invasiveness, metastatic ability, and drug resistance[97,98]. Therefore, the inhibition of EMT is a key factor in the treatment of GC. Wang et al[99] found that Poria acid can inhibit the EMT process by significantly increasing the expression of E-cadherin and inhibiting the expression of N-cadherin and Vimentin, thereby inhibiting the invasion and metastasis of GC cells. Babaodan (BBD) is a TCM compound that has been used in clinical treatment since the Ming Dynasty, (more than 400 years ago). Modern research has found that Babaodan has significant anti-tumor, anti-inflammatory, immune regulatory effects, as well as other effects. Liu et al[100] found that BBD can inhibit the TGF-β/Smad signaling pathway, thereby inhibiting TGF-β-induced EMT.
Compared with other therapies, immunotherapy has the characteristics of lasting remission, improving the quality of life of patients, and prolonging survival[101], which brings new hope to most patients with GC[102]. Modern experimental studies have found that many TCMs regulate immunity and eliminate immune disorders[103]. Oleanolic acid is widely found in many TCMs, such as hawthorns and black plums. Lu et al[104] found that OA destroyed IL-1 in GC cells β/NF-κB/TET3 axis, leading to DNA hypomethylation and downregulation of PD-L1. This suggests that OA can be used as an epigenetic modulator in GC immunotherapy. In addition, sophoridine, a monomer of TCM, regulates immune function and can act on macrophages and CD8+ T cells, thus reshaping the immune microenvironment of GC[105].
The invasion and migration of cancer cells play an important role in tumor metastasis, and distant metastasis of GC is a direct cause of high mortality[106,107]. A variety of Chinese herbal monomers and compounds have been used, including crocin[108], betulinic acid[109], ALO[77], 18 β-glycyrrhetinic acid[110], baicalein[111], and YangZheng Sanjie decoction[112].
As early as 1994, H. pylori was identified as a carcinogen in GC[113]. Currently, it is the first type of carcinogen to be identified in GC. Reducing H. pylori infections is an important means of preventing and treating GC[114]. The TCM monomers piperine[115], ART[53] and SC[55] have been proven to have corresponding therapeutic potentials.
As early as 40 years ago, GC was recognized as the end result of further development of GPL[116]. Recently, knowledge of the molecular basis of GC and GPL has been accumulating rapidly[117]. However, the molecular mechanism of the transformation from GPL to GC remains unclear[118]. In this study, we reviewed the progress of TCM in treating GPL and GC, while aiming to investigate the potential therapeutic treatment of TCM on the transformation from GPL to GC.
In this review (Tables 1-3, Figure 1), we found that multiple mechanisms of TCM can be identified in the treatment of both GPL and GC. The abnormal activation of the PI3K/ATK, NF-κB, IL-6/STAT3, and HIF-1α/VEGF signaling pathways in both GPL and GC indicated that some pathological changes in GC occurred as early as in the GPL stage. Therefore, in the treatment of GC, secondary prevention should be moved to the GPL stage[119]. According to these studies, active components of TCM, such as Epigallocatechin Gallate, GRg3, AT-III and AS-IV, showed multiple therapeutic effects on both GPL and GC via different targets and signaling pathways. This suggests that these active ingredients may have the therapeutic potential to block the transition from GPL to GC through these targets and signaling pathways. For example, GRg3 has a significant anti-angiogenic effect in both the GPL and GC processes. In the GPL stage, GRg3 can inhibit angiogenesis by downregulating GLUT1 and GLUT4[120], and suppressing the PI3K/Akt/mTOR pathway and downstream HIF-1α[63]. In GC, GRg3 can also reduce HIF-1α, thereby reducing tumor angiogenesis[121]. This has revealed that in the process of progression from GPL to GC, the pro angiogenic effect of HIF-1α may be a theme throughout the two pathological stages. GLUT1, GLUT4, and other proteins may be potential targets for the progression of GPL to GC. In a broader perspective, the existence of the same TCM compound with obvious therapeutic effect both on GPL and GC indicate that “dual effects” in treating GC and its precancerous lesions: when TCM is used to treat GPL, it also eliminates the possibility of GC as a malignant progression.
Many cancers, including GCs, are preceded by precancers. Treating precancers to prevent GC is essential for reducing GC-associated morbidity and mortality. Effective cancer prevention is the best way to stop cancer, and TCM have been shown to be effective in preventing cancer[122]. GRb1, Notoginsenoside R1, AS-IV, GRg3, AT-III, Calycosin, and other active ingredients have shown a variety of therapeutic effects in the treatment of precancerous lesions, including anti-proliferation and apoptosis induction, anti-angiogenesis, inhibition of glycolysis, and anti-inflammatory activities, including PI3K/Akt, Wnt/β-catenin, NF-κB, and STAT3 signaling. Clinically, Chinese herbal medicines containing these active ingredients are often used to treat precancerous lesions, such as ginseng, Panax notoginseng, Atractylodes, Astragalus membranaceus, and Pseudostellariae radix. These TCMs are usually combined to form a TCM compound for the clinical treatment of precancerous lesions, such as WPX, Sancao Tiaowei decoction, and Guiqi Baizhu prescriptions. Interestingly, the mechanisms of action of these active ingredients and TCM prescriptions in the treatment of precancerous lesions are not the same, which suggests that the curative effects of TCM are not caused by single chemical entities but result from their multi-ingredient prescription[123]. Chinese medicine differs from Western medicine in that many compounds in Chinese medicine act on multiple targets simultaneously, producing significant therapeutic effects[124]. For example, WPX can regulate proliferation and apoptosis by regulating the Wnt/β-catenin and Wnt/GSK3β pathways, playing an anti-angiogenic role by inhibiting the angiogenic factors HIF-1α, VEGF, and ERK1/CylinD1 pathway, and inhibiting glycolysis by regulating the miRNA-34a/PI3K/AkT/mTOR pathway. Interestingly, the Chinese herbal monomers contained in this formula, such as GRb1, AS-IV, AT-III, and Calycosin, can also regulate proliferation, apoptosis, angiogenesis, and glycolysis, and the targets of these monomers from WPX are not exactly same as those of WPX. In this comparison, TCM compounds change the mechanism of action of a single compound through the combination of a variety of TCMs and lead the creation of a new mechanism of action. This feature is precisely an advantage of TCM in treating GPL, as these medicines block its progression to GC, and fill the gap of Western medicine in treating GPL.
Clinical trials are one of the most reliable sources of evidence that guide medical practice. Current western medicine therapy for GPL generally includes the eradication of H. pylori, vitamin supplements, and other treatments[10]. However, for patients with advanced GPL, such as the IM stage, whether eradication of H. pylori have therapeutic effects remain controversial[125]. Compared with Western medicine, TCM has a curative effect at all stages of GPL. Currently, clinical trials have confirmed that TCM can block the progression of GPL to GC[126,127]. Taking WFC as an example, compared with vitacoenzyme (Vit), the total effective rates of the WFC and Vit groups in alleviating the degree of atrophy were 80.00% and 23.33%, respectively. The total effective rates of relieving IM in the WFC and Vit groups were 73.33% and 26.67%, respectively[16]. Notably, primary outcome measures, such as overall survival and 5-year survival rates, were employed in majority of these trials. These “head-to-head” trials demonstrated the efficacy of TCM in preventing the transformation of GPL to GC. Nevertheless, compared to the various mechanisms of TCM against GPL and GC reported by experiments, the development of relevant clinical trials is still insufficient. In the future, more attention should be paid to the development of clinical trials of GPL and GC with TCM.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng TH, Taiwan; Zhang GL, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Zhang C, Chen Z, Zhou X, Xu W, Wang G, Tang X, Luo L, Tu J, Zhu Y, Hu W, Xu X, Pan W. Cantharidin induces G(2)/M phase arrest and apoptosis in human gastric cancer SGC-7901 and BGC-823 cells. Oncol Lett. 2014;8:2721-2726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Shin VY, Ng EK, Chan VW, Kwong A, Chu KM. A three-miRNA signature as promising non-invasive diagnostic marker for gastric cancer. Mol Cancer. 2015;14:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11272] [Article Influence: 490.1] [Reference Citation Analysis (2)] |
| 5. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 6. | You WC, Li JY, Blot WJ, Chang YS, Jin ML, Gail MH, Zhang L, Liu WD, Ma JL, Hu YR, Mark SD, Correa P, Fraumeni JF Jr, Xu GW. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int J Cancer. 1999;83:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Kim YI, Park JY, Kim BJ, Hwang HW, Hong SA, Kim JG. Risk of metachronous gastric neoplasm occurrence during intermediate-term follow-up period after endoscopic submucosal dissection for gastric dysplasia. Sci Rep. 2020;10:6747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Lin Z, Luo M, Chen X, He X, Qian Y, Lai S, Si J, Chen S. Combined Detection of Plasma ZIC1, HOXD10 and RUNX3 Methylation is a Promising Strategy for Early Detection of Gastric Cancer and Precancerous Lesions. J Cancer. 2017;8:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Zhao S, Zhang X, Wang J, Ge J, Liu J. Endoscopic resection versus surgery for early gastric cancer and precancerous lesions: a meta-analysis. Springerplus. 2016;5:678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 11. | Dawsey SP, Hollenbeck A, Schatzkin A, Abnet CC. A prospective study of vitamin and mineral supplement use and the risk of upper gastrointestinal cancers. PLoS One. 2014;9:e88774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5763] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 13. | Zheng J, Cai W, Lu X, He W, Li D, Zhong H, Yang L, Li S, Li H, Rafee S, Zhao Z, Wang Q, Pan H. Chronic stress accelerates the process of gastric precancerous lesions in rats. J Cancer. 2021;12:4121-4133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Scotiniotis IA, Rokkas T, Furth EE, Rigas B, Shiff SJ. Altered gastric epithelial cell kinetics in Helicobacter pylori-associated intestinal metaplasia: implications for gastric carcinogenesis. Int J Cancer. 2000;85:192-200. [PubMed] |
| 15. | Bockerstett KA, DiPaolo RJ. Regulation of Gastric Carcinogenesis by Inflammatory Cytokines. Cell Mol Gastroenterol Hepatol. 2017;4:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Bian Y, Chen X, Cao H, Xie D, Zhu M, Yuan N, Lu L, Lu B, Wu C, Bahaji Azami NL, Wang Z, Wang H, Zhang Y, Li K, Ye G, Sun M. A correlational study of Weifuchun and its clinical effect on intestinal flora in precancerous lesions of gastric cancer. Chin Med. 2021;16:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Subotički T, Mitrović Ajtić O, Živković E, Diklić M, Đikić D, Tošić M, Beleslin-Čokić B, Dragojević T, Gotić M, Santibanez JF, Čokić V. VEGF Regulation of Angiogenic Factors via Inflammatory Signaling in Myeloproliferative Neoplasms. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457-465. [PubMed] |
| 19. | Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 492] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 20. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8320] [Article Influence: 489.4] [Reference Citation Analysis (0)] |
| 21. | Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1845] [Cited by in RCA: 1937] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 22. | Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3471] [Cited by in RCA: 4023] [Article Influence: 287.4] [Reference Citation Analysis (0)] |
| 23. | Cao Y. Tumor angiogenesis and molecular targets for therapy. Front Biosci (Landmark Ed). 2009;14:3962-3973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, Li X, Cao K, Deng H, He Y, Liao Q, Xiang B, Zhou M, Guo C, Zeng Z, Li G, Xiong W. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 25. | Zhong WR, Huang YX, Cui JP. [Clinical study on modified sijunzi decoction in treating intestinal metaplasia of gastric mucosa]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1997;17:462-464. [PubMed] |
| 26. | Gan D, Xu A, Du H, Ye Y. Chinese Classical Formula Sijunzi Decoction and Chronic Atrophic Gastritis: Evidence for Treatment Approach? Evid Based Complement Alternat Med. 2017;2017:9012929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Li YJ, Liao LL, Liu P, Tang P, Wang H, Peng QH. Sijunzi Decoction Inhibits Stemness by Suppressing β-Catenin Transcriptional Activity in Gastric Cancer Cells. Chin J Integr Med. 2022;28:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Qian J, Li J, Jia J, Jin X, Yu D, Guo C, Xie B, Qian L. DIFFERENT CONCENTRATIONS OF SIJUNZI DECOCTION INHIBIT PROLIFERATION AND INDUCE APOPTOSIS OF HUMAN GASTRIC CANCER SGC-7901 SIDE POPULATION. Afr J Tradit Complement Altern Med. 2016;13:145-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Yin J, Yi J, Yang C, Xu B, Lin J, Hu H, Wu X, Shi H, Fei X. Weiqi Decoction Attenuated Chronic Atrophic Gastritis with Precancerous Lesion through Regulating Microcirculation Disturbance and HIF-1α Signaling Pathway. Evid Based Complement Alternat Med. 2019;2019:2651037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Hao X, Liu Y, Zhou P, Jiang Q, Yang Z, Xu M, Liu S, Zhang S, Wang Y. Integrating Network Pharmacology and Experimental Validation to Investigate the Mechanisms of Huazhuojiedu Decoction to Treat Chronic Atrophic Gastritis. Evid Based Complement Alternat Med. 2020;2020:2638362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Zeng JH, Pan HF, Liu YZ, Xu HB, Zhao ZM, Li HW, Ren JL, Chen LH, Hu X, Yan Y. Effects of Weipixiao on Wnt pathway-associated proteins in gastric mucosal epithelial cells from rats with gastric precancerous lesions. Chin J Integr Med. 2016;22:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Wang H, Wu R, Xie D, Ding L, Lv X, Bian Y, Chen X, Nisma Lena BA, Wang S, Li K, Chen W, Ye G, Sun M. A Combined Phytochemistry and Network Pharmacology Approach to Reveal the Effective Substances and Mechanisms of Wei-Fu-Chun Tablet in the Treatment of Precancerous Lesions of Gastric Cancer. Front Pharmacol. 2020;11:558471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Zeng J, Ma X, Zhao Z, Chen Y, Wang J, Hao Y, Yu J, Zeng Z, Chen N, Zhao M, Tang J, Gong D. Ginsenoside Rb1 Lessens Gastric Precancerous Lesions by Interfering With β-Catenin/TCF4 Interaction. Front Pharmacol. 2021;12:682713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Brenes F, Ruiz B, Correa P, Hunter F, Rhamakrishnan T, Fontham E, Shi TY. Helicobacter pylori causes hyperproliferation of the gastric epithelium: pre- and post-eradication indices of proliferating cell nuclear antigen. Am J Gastroenterol. 1993;88:1870-1875. [PubMed] |
| 35. | Zhang Y, Kwok-Shing Ng P, Kucherlapati M, Chen F, Liu Y, Tsang YH, de Velasco G, Jeong KJ, Akbani R, Hadjipanayis A, Pantazi A, Bristow CA, Lee E, Mahadeshwar HS, Tang J, Zhang J, Yang L, Seth S, Lee S, Ren X, Song X, Sun H, Seidman J, Luquette LJ, Xi R, Chin L, Protopopov A, Westbrook TF, Shelley CS, Choueiri TK, Ittmann M, Van Waes C, Weinstein JN, Liang H, Henske EP, Godwin AK, Park PJ, Kucherlapati R, Scott KL, Mills GB, Kwiatkowski DJ, Creighton CJ. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell. 2017;31:820-832.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 451] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 36. | Bonizzi A, Truffi M, Sevieri M, Allevi R, Sitia L, Ottria R, Sorrentino L, Sottani C, Negri S, Grignani E, Mazzucchelli S, Corsi F. Everolimus Nanoformulation in Biological Nanoparticles Increases Drug Responsiveness in Resistant and Low-Responsive Breast Cancer Cell Lines. Pharmaceutics. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Wang Y, Chu F, Lin J, Li Y, Johnson N, Zhang J, Gai C, Su Z, Cheng H, Wang L, Ding X. Erianin, the main active ingredient of Dendrobium chrysotoxum Lindl, inhibits precancerous lesions of gastric cancer (PLGC) through suppression of the HRAS-PI3K-AKT signaling pathway as revealed by network pharmacology and in vitro experimental verification. J Ethnopharmacol. 2021;279:114399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Zhu F, Xu Y, Pan J, Li M, Chen F, Xie G. Epigallocatechin Gallate Protects against MNNG-Induced Precancerous Lesions of Gastric Carcinoma in Rats via PI3K/Akt/mTOR Pathway. Evid Based Complement Alternat Med. 2021;2021:8846813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Silva-García O, Valdez-Alarcón JJ, Baizabal-Aguirre VM. Wnt/β-Catenin Signaling as a Molecular Target by Pathogenic Bacteria. Front Immunol. 2019;10:2135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 40. | Seckin Y, Arici S, Harputluoglu M, Yonem O, Yilmaz A, Ozer H, Karincaoglu M, Demirel U. Expression of claudin-4 and beta-catenin in gastric premalignant lesions. Acta Gastroenterol Belg. 2009;72:407-412. [PubMed] |
| 42. | Chen Y, Henson ES, Xiao W, Huang D, McMillan-Ward EM, Israels SJ, Gibson SB. Tyrosine kinase receptor EGFR regulates the switch in cancer cells between cell survival and cell death induced by autophagy in hypoxia. Autophagy. 2016;12:1029-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 43. | Zhao E, Feng L, Bai L, Cui H. NUCKS promotes cell proliferation and suppresses autophagy through the mTOR-Beclin1 pathway in gastric cancer. J Exp Clin Cancer Res. 2020;39:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Cai T, Zhang C, Zhao Z, Li S, Cai H, Chen X, Cai D, Liu W, Yan Y, Xie K, Pan H, Zeng X. The gastric mucosal protective effects of astragaloside IV in mnng-induced GPL rats. Biomed Pharmacother. 2018;104:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Zhao B, Wang Y, Tan X, Ke K, Zheng X, Wang F, Lan S, Liao N, Cai Z, Shi Y, Zheng Y, Lai Y, Wang L, Li Q, Liu J, Huang A, Liu X. Inflammatory Micro-environment Contributes to Stemness Properties and Metastatic Potential of HCC via the NF-κB/miR-497/SALL4 Axis. Mol Ther Oncolytics. 2019;15:79-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 557] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 47. | Moser B, Hochreiter B, Basílio J, Gleitsmann V, Panhuber A, Pardo-Garcia A, Hoesel B, Salzmann M, Resch U, Noreen M, Schmid JA. The inflammatory kinase IKKα phosphorylates and stabilizes c-Myc and enhances its activity. Mol Cancer. 2021;20:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | Zheng C, Yin Q, Wu H. Structural studies of NF-κB signaling. Cell Res. 2011;21:183-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 49. | DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1230] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 50. | Liu K, Zhao F, Yan J, Xia Z, Jiang D, Ma P. Hispidulin: A promising flavonoid with diverse anti-cancer properties. Life Sci. 2020;259:118395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Agharahimi M, Badisa RB, Mazzio E, Soliman KF, Goodman CB. Cocaine potentiates an inflammatory response in C6 astroglia-like cells. Biomed Rep. 2021;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 52. | Yang H, Zhao H, Dong X, Yang Z, Chang W. Tomentosin induces apoptotic pathway by blocking inflammatory mediators via modulation of cell proteins in AGS gastric cancer cell line. J Biochem Mol Toxicol. 2020;34:e22501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Su T, Li F, Guan J, Liu L, Huang P, Wang Y, Qi X, Liu Z, Lu L, Wang D. Artemisinin and its derivatives prevent Helicobacter pylori-induced gastric carcinogenesis via inhibition of NF-κB signaling. Phytomedicine. 2019;63:152968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Han S, Yang S, Cai Z, Pan D, Li Z, Huang Z, Zhang P, Zhu H, Lei L, Wang W. Anti-Warburg effect of rosmarinic acid via miR-155 in gastric cancer cells. Drug Des Devel Ther. 2015;9:2695-2703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Sun J, Meng M. Chemoprotective Effect of Scutellarin against Gastric Cancer in Rats: An in vitro and in vivo Study. J Oleo Sci. 2022;71:1003-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 56. | Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 721] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 57. | Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res Int. 2015;2015:549412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 417] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 58. | Gao Y, Wang J, Zhao M, Xia T, Liu Q, Chen N, Liao W, Zeng Z, You F, Zeng J. Atractylenolide III Attenuates Angiogenesis in Gastric Precancerous Lesions Through the Downregulation of Delta-Like Ligand 4. Front Pharmacol. 2022;13:797805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Zeng J, Yan R, Pan H, You F, Cai T, Liu W, Zheng C, Zhao Z, Gong D, Chen L, Zhang Y. Weipixiao attenuate early angiogenesis in rats with gastric precancerous lesions. BMC Complement Altern Med. 2018;18:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1704] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 61. | Flaveny CA, Griffett K, El-Gendy Bel-D, Kazantzis M, Sengupta M, Amelio AL, Chatterjee A, Walker J, Solt LA, Kamenecka TM, Burris TP. Broad Anti-tumor Activity of a Small Molecule that Selectively Targets the Warburg Effect and Lipogenesis. Cancer Cell. 2015;28:42-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 62. | Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, Esumi H, Soga T. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918-4925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 775] [Article Influence: 48.4] [Reference Citation Analysis (1)] |
| 63. | Liu W, Pan HF, Yang LJ, Zhao ZM, Yuan DS, Liu YL, Lin LZ. Panax ginseng C.A. Meyer (Rg3) Ameliorates Gastric Precancerous Lesions in Atp4a(-/-) Mice via Inhibition of Glycolysis through PI3K/AKT/miRNA-21 Pathway. Evid Based Complement Alternat Med. 2020;2020:2672648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Cai T, Zhang C, Zeng X, Zhao Z, Yan Y, Yu X, Wu L, Lin L, Pan H. Protective effects of Weipixiao decoction against MNNG-induced gastric precancerous lesions in rats. Biomed Pharmacother. 2019;120:109427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Zhang C, Cai T, Zeng X, Cai D, Chen Y, Huang X, Gan H, Zhuo J, Zhao Z, Pan H, Li S. Astragaloside IV reverses MNNG-induced precancerous lesions of gastric carcinoma in rats: Regulation on glycolysis through miRNA-34a/LDHA pathway. Phytother Res. 2018;32:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 2520] [Article Influence: 420.0] [Reference Citation Analysis (0)] |
| 67. | Roussos ET, Keckesova Z, Haley JD, Epstein DM, Weinberg RA, Condeelis JS. AACR special conference on epithelial-mesenchymal transition and cancer progression and treatment. Cancer Res. 2010;70:7360-7364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 68. | Liao W, Wen Y, Wang J, Zhao M, Lv S, Chen N, Li Y, Wan L, Zheng Q, Mou Y, Zhao Z, Tang J, Zeng J. Gallic acid alleviates gastric precancerous lesions through inhibition of epithelial mesenchymal transition via Wnt/β-catenin signaling pathway. J Ethnopharmacol. 2023;302:115885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 69. | Li Y, Li T, Chen J, Zheng H, Li Y, Chu F, Wang S, Li P, Lin J, Su Z, Ding X. Manpixiao Decoction Halted the Malignant Transformation of Precancerous Lesions of Gastric Cancer: From Network Prediction to In-Vivo Verification. Front Pharmacol. 2022;13:927731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 70. | Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3672] [Cited by in RCA: 4507] [Article Influence: 643.9] [Reference Citation Analysis (0)] |
| 71. | Tao W, Li Y, Zhu M, Li C, Li P. LncRNA NORAD Promotes Proliferation And Inhibits Apoptosis Of Gastric Cancer By Regulating miR-214/Akt/mTOR Axis. Onco Targets Ther. 2019;12:8841-8851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 72. | Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, Muro K, Kim YH, Ferry D, Tebbutt NC, Al-Batran SE, Smith H, Costantini C, Rizvi S, Lebwohl D, Van Cutsem E. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 73. | Will M, Qin AC, Toy W, Yao Z, Rodrik-Outmezguine V, Schneider C, Huang X, Monian P, Jiang X, de Stanchina E, Baselga J, Liu N, Chandarlapaty S, Rosen N. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov. 2014;4:334-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 74. | Xu C, Huang X, Huang Y, Liu X, Wu M, Wang J, Duan X. Naringin induces apoptosis of gastric carcinoma cells via blocking the PI3K/AKT pathway and activating prodeath autophagy. Mol Med Rep. 2021;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Lee HJ, Venkatarame Gowda Saralamma V, Kim SM, Ha SE, Raha S, Lee WS, Kim EH, Lee SJ, Heo JD, Kim GS. Pectolinarigenin Induced Cell Cycle Arrest, Autophagy, and Apoptosis in Gastric Cancer Cell via PI3K/AKT/mTOR Signaling Pathway. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 76. | He JQ, Zhang SR, Li DF, Tang JY, Wang YQ, He X, Li YM, Wu H, Zhou M, Jiao J, Xiao PL. Experimental Study on the Effect of a Weifufang on Human Gastric Adenocarcinoma Cell Line BGC-823 Xenografts and PTEN Gene Expression in Nude Mice. Cancer Biother Radiopharm. 2020;35:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Wang Z, Tang T, Wang S, Cai T, Tao H, Zhang Q, Qi S, Qi Z. Aloin Inhibits the Proliferation and Migration of Gastric Cancer Cells by Regulating NOX2-ROS-Mediated Pro-Survival Signal Pathways. Drug Des Devel Ther. 2020;14:145-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 78. | Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr. Stat3 as an oncogene. Cell. 1999;98:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2236] [Cited by in RCA: 2345] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 79. | Hackermüller J, Reiche K, Otto C, Hösler N, Blumert C, Brocke-Heidrich K, Böhlig L, Nitsche A, Kasack K, Ahnert P, Krupp W, Engeland K, Stadler PF, Horn F. Cell cycle, oncogenic and tumor suppressor pathways regulate numerous long and macro non-protein-coding RNAs. Genome Biol. 2014;15:R48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Piao JY, Kim SJ, Kim DH, Park JH, Park SA, Han HJ, Na HK, Yoon K, Lee HN, Kim N, Hahm KB, Surh YJ. Helicobacter pylori infection induces STAT3 phosphorylation on Ser727 and autophagy in human gastric epithelial cells and mouse stomach. Sci Rep. 2020;10:15711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Liu YF, Lu YM, Qu GQ, Liu Y, Chen WX, Liao XH, Kong WM. Ponicidin induces apoptosis via JAK2 and STAT3 signaling pathways in gastric carcinoma. Int J Mol Sci. 2015;16:1576-1589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Tang X, Ding Q, Chen C, Chen F, Zhou X, Hong CJ, Pan W. Micheliolide inhibits gastric cancer growth in vitro and in vivo via blockade of the IL-6/STAT3 pathway. Pharmazie. 2019;74:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 83. | Crone SG, Jacobsen A, Federspiel B, Bardram L, Krogh A, Lund AH, Friis-Hansen L. microRNA-146a inhibits G protein-coupled receptor-mediated activation of NF-κB by targeting CARD10 and COPS8 in gastric cancer. Mol Cancer. 2012;11:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 84. | Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR, Marcusson EG, Karras JG, Na S, Sedgwick JD, Hertzog PJ, Jenkins BJ. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 85. | Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 3393] [Article Influence: 212.1] [Reference Citation Analysis (0)] |
| 86. | Tang S, Wang D, Zhang Q, Li L. miR-218 suppresses gastric cancer cell proliferation and invasion via regulation of angiopoietin-2. Exp Ther Med. 2016;12:3837-3842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 87. | Xu J, Zheng X, Cheng KK, Chang X, Shen G, Liu M, Wang Y, Shen J, Zhang Y, He Q, Dong J, Yang Z. NMR-based metabolomics Reveals Alterations of Electro-acupuncture Stimulations on Chronic Atrophic Gastritis Rats. Sci Rep. 2017;7:45580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Yang J, Wang Q, Qiao C, Lin Z, Li X, Huang Y, Zhou T, Li Y, Shen B, Lv M, Feng J. Potent anti-angiogenesis and anti-tumor activity of a novel human anti-VEGF antibody, MIL60. Cell Mol Immunol. 2014;11:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Li B, Qu G. Inhibition of the hypoxia-induced factor-1α and vascular endothelial growth factor expression through ginsenoside Rg3 in human gastric cancer cells. J Cancer Res Ther. 2019;15:1642-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Palazon A, Tyrakis PA, Macias D, Veliça P, Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk I, Hatschek T, Lövrot J, Foukakis T, Goldrath AW, Bergh J, Johnson RS. An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell. 2017;32:669-683.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 394] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 91. | Zhang Y, Coleman M, Brekken RA. Perspectives on Hypoxia Signaling in Tumor Stroma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 92. | Deng X, Liang X, Zhou X, Jiang M, Zhao X, Fu L, Liang M, Wang X, Liang J. Protective effect and mechanisms of Weining granule on N-methyl-N'-nitro-N- nitrosoguanidine-induced gastric cancer in rats. J Tradit Chin Med. 2019;39:393-401. [PubMed] |
| 93. | Ohshima K, Morii E. Metabolic Reprogramming of Cancer Cells during Tumor Progression and Metastasis. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 94. | Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 2456] [Article Influence: 175.4] [Reference Citation Analysis (0)] |
| 95. | Wu J, Zhang X, Wang Y, Sun Q, Chen M, Liu S, Zou X. Licochalcone A suppresses hexokinase 2-mediated tumor glycolysis in gastric cancer via downregulation of the Akt signaling pathway. Oncol Rep. 2018;39:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Chen F, Zhuang M, Zhong C, Peng J, Wang X, Li J, Chen Z, Huang Y. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1α signaling pathway. Oncol Rep. 2015;33:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 97. | Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1600] [Cited by in RCA: 1928] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 98. | Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1889] [Article Influence: 269.9] [Reference Citation Analysis (0)] |
| 99. | Wang H, Luo Y, Chu Z, Ni T, Ou S, Dai X, Zhang X, Liu Y. Poria Acid, Triterpenoids Extracted from Poria cocos, Inhibits the Invasion and Metastasis of Gastric Cancer Cells. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 100. | Liu J, Chen Y, Cao Z, Guan B, Peng J, Zhan Z, Sferra TJ, Sankararaman S, Lin J. Babao Dan inhibits the migration and invasion of gastric cancer cells by suppressing epithelial-mesenchymal transition through the TGF-β/Smad pathway. J Int Med Res. 2020;48:300060520925598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2905] [Article Influence: 207.5] [Reference Citation Analysis (0)] |
| 102. | Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20:1657-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 103. | Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front Immunol. 2020;11:609705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 104. | Lu X, Li Y, Yang W, Tao M, Dai Y, Xu J, Xu Q. Inhibition of NF-κB is required for oleanolic acid to downregulate PD-L1 by promoting DNA demethylation in gastric cancer cells. J Biochem Mol Toxicol. 2021;35:e22621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 105. | Zhuang H, Dai X, Zhang X, Mao Z, Huang H. Sophoridine suppresses macrophage-mediated immunosuppression through TLR4/IRF3 pathway and subsequently upregulates CD8(+) T cytotoxic function against gastric cancer. Biomed Pharmacother. 2020;121:109636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 106. | Kanojia D, Garg M, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9 is a novel biomarker for colorectal cancer and is involved in tumor growth and tumorigenicity. Am J Pathol. 2011;178:1009-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 107. | Tian SB, Yu JC, Liu YQ, Kang WM, Ma ZQ, Ye X, Yan C. MiR-30b suppresses tumor migration and invasion by targeting EIF5A2 in gastric cancer. World J Gastroenterol. 2015;21:9337-9347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 108. | Zhou Y, Xu Q, Shang J, Lu L, Chen G. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J Cell Physiol. 2019;234:17876-17885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 109. | Chen X, Yuan XN, Zhang Z, Gong PJ, Yin WN, Jiang Q, Xu J, Xu XL, Gao Y, Chen WL, Chen FF, Tian YH, Wei L, Zhang JW. Betulinic acid inhibits cell proliferation and migration in gastric cancer by targeting the NF-κB/VASP pathway. Eur J Pharmacol. 2020;889:173493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 110. | Cai H, Chen X, Zhang J, Wang J. 18β-glycyrrhetinic acid inhibits migration and invasion of human gastric cancer cells via the ROS/PKC-α/ERK pathway. J Nat Med. 2018;72:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 111. | Yan X, Rui X, Zhang K. Baicalein inhibits the invasion of gastric cancer cells by suppressing the activity of the p38 signaling pathway. Oncol Rep. 2015;33:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Chen W, Yu Y, Yang N, Zhu J, Li K, Li R, Su W, Luo L, Hu L, Chen G, Deng H. Effects of Yangzheng Sanjie Decoction-containing serum mediated by microRNA-7 on cell proliferation and apoptosis in gastric cancer. Oncol Lett. 2018;15:3621-3629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 113. | Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 114. | Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MW, Lu KH, Roach N, Limburg PJ; American Society of Clinical Oncology; European Society of Clinical Oncology. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015;33:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 115. | Tharmalingam N, Park M, Lee MH, Woo HJ, Kim HW, Yang JY, Rhee KJ, Kim JB. Piperine treatment suppresses Helicobacter pylori toxin entry in to gastric epithelium and minimizes β-catenin mediated oncogenesis and IL-8 secretion in vitro. Am J Transl Res. 2016;8:885-898. [PubMed] |
| 116. | Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 733] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 117. | Koulis A, Buckle A, Boussioutas A. Premalignant lesions and gastric cancer: Current understanding. World J Gastrointest Oncol. 2019;11:665-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 118. | Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 119. | Malik TH, Sayahan MY, Al Ahmed HA, Hong X. Gastric Intestinal Metaplasia: An Intermediate Precancerous Lesion in the Cascade of Gastric Carcinogenesis. J Coll Physicians Surg Pak. 2017;27:166-172. [PubMed] |
| 120. | Zeng Z, Nian Q, Chen N, Zhao M, Zheng Q, Zhang G, Zhao Z, Chen Y, Wang J, Zeng J, Gong D, Tang J. Ginsenoside Rg3 inhibits angiogenesis in gastric precancerous lesions through downregulation of Glut1 and Glut4. Biomed Pharmacother. 2022;145:112086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Aziz F, Wang X, Liu J, Yan Q. Ginsenoside Rg3 induces FUT4-mediated apoptosis in H. pylori CagA-treated gastric cancer cells by regulating SP1 and HSF1 expressions. Toxicol In Vitro. 2016;31:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 122. | Zhang W, Shu H, Fang L, Tang N, Li Y, Guo B, Meng F. Cancer inhibition mechanism of lung cancer mouse model based on dye trace method. Saudi J Biol Sci. 2020;27:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 123. | Cui X, Shen YM, Jiang S, Qian DW, Shang EX, Zhu ZH, Duan JA. Comparative analysis of the main active components and hypoglycemic effects after the compatibility of Scutellariae Radix and Coptidis Rhizoma. J Sep Sci. 2019;42:1520-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 124. | Dong LC, Fan YX, Yu Q, Ma J, Dong X, Li P, Li HJ. Synergistic effects of rhubarb-gardenia herb pair in cholestatic rats at pharmacodynamic and pharmacokinetic levels. J Ethnopharmacol. 2015;175:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Deng X, Liu ZW, Wu FS, Li LH, Liang J. A clinical study of weining granules in the treatment of gastric precancerous lesions. J Tradit Chin Med. 2012;32:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Li HZ, Wang H, Wang GQ, Liu J, Zhao SM, Chen J, Song QW, Gao W, Qi XZ, Gao Q. Treatment of gastric precancerous lesions with Weiansan. World J Gastroenterol. 2006;12:5389-5392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 127. | Wang J, Xu L, Shi R, Huang X, Li SW, Huang Z, Zhang G. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. 2011;83:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 128. | Pan X, Tao H, Nie M, Liu Y, Huang P, Liu S, Sun W, Wu J, Ma T, Dai A, Lu J, Liu B, Zou X, Sun Q. A clinical study of traditional Chinese medicine prolonging the survival of advanced gastric cancer patients by regulating the immunosuppressive cell population: A study protocol for a multicenter, randomized controlled trail. Medicine (Baltimore). 2020;99:e19757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 129. | Xu Y, Zhao AG, Li ZY, Zhao G, Cai Y, Zhu XH, Cao ND, Yang JK, Zheng J, Gu Y, Han YY, Zhu YJ, Yang JZ, Gao F, Wang Q. Survival benefit of traditional Chinese herbal medicine (a herbal formula for invigorating spleen) for patients with advanced gastric cancer. Integr Cancer Ther. 2013;12:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 130. | Shu P, Tang H, Zhou B, Wang R, Xu Y, Shao J, Qi M, Xia Y, Huang W, Liu S. Effect of Yiqi Huayu Jiedu decoction on stages II and III gastric cancer: A multicenter, prospective, cohort study. Medicine (Baltimore). 2019;98:e17875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Lv S, Chen X, Chen Y, Gong D, Mao G, Shen C, Xia T, Cheng J, Luo Z, Cheng Y, Li W, Zeng J. Ginsenoside Rg3 induces apoptosis and inhibits proliferation by down-regulating TIGAR in rats with gastric precancerous lesions. BMC Complement Med Ther. 2022;22:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 132. | Yang C, Du W, Yang D. Inhibition of green tea polyphenol EGCG((-)-epigallocatechin-3-gallate) on the proliferation of gastric cancer cells by suppressing canonical wnt/β-catenin signalling pathway. Int J Food Sci Nutr. 2016;67:818-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 133. | Fu JD, Yao JJ, Wang H, Cui WG, Leng J, Ding LY, Fan KY. Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state. Eur Rev Med Pharmacol Sci. 2019;23:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 134. | Geng X, Zhang X, Zhou B, Zhang C, Tu J, Chen X, Wang J, Gao H, Qin G, Pan W. Usnic Acid Induces Cycle Arrest, Apoptosis, and Autophagy in Gastric Cancer Cells In Vitro and In Vivo. Med Sci Monit. 2018;24:556-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 135. | Xu J, Wang Z, Huang Y, Wang Y, Xiang L, He X. A spirostanol saponin isolated from Tupistra chinensis Baker simultaneously induces apoptosis and autophagy by regulating the JNK pathway in human gastric cancer cells. Steroids. 2020;164:108737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 136. | Chen J, Shi DY, Liu SL, Zhong L. Tanshinone IIA induces growth inhibition and apoptosis in gastric cancer in vitro and in vivo. Oncol Rep. 2012;27:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 137. | Sun JY, Zhu MZ, Wang SW, Miao S, Xie YH, Wang JB. Inhibition of the growth of human gastric carcinoma in vivo and in vitro by swainsonine. Phytomedicine. 2007;14:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Li SG, Wang YY, Ye ZY, Shao QS, Tao HQ, Shu LS, Zhao YF, Yang YJ, Yang J, Peng T, Han B, Huang D. Proliferative and apoptotic effects of andrographolide on the BGC-823 human gastric cancer cell line. Chin Med J (Engl). 2013;126:3739-3744. [PubMed] |
| 139. | Liu G, Xiang T, Wu QF, Wang WX. Curcumin suppresses the proliferation of gastric cancer cells by downregulating H19. Oncol Lett. 2016;12:5156-5162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 140. | Lee HH, Lee S, Shin YS, Cho M, Kang H, Cho H. Anti-Cancer Effect of Quercetin in Xenograft Models with EBV-Associated Human Gastric Carcinoma. Molecules. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 141. | Saralamma VV, Nagappan A, Hong GE, Lee HJ, Yumnam S, Raha S, Heo JD, Lee SJ, Lee WS, Kim EH, Kim GS. Poncirin Induces Apoptosis in AGS Human Gastric Cancer Cells through Extrinsic Apoptotic Pathway by up-Regulation of Fas Ligand. Int J Mol Sci. 2015;16:22676-22691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 142. | Wang P, Jin JM, Liang XH, Yu MZ, Yang C, Huang F, Wu H, Zhang BB, Fei XY, Wang ZT, Xu R, Shi HL, Wu XJ. Helichrysetin inhibits gastric cancer growth by targeting c-Myc/PDHK1 axis-mediated energy metabolism reprogramming. Acta Pharmacol Sin. 2022;43:1581-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 143. | Zang MD, Hu L, Fan ZY, Wang HX, Zhu ZL, Cao S, Wu XY, Li JF, Su LP, Li C, Zhu ZG, Yan M, Liu BY. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J Transl Med. 2017;15:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 144. | Zhu J, Wen K. Astragaloside IV inhibits TGF-β1-induced epithelial-mesenchymal transition through inhibition of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother Res. 2018;32:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 145. | Zeng J, Guo J, Gong D, Zhang Y, You F, Liang C, Pan H, Cai T, Chen X, Chen L, Zhao Z. Weipixiao ameliorates gastric precancerous lesions in a rat's model by regulating GSK3¦Â and C-myc. J Tradit Chin Med. 2018;38:705-713. [PubMed] |
| 146. | Cai Y, Cao Y, Cheng S, Zou L, Yang T, Zhang Y, Shou Q, Chen B, Chen W. Study on the Mechanism of Sancao Tiaowei Decoction in the Treatment of MNNG-Induced Precancerous Lesions of Gastric Carcinoma Through Hedgehog Signaling Pathway. Front Oncol. 2022;12:841553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 147. | Hao X, Zhou P, Yang Z, Yang T, Wang Y. The therapeutic effect of Huazhuojiedu decoction on precancerous lesions in a gastric cancer model via the regulation of lnc 517368. J Ethnopharmacol. 2022;283:114635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 148. | Xu J, Shen W, Pei B, Wang X, Sun D, Li Y, Xiu L, Liu X, Lu Y, Zhang X, Yue X. Xiao Tan He Wei Decoction reverses MNNG-induced precancerous lesions of gastric carcinoma in vivo and vitro: Regulation of apoptosis through NF-κB pathway. Biomed Pharmacother. 2018;108:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 149. | Shen SW, Yuwen Y, Zhang ZL, Dong S, Liu JT, Wang XM. Effect of Jinguo Weikang Capsule on proto-oncogene expression of gastric mucosa in rats with gastric precancerous lesions. Chin J Integr Med. 2008;14:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 150. | Wang X, Xu J, Zhang X, Zhang C, Zheng W, Jiao J, Liu X, Yue X. Effects of Jinlongshe granules on gastric precancerous lesions in rats and its mechanism. Int J Clin Exp Pathol. 2020;13:846-853. [PubMed] |
| 151. | Yi Z, Jia Q, Lin Y, Wang Y, Cong J, Gu Z, Ling J, Cai G. Mechanism of Elian granules in the treatment of precancerous lesions of gastric cancer in rats through the MAPK signalling pathway based on network pharmacology. Pharm Biol. 2022;60:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 152. | Liu W, Zhao ZM, Liu YL, Pan HF, Lin LZ. Weipiling ameliorates gastric precancerous lesions in Atp4a(-/-) mice. BMC Complement Altern Med. 2019;19:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 153. | Fang SQ, Liu YH, Zhao KP, Zhang HX, Wang HW, Deng YH, Zhou YX, Ge GB, Ni HM, Chen QL. Transcriptional profiling and network pharmacology analysis identify the potential biomarkers from Chinese herbal formula Huosu Yangwei Formula treated gastric cancer in vivo. Chin J Nat Med. 2021;19:944-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 154. | Yuan M, Zou X, Liu S, Xu X, Wang H, Zhu M, Xie X, Wu J, Sun Q. Modified Jian-pi-yang-zheng decoction inhibits gastric cancer progression via the macrophage immune checkpoint PI3Kγ. Biomed Pharmacother. 2020;129:110440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 155. | Li L, Jin XJ, Li JW, Li CH, Zhou SY, Li JJ, Feng CQ, Liu DL, Liu YQ. Systematic insight into the active constituents and mechanism of Guiqi Baizhu for the treatment of gastric cancer. Cancer Sci. 2021;112:1772-1784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |