Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1699
Peer-review started: April 19, 2022
First decision: May 11, 2022
Revised: May 18, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: September 15, 2022
Processing time: 143 Days and 2.3 Hours
In colorectal cancer, tumor deposits (TDs) are considered to be a prognostic factor in the current staging system, and are only considered in the absence of lymph node metastases (LNMs). However, this definition and the subsequent prognostic value based on it is controversial, with various hypotheses. TDs may play an independent role when it comes to survival and addition of TDs to LNM count may predict the prognosis of patients more accurately.
To assess the prognostic impact of TDs and evaluate the effect of their addition to the LNM count.
The patients are derived from the Surveillance, Epidemiology, and End Results database. A prognostic analysis regarding impact of TDs on overall survival (OS) was performed using Cox regression model, and other covariates associating with OS were adjusted. The effect of addition of TDs to LNM count on N restaging was also evaluated. The subgroup analysis was performed to explore the different profile of risk factors between patients with and without TDs.
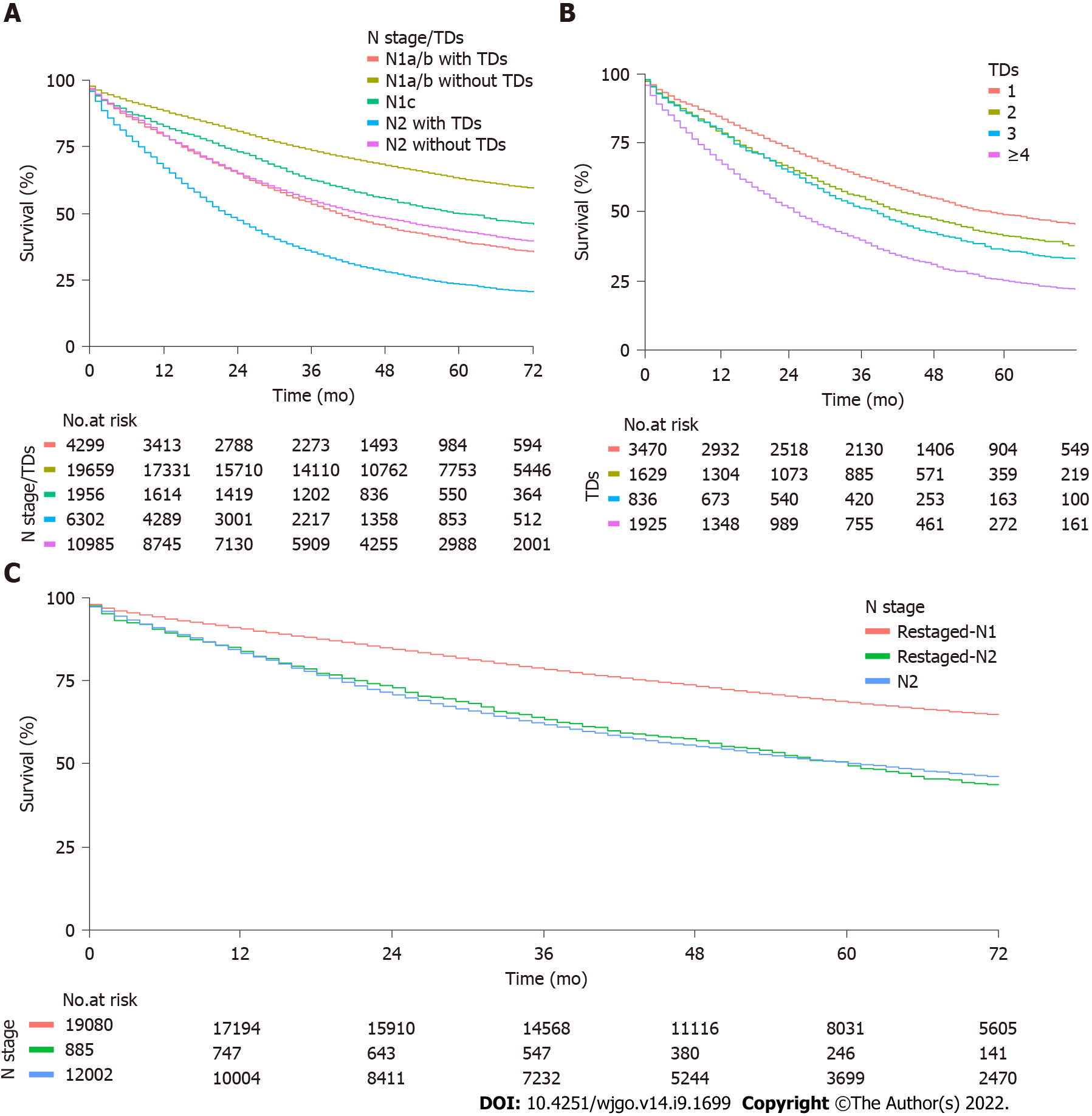
Overall, 103755 patients were enrolled with 14131 (13.6%) TD-positive and 89624 (86.4%) TD-negative tumors. TD-positive patients had worse prognosis compared with TD-negative patients, with 3-year OS rates of 47.3% (95%CI, 46.5%-48.1%) and 77.5% (95%CI, 77.2%-77.8%, P < 0.0001), respectively. On multivariable analysis, TDs were associated poorer OS (hazard ratio, 1.35; 95%CI, 1.31-1.38; P < 0.0001). Among TD-positive patients, the number of TDs had a linear negative effect on disease-free survival and OS. After reclassifying patients by adding TDs to the LNM count, 885 of 19 965 (4.4%) N1 patients were restaged as pN2, with worse outcomes than patients restaged as pN1 (3-year OS rate: 78.5%, 95%CI, 77.9%-79.1% vs 63.2%, 95%CI, 60.1%-66.5%, respectively; P < 0.0001).
TDs are an independent prognostic factor for OS in colorectal cancer. The addition of TDs to LNM count improved the prognostic accuracy of tumor, node and metastasis staging.
Core Tip: We evaluated the predictive value of tumor deposits (TDs) for overall survival (OS) in patients with colorectal cancer based on a collection of 103755 patients derived from Surveillance, Epidemiology, and End Results database, including TD-negative and TD-positive subpopulations with Cox proportional hazard model. The sensitivity analyses were performed to detect outcome robustness. TD was an independent prognostic factor for OS. We also performed exploratory analysis to evaluate the effect of TD addition to the lymph node metastases count in tumor, node and metastasis-stage III subpopulations. The outcomes of subgroup analysis investigating the different risk factor profiles indicated that TDs may affect survival through more than one approach.
- Citation: Wu WX, Zhang DK, Chen SX, Hou ZY, Sun BL, Yao L, Jie JZ. Prognostic impact of tumor deposits on overall survival in colorectal cancer: Based on Surveillance, Epidemiology, and End Results database. World J Gastrointest Oncol 2022; 14(9): 1699-1710
- URL: https://www.wjgnet.com/1948-5204/full/v14/i9/1699.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i9.1699
Colorectal cancer was the third most common cancer and second leading cause of death among all types of cancer with 1.93 million new cases and 0.94 million deaths in 2020[1]. The key point of treatment for colorectal cancer is to determine the stage on which we depend when carrying out treatment strategies. The American Joint Committee on Cancer (AJCC) tumor, node and metastasis (TNM) staging system is the standard tool for staging. Staging systems for colorectal cancer are evolving as more information regarding predictors of outcome emerges; among which, tumor deposits (TDs) have been debated and investigated. Previous studies have shown that TDs are associated with poor survival and earlier development of metastasis[2-4]. However, the definition and prognostic value of TDs remains controversial. TDs first appeared in the fifth edition of TNM staging system in 1997 and the definition of TDs has been evolving since then. The distinction of a TD from involved lymph nodes (LNs) has progressed from a reliance on size, to contours, to only features of residual LN structure[2,5]. The latest TNM 8th staging system was released in 2016, aiming to exclude any lesion with identifiable structures pointing towards LN metastasis (LNM), extramural venous invasion or perineural invasion[6]. However, some researchers have proposed that nodules with evidence of origin should still be categorized as TDs and the exclusion of lesions of vascular, lymphatic and perineural origin by TNM 8th has no evidence base[7,8]. Another controversial issue is the introduction of a new category of N1c in the TNM staging system. In the 7th edition, if TDs are observed with lesions that would otherwise be classified as T1 or T2, then T classification is not changed but nodules are recorded as N1c in the absence of LN involvement. The prognostic value of N1c remains unclear. Some researchers suggest that TDs should be taken into consideration for N staging, while others propose that N1c is not by definition worse than N1a or N1b and the use of N1c was chosen because the letter c was the subsequent letter in the alphabet[5]. The post hoc analyses of the IDEA France and GALGB/SWOG 80702 studies have suggested addition of TDs to the LNM count. The results of these studies require validation, as the potential bias may derive from the post hoc analysis and some information related to the analysis was not recorded in the primary clinical trial. Moreover, the outcomes of these study could only represent a part of patients due to the rigorous inclusion criteria. As a result of these controversies and the fact that the TNM stage can affect the therapeutic decision, this analysis aimed to assess the prognostic impact of TDs in colorectal cancer and to evaluate the effect of their addition to the LNM count.
The patients in the current study are derived from the Surveillance, Epidemiology, and End Results (SEER) database (November 2020). We enrolled patients diagnosed with colorectal cancer between 2010 and 2015. SEER used a study cutoff date for data submission and the study cutoff was 12/31/2018 for the November 2020 data submission. All deaths up to this point had been recorded in the data through death clearance linkages. The survival time was recorded as the interval between the time of diagnosis and the date of last contact. For cancer registries that did not conduct active patient follow-up, the presumed-alive method was used by which the survival time was calculated based on the assumption that the registry has ascertained all available deaths, and persons not known to be deceased were presumed to be alive on the last date for which complete death ascertainment was available. The inclusion criteria were: histological confirmed colorectal cancer, malignant behavior, known age, without other in situ or malignant tumors. Exclusion criteria were: patients without available TNM stage, TDs indeterminate or not documented, last contact date was the date of diagnosis, and survival time not documented.
The histopathological characteristics, including survival months, survival status, race, tumor site, carcinoembryonic antigen (CEA), perineural invasion, sex, age, TNM stage, liver metastasis, lung metastasis and TDs, were derived from the SEER database. Patients were allocated into White, Black and Others according to race. Tumor site was reclassified as colon and rectum. Age was pooled into three groups of < 45, 45-75 and ≥ 75 years. Patients were divided into two categories based on the presence or absence of TDs. The TNM stage for patients was derived from the 7th AJCC TNM staging system. The outcome included OS, defined as the time from diagnosis to any cause of death, and patients lost to follow-up were treated as censored, which is equivalent to the record of survival months derived from the SEER database. This study was based on the public data derived from SEER database in which the private information related to patients was not available. Therefore, this study was exempt from institutional review board approval and informed consent.
The primary objective of the current study was to assess the association between the presence of TDs and OS. As exploratory outcomes, the impact of number of TDs on OS was investigated in patients with available record for number of tumor deposits and the N stage was reclassified to the novel N category by the addition of TDs to the LNM count. A score of 2 was assigned for the number of LNMs of cases with stage N1b. Finally, survival was estimated according to this reclassification.
Continuous and categorical variables were summarized as median values with interquartile ranges and frequencies with percentages. Proportions were compared using the χ2 test. Cox proportional hazards models were performed to estimate hazard ratio (HR) and 95%CIs for factors associated with OS. Parameters with P < 0.1 in the univariable Cox analysis were entered into a final multivariable Cox regression model including TDs, with stepwise selection for both directions with respect to collinearity among covariates after excluding variables with > 10% missing data. To assess robustness of the association between TDs and OS evaluated in the primary Cox multivariable analysis, multiple imputation was performed to limit the bias as a result of missing data for sensitivity analysis. With regard to potential heterogeneity between patients with and without TDs, a propensity score approach with inverse probability of treatment weighting (IPTW) method was applied. Survival curves were constructed using the Kaplan–Meier method. Curves adjusted for covariates associated with OS in Cox regression model were also performed. The difference of HRs between subgroups was tested[9]. The statistical methods were reviewed by Wen-Quan Niu from the Institute of Clinical Medical Science of the China–Japan Friendship Hospital.
Using data from 18 SEER registries between 2010 and 2015, 162328 patients were diagnosed with colorectal cancer and 103755 patients were enrolled in the current study. Baseline characteristics with respect to the presence or absence of TDs are listed in Table 1: 14131 patients (13.6%) had TDs and 89624 patients (86.4%) had no TDs. Patients with TDs were more likely to have advanced-stage tumors (linear-by-linear association P < 0.0001). Similar trends were also observed as for T-stage and N-stage. Patients with TDs had more extensive T-stage and higher nodal stage. In the TD-positive subpopulation, patients had more metastatic disease including liver (25.7% vs 7.2% in TD-negative patients; P < 0.001) and lungs (6.3% vs in 1.7% TD-negative patients; P < 0.001), more perineural invasion (33.0% vs 8.2% in TD-negative patients; P < 0.001) and elevated CEA (40.4% vs 23.4% in TD-negative patients; P < 0.001). The presence of TDs was associated with tumors in the colon and in younger patients (Table 1).
| Tumor deposits | P value | ||
| No (89624) | Yes (14131) | ||
| Sex | 0.827 | ||
| Female | 43569 (48.6%) | 6855 (48.5%) | |
| Male | 46055 (51.4%) | 7276 (51.5%) | |
| Race | 0.420 | ||
| White | 70019 (78.1%) | 11015 (77.9%) | |
| Black | 10345 (11.5%) | 1681 (11.9%) | |
| Others | 9260 (10.3%) | 1435 (10.2%) | |
| Age group, yr | < 0.001a | ||
| < 45 | 5740 (6.40%) | 1231 (8.71%) | |
| 45-75 | 58860 (65.7%) | 9387 (66.4%) | |
| ≥ 75 | 25024 (27.9%) | 3513 (24.9%) | |
| TNM-stage | < 0.001a | ||
| Ι | 24816 (27.7%) | 111 (0.79%) | |
| ΙΙ | 29374 (32.8%) | 825 (5.84%) | |
| ΙΙΙ | 26627 (29.7%) | 7697 (54.5%) | |
| ΙV | 8807 (9.83%) | 5498 (38.9%) | |
| T-stage | < 0.001a | ||
| T1 | 15480 (17.3%) | 181 (1.28%) | |
| T2 | 14312 (16.0%) | 482 (3.41%) | |
| T3 | 47654 (53.2%) | 7890 (55.8%) | |
| T4 | 12178 (13.6%) | 5578 (39.5%) | |
| N-stage | < 0.001a | ||
| N0 | 56512 (63.1%) | 1247 (8.82%) | |
| N1 | 22127 (24.7%) | 6582 (46.6%) | |
| N2 | 10985 (12.3%) | 6302 (44.6%) | |
| M-stage | < 0.001a | ||
| M0 | 80817 (90.2%) | 8633 (61.1%) | |
| M1 | 8807 (9.83%) | 5498 (38.9%) | |
| Liver metastasis | < 0.001a | ||
| No | 82821 (92.4%) | 10377 (73.4%) | |
| Yes | 6443 (7.19%) | 3627 (25.7%) | |
| Unknown | 360 (0.40%) | 127 (0.90%) | |
| Lung metastasis | < 0.001a | ||
| No | 87677 (97.8%) | 13053 (92.4%) | |
| Yes | 1526 (1.70%) | 893 (6.32%) | |
| Unknown | 421 (0.47%) | 185 (1.31%) | |
| Site | < 0.001a | ||
| Colon | 71779 (80.1%) | 11697 (82.8%) | |
| Rectum | 16603 (18.5%) | 2148 (15.2%) | |
| Unknown | 1242 (1.39%) | 286 (2.02%) | |
| CEA | < 0.001a | ||
| Normal | 32166 (35.9%) | 3543 (25.1%) | |
| Elevated | 20936 (23.4%) | 5705 (40.4%) | |
| Borderline | 279 (0.31%) | 48 (0.34%) | |
| Unknown | 36243 (40.4%) | 4835 (34.2%) | |
| Perineural invasion | < 0.001a | ||
| Negative | 75025 (83.7%) | 8437 (59.7%) | |
| Positive | 7301 (8.15%) | 4659 (33.0%) | |
| Unknown | 7298 (8.14%) | 1035 (7.32%) | |
The median overall follow-up was 68 (31.0-74.0) mo. Median OS was 34.0 (33.0–36.0) mo for TD-positive patients and not reached in the TD-negative patients. According to the presence or absence of TDs, TD-positive patients had a worse prognosis than TD-negative patients. The 3-year OS rates were 47.3% (95%CI, 46.5%-48.1%) and 77.5% (95%CI, 77.2%-77.8%, log rank P < 0.0001), respectively. The negative effect of TDs on OS was observed for both N1 and N2 subgroups. Three-year OS rates for N1a/b patients with or without TDs were 53.5% (95%CI, 52.0%-55.0%) and 73.6% (95%CI, 73.0%-74.2%, log rank P < 0.0001), respectively. For N2 patients with or without TDs, 3-year OS rates were 35.5% (95%CI, 34.3%-36.7%) and 54.7% (95%CI, 53.7%-55.6%, log rank P < 0.0001) (Figure 1A).
In a univariable Cox model, the presence of TDs was associated with poor OS (HR, 2.73; 95%CI, 2.67-2.80; P < 0.0001). Other variables significantly associated with OS were TNM, T, N, M, race, age, tumor site, CEA, perineural invasion, liver metastasis and lung metastasis. In multivariable analysis including TNM-stage, T-stage, N-stage, TDs, liver metastasis, lung metastasis, age, perineural invasion and race, the negative prognostic impact of TD remained significant (HR, 1.35; 95%CI, 1.31-1.38; P < 0.0001) (Table 2). Because of unavailable records, 10291 patients were excluded in a multivariable Cox model analysis and the factor CEA with 39.6% missing data was excluded. The analysis outcome for the complete dataset was robust with multiple imputation (HR, 1.39; 95%CI, 1.35-1.42; P < 0.0001) and propensity score approach with IPTW method (HR, 1.29; 95%CI, 1.19-1.39; P < 0.0001) (Table 3). After adjusting for other covariates, the HR value of TDs was lowered. In the subgroup analysis, T-stage, N-stage, M-stage, CEA, perineural invasion, liver metastasis and lung metastasis were associated with poor OS both in patients with and without TDs, but these risk factors had less impact on survival in patients with than those without TDs, which may partly explain the lower HR value in multivariable analysis (Table 4).
| Univariate Cox models | Multivariate Cox model | |||||
| Events/total | HR (95%CI) | P value | Events/total | HR (95%CI) | P value | |
| Sex | 0.3665 | |||||
| Female | 19008/50424 | Reference | ||||
| Male | 20358/53331 | 1.01 (0.99-1.03) | 0.3665 | |||
| Race | < 0.0001a | < 0.0001a | ||||
| White | 30958/81034 | Reference | 27279/73091 | Reference | ||
| Black | 5049/12026 | 1.14 (1.11-1.17) | < 0.0001a | 4440/10821 | 1.19 (1.16-1.23) | < 0.0001a |
| Others | 3359/10695 | 0.80(0.77-0.83) | <.0001a | 2926/9552 | 0.83(0.80-0.86) | <.0001a |
| Age group, yr | < 0.0001a | < 0.0001a | ||||
| < 45 | 1866/6971 | Reference | 1627/6276 | Reference | ||
| 45-75 | 20956/68247 | 1.17 (1.11-1.22) | < 0.0001a | 18394/61529 | 1.49 (1.42-1.57) | < 0.0001a |
| ≥ 75 | 16544/28537 | 2.78 (2.65-2.92) | < 0.0001a | 14624/25659 | 4.40 (4.17-4.63) | < 0.0001a |
| TNM-stage | < 0.0001a | < 0.0001a | ||||
| Ι | 4709/24927 | Reference | 4154/22321 | Reference | ||
| ΙΙ | 9334/30199 | 1.79 (1.73-1.85) | < 0.0001a | 8362/27688 | 0.96 (0.89-1.02) | 0.2060 |
| ΙΙΙ | 13498/34324 | 2.46 (2.38-2.55) | < 0.0001a | 12086/31183 | 1.32 (1.21-1.43) | < 0.0001a |
| ΙV | 11825/14305 | 9.08 (8.77-9.40) | < 0.0001a | 10043/12272 | 3.27 (2.99-3.56) | < 0.0001a |
| T-stage | < 0.0001a | < 0.0001a | ||||
| T1 | 2794/15661 | Reference | 2259/13476 | Reference | ||
| T2 | 3477/14794 | 1.35 (1.29-1.42) | < 0.0001a | 3167/13728 | 1.18 (1.12-1.25) | < 0.0001a |
| T3 | 21562/55544 | 2.52 (2.42-2.62) | < 0.0001a | 19171/50529 | 1.71 (1.59-1.83) | < 0.0001a |
| T4 | 11533/17756 | 5.80 (5.57-6.05) | < 0.0001a | 10048/15731 | 2.83 (2.63-3.05) | < 0.0001a |
| N-stage | < 0.0001a | < 0.0001a | ||||
| N0 | 16014/57759 | Reference | 14081/52146 | Reference | ||
| N1 | 12199/28709 | 1.73 (1.69-1.78) | < 0.0001a | 10718/25885 | 0.96 (0.91-1.02) | 0.1880 |
| N2 | 11153/17287 | 3.38 (3.30-3.47) | < 0.0001a | 9846/15433 | 1.44 (1.36-1.53) | < 0.0001a |
| M-stage | < 0.0001a | |||||
| M0 | 27541/89450 | Reference | ||||
| M1 | 11825/14305 | 5.05 (4.94-5.16) | < 0.0001a | |||
| Liver metastasis | < 0.0001a | < 0.0001a | ||||
| No | 30670/93198 | Reference | 27418/84728 | Reference | ||
| Yes | 8406/10070 | 4.67(4.56-4.79) | < 0.0001a | 7227/8736 | 1.33(1.28-1.39) | < 0.0001a |
| Lung metastasis | < 0.0001a | < 0.0001a | ||||
| No | 36847/100730 | Reference | 32838/91390 | Reference | ||
| Yes | 2119/2419 | 4.62 (4.42-4.83) | < 0.0001a | 1807/2074 | 1.32 (1.25-1.38) | < 0.0001a |
| Site | < 0.0001a | |||||
| Colon | 32774/83476 | Reference | ||||
| Rectum | 5782/18751 | 0.71 (0.69-0.73) | < 0.0001a | |||
| CEA | < 0.0001a | |||||
| Normal | 9708/35709 | Reference | ||||
| Elevated | 14098/26641 | 2.46 (2.40-2.53) | < 0.0001a | |||
| Borderline | 125/327 | 1.51 (1.26-1.80) | 0.0001a | |||
| Perineural invasion | < 0.0001a | < 0.0001a | ||||
| Negative | 28478/83462 | Reference | 27655/81868 | Reference | ||
| Positive | 7260/11960 | 2.32 (2.26-2.38) | < 0.0001a | 6990/11596 | 1.22 (1.19-1.26) | < 0.0001a |
| Tumor deposits | < 0.0001a | < 0.0001a | ||||
| No | 30165/89624 | Reference | 26523/80813 | Reference | ||
| Yes | 9201/14131 | 2.73 (2.67-2.80) | < 0.0001a | 8122/12651 | 1.35 (1.31-1.38) | < 0.0001a |
| TDs (no) | TDs (yes) | RHR | P value | |||||
| Events/total | HR (95%CI) | P value | Events/total | HR (95%CI) | P value | |||
| Sex | 0.1265 | 0.0528 | ||||||
| Female | 14534/43569 | Reference | 4474/6855 | Reference | ||||
| Male | 15631/46055 | 1.02 (1.00-1.04) | 0.1265 | 4727/7276 | 0.96 (0.92-1.00) | 0.0528 | ||
| Race | < 0.0001a | < 0.0001 | ||||||
| White | 23736/70019 | Reference | 7222/11015 | Reference | ||||
| Black | 3904/10345 | 1.16 (1.12-1.20) | < 0.0001a | 1145/1681 | 1.06 (1.00-1.13) | 0.0536 | 0.91 (0.85-0.98) | 0.0118a |
| Others | 2525/9260 | 0.79 (0.76-0.82) | < 0.0001a | 834/1435 | 0.83 (0.77-0.89) | < 0.0001a | 1.05 (0.97-1.14) | 0.2380 |
| Age group, yr | < 0.0001a | < 0.0001a | ||||||
| < 45 | 1201/5740 | Reference | 665/1231 | Reference | ||||
| 45-75 | 15238/58860 | 1.26 (1.19-1.34) | < 0.0001a | 5718/9387 | 1.23 (1.13-1.33) | < 0.0001a | 0.98 (0.88-1.08) | 0.6384 |
| ≥ 75 | 13726/25024 | 3.37 (3.18-3.58) | < 0.0001a | 2818/3513 | 2.34 (2.15-2.55) | < 0.0001a | 0.69 (0.63-0.77) | < 0.0001a |
| TNM-stage | < 0.0001a | < 0.0001a | ||||||
| Ι | 4683/24816 | Reference | 26/111 | Reference | ||||
| ΙΙ | 8962/29374 | 1.77 (1.70-1.83) | < 0.0001a | 372/825 | 2.40 (1.61-3.57) | < 0.0001a | 1.36 (0.91-2.02) | 0.1362 |
| ΙΙΙ | 9545/26627 | 2.17 (2.09-2.24) | < 0.0001a | 3953/7697 | 3.05 (2.08-4.49) | < 0.0001a | 1.41 (0.96-2.07) | 0.0836 |
| ΙV | 6975/8807 | 8.05 (7.76-8.36) | < 0.0001a | 4850/5498 | 9.25 (6.29-13.6) | < 0.0001a | 1.15 (0.78-1.69) | 0.4840 |
| T-stage | < 0.0001a | < 0.0001a | ||||||
| T1 | 2726/15480 | Reference | 68/181 | Reference | ||||
| T2 | 3321/14312 | 1.35 (1.29-1.42) | < 0.0001a | 156/482 | 0.84 (0.63-1.11) | 0.2246 | 0.62 (0.47-0.83) | 0.0012a |
| T3 | 17029/47654 | 2.30 (2.20-2.39) | < 0.0001a | 4533/7890 | 1.84 (1.45-2.34) | < 0.0001a | 0.80 (0.63-1.02) | 0.0602 |
| T4 | 7089/12178 | 4.84 (4.63-5.05) | < 0.0001a | 4444/5578 | 3.69 (2.90-4.68) | < 0.0001a | 0.76 (0.60-0.97) | 0.0286a |
| N-stage | < 0.0001a | < 0.0001a | ||||||
| N0 | 15350/56512 | Reference | 664/1247 | Reference | ||||
| N1 | 8434/22127 | 1.53 (1.49-1.57) | < 0.0001a | 3765/6582 | 1.14 (1.05-1.24) | 0.0014a | 0.75 (0.68-0.81) | < 0.0001a |
| N2 | 6381/10985 | 2.86 (2.77-2.94) | < 0.0001a | 4772/6302 | 1.99 (1.83-2.16) | < 0.0001a | 0.70 (0.64-0.76) | < 0.0001a |
| M-stage | < 0.0001a | < 0.0001a | ||||||
| M0 | 23190/80817 | Reference | 4351/8633 | Reference | ||||
| M1 | 6975/8807 | 4.90 (4.77-5.04) | < 0.0001a | 4850/5498 | 3.13 (3.00-3.27) | < 0.0001a | 0.64 (0.61-0.67) | < 0.0001a |
| Site | < 0.0001a | < 0.0001a | ||||||
| Colon | 24983/71779 | Reference | 7791/11679 | Reference | ||||
| Rectum | 4595/16603 | 0.74 (0.71-0.76) | < 0.0001a | 1187/2148 | 0.66 (0.62-0.71) | < 0.0001a | 0.89 (0.83-0.96) | 0.0030a |
| CEA | < 0.0001a | < 0.0001a | ||||||
| Normal | 7926/32166 | Reference | 1782/3543 | Reference | ||||
| Elevated | 9908/20936 | 2.34 (2.27-2.41) | < 0.0001a | 4190/5705 | 1.95 (1.84-2.06) | < 0.0001a | 0.83 (0.78-0.89) | < 0.0001a |
| Borderline | 95/279 | 1.47 (1.20-1.80) | 0.0002a | 30/48 | 1.32 (0.92-1.89) | 0.1343 | 0.90 (0.59-1.36) | 0.6100 |
| Perineural invasion | < 0.0001a | < 0.0001a | ||||||
| Negative | 23417/75025 | Reference | 5061/8437 | Reference | ||||
| Positive | 3845/7301 | 2.05 (1.98-2.12) | < 0.0001a | 3415/4659 | 1.46 (1.40-1.52) | < 0.0001a | 0.71 (0.68-0.75) | < 0.0001a |
| Liver metastasis | < 0.0001a | < 0.0001a | ||||||
| No | 24811/82821 | Reference | 5859/10377 | Reference | ||||
| Yes | 5164/6443 | 4.73 (4.59-4.88) | < 0.0001a | 3242/3627 | 2.66 (2.54-2.78) | < 0.0001a | 0.56 (0.53-0.59) | < 0.0001a |
| Lung metastasis | < 0.0001a | < 0.0001a | ||||||
| No | 28622/87677 | Reference | 8225/13053 | Reference | ||||
| Yes | 1297/1526 | 4.97 (4.70-5.25) | < 0.0001a | 822/893 | 2.40 (2.23-2.58) | < 0.0001a | 0.48 (0.44-0.53) | < 0.0001a |
In the exploratory analysis, there were 7860 patients with records of numbers of TDs. Among these, the number of TDs was subdivided into four groups with 1, 2, 3 and ≥ 4 TDs. The 3-year OS rates were 62.8% (95%CI, 61.2%–64.5%), 55.6% (95%CI, 53.2%–58.1%), 51.6%, (95%CI, 48.3%-55.1%), and 39.7% (95%CI, 37.6%–42.0%; P < 0.0001), respectively (Figure 1B). The 3-year OS rates were linearly associated with the number of TDs (P for trend < 0.0001).
There were 19965 N1-staged patients with records of numbers of TDs, in TNM-stage III subpopulations. Among these, 885 were restaged as N2 by the addition of TDs to the LNM count (Table 5). Patients with tumors restaged as N2 had a lower 3-year OS rate than those with tumors remaining as N1 despite the addition of TDs to the LNM count (78.5%, 95%CI, 77.9%-79.1% vs 63.2%, 95%CI, 60.1%-66.5%, respectively; P < 0.0001). OS was not different between patients restaged as N2 and those initially staged as N2 (63.2%, 95%CI, 60.1%-66.5% vs 61.7%, 95%CI, 60.8%-62.6%, respectively; P = 0.8) (Figure 1C).
| Initial N stage | Restaged N1 | Restaged N2 | Total |
| N1a/b | 18077 | 752 | 18820 |
| N1c | 1003 | 133 | 1136 |
| Total | 19080 | 885 | 19965 |
In the current TNM staging system for colorectal cancer, neither the presence nor the number of TDs is considered in the N staging in case of concomitant LNM, and the N1c category is only used if no LNM is present.
Our study demonstrated that the presence of TDs was associated with significantly poorer survival outcomes and the negative impact of TDs remained significant across all N stages, indicating that TDs should be considered when performing N staging. The number of TDs had a linear effect on OS. Thus, valuable prognostic information is lost when ignoring the number of TDs. Given the prognostic value of TDs both qualitatively and quantitatively, we went further in our analysis by adding the number of TDs to the LNM count. The current study is, to our knowledge, the largest comparative effectiveness research to investigate reclassification of the TNM staging system by incorporation of TDs into the LNM count. We showed that N1-staged patients who were reclassified as N2 through the integration of the number of TDs into LNM count had poorer outcomes than those who remained as N1, despite the addition of TDs to the LNM count and outcomes similar to those of patients initially staged as N2. Therefore, our results, in agreement with other studies[3,4,10], suggest that both TDs and their numbers should be integrated into N staging and that the N1c category in TNM staging was inappropriate because there were subpopulations with ≥ 4 TDs whose survival was similar to that in patients with ≥ 4 LNMs. Moreover, the results were similar in subgroup analysis when considering the different tumor sites. Our study is, to our knowledge, the first to investigate the outcomes of reclassification in patients with rectal cancer.
Advanced TNM stage, extensive T-stage, higher nodal stage, metastatic disease, perineural invasion and elevated CEA were more often present among TD-positive patients. Although these correlations may partly explain the pejorative prognosis of TD-positive tumors, the poor prognostic value of TDs remains when the imbalance of these covariates is taken into account in the propensity score approach analysis. The different HR values of these covariates between TD-positive and -negative subpopulations remain to be clarified, which may indicate more than one way through which TDs influence survival[10]. In light of these results, we propose that the presence of TDs is an independent prognostic factor for OS in colorectal cancer and the origin and formation of TDs need to be further investigated.
Although there were multiple origins reported in previous studies of TDs, including perineural, perivascular, intravascular and a mixture of them[10-13], the definition of TDs is still ambiguous with regard to the inclusion of recognized structures of vascular, lymphatic and perineural TDs[8]. The hypotheses of mechanisms through which the TDs affect survival are diverse. A previous study demonstrated that TD-positive patients was more likely to present vascular and perineural invasion[14]. Certain groups showed that the prognostic value of TDs and extra nodal extension of which the negative effect towards survival has been demonstrated previously was similar with regard to HR values for OS and DFS. Thus, some researchers suggested that TDs could be complete replacement of an lymph node by metastatic tumor and represent the advanced stage of extra nodal extension[8,15-18]. Some authors hypothesize that TDs may reflect blood-borne spread associated with poor prognosis and may be included in M category[19], while others consider TDs as in-transit metastases, where tumor cells spread through lymphatic channels and form tumors before reaching LNs[20]. In addition, the biological behavior of TDs is considered to be similar to tumor budding in the leading area of colorectal cancer, which represents migration over and crossing through histological boundaries[11]. The TDs may migrate and metastasize after undergoing epithelial-to-mesenchymal transition[21].
There were two major limitations to the current study. First, the results of the exploratory analysis may reflect potential bias due to the missing data of TDs. However, it does lend support to the TD-based staging approach. Second, we did not take into consideration that novel adjuvant therapy has already been the standard regimen in some settings. Further studies are needed to investigate patients with and without novel adjuvant therapy, especially when patients achieve substantial downstaging, to substantiate the definition and demonstrate the pathogenesis of TDs[22]. In the exploratory analysis, we chose a worse-case scenario by assigning a value of 2 for the number of LNs involved for cases with N1b stage, by which some patients were confirmed as N1 who should in fact be restaged as N2. Despite this, the outcome still indicated the addition of TDs to LNM count. Therefore, we do not believe that this compromises the accuracy of our results. Our analysis shows that TDs play an important role in the survival of patients. The N1c category is not optimal in the current staging system and adding the number of TDs to LN count may improve the prognostic accuracy. In addition, more investigations are needed with respect to the origin and pathophysiological mechanism of development of TDs, by which a more reproducible and scientific definition can be developed.
Addition of TDs to the LNM count improves the prognostic accuracy of current TNM staging. However, the origin and pathogenesis of TDs remain to be clarified.
Tumor deposits (TDs) plays an important role in The American Joint Committee on Cancer (AJCC) tumor, node and metastasis (TNM) staging system. However, the definition of TDs as well as N1c remains controversial. Just taking the quantitative information of TDs into consideration may be suboptimal in the current staging system while adding TDs into lymph node metastases (LNMs) count may improve accuracy and N1c category may represents patients with heterogeneous survival.
AJCC TNM staging system is the standard tool for tumor staging and the treatment strategies for patients mostly depend on tumor stage. To guarantee more appropriate treatment strategies can be received by patients and to predict prognosis of patients better, developing an optimal staging system is crucial.
The main objective of this study is to assess the association between the presence of TDs and overall survival (OS). As exploratory outcomes, the impact of number of TDs on OS was investigated and the N stage was reclassified to the novel N category by the addition of TDs to the LNM count. The outcome indicated that TDs are an independent prognostic factor for OS in colorectal cancer and the addition of TDs to LNM count improved the prognostic accuracy of TNM staging. Therefore, a part of patients staged as N1 previously would be N2 after the addition of TDs to LNM count and the prognosis would change subsequently.
Patients with colorectal cancer including TD-negative and TD-positive subpopulations were derived from Surveillance, Epidemiology, and End Results database (SEER). Cox proportional hazard model was used for survival analysis and the sensitivity analyses were performed to detect outcome robustness. The subgroup analysis was also performed to explore the different profile of risk factors between patients with and without TDs. Comparative effectiveness research was used in current study.
The presence of TDs is an independent prognostic factor for OS in colorectal cancer and there may be more than one way through which TDs influence survival. Both TDs and their numbers should be integrated into N staging and the N1c category in TNM staging was inappropriate. Given that novel adjuvant therapy has already been the standard regimen in some settings and there is no evidence whether TDs in patients with novel adjuvant therapy should be regarded the same as patients without novel adjuvant therapy, further investigations need to be conducted.
The presence of TDs is an independent prognostic factor for OS in colorectal cancer and addition of TDs to the LNM count improves the prognostic accuracy of current TNM staging.
The origin as well as formation of TDs remains ambiguous and further studies are needed to substantiate the definition and demonstrate the pathogenesis of TDs. Patients with and without novel adjuvant therapy need to be investigated separately, especially when patients achieve substantial downstaging.
We wish to thank the useful suggestions given by Professor Wen-Quan Niu from the Institute of Clinical Medical Science of the China–Japan Friendship Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lunkka P, Finland; Rajagopalan A, Australia; Shinozaki E, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64590] [Article Influence: 16147.5] [Reference Citation Analysis (176)] |
| 2. | Nagtegaal ID, Tot T, Jayne DG, McShane P, Nihlberg A, Marshall HC, Påhlman L, Brown JM, Guillou PJ, Quirke P. Lymph nodes, tumor deposits, and TNM: are we getting better? J Clin Oncol. 2011;29:2487-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Cohen R, Shi Q, Meyers J, Jin Z, Svrcek M, Fuchs C, Couture F, Kuebler P, Ciombor KK, Bendell J, De Jesus-Acosta A, Kumar P, Lewis D, Tan B, Bertagnolli MM, Philip P, Blanke C, O'Reilly EM, Shields A, Meyerhardt JA. Combining tumor deposits with the number of lymph node metastases to improve the prognostic accuracy in stage III colon cancer: a post hoc analysis of the CALGB/SWOG 80702 phase III study (Alliance)☆. Ann Oncol. 2021;32:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 4. | Delattre JF, Cohen R, Henriques J, Falcoz A, Emile JF, Fratte S, Chibaudel B, Dauba J, Dupuis O, Bécouarn Y, Bibeau F, Taieb J, Louvet C, Vernerey D, André T, Svrcek M. Prognostic Value of Tumor Deposits for Disease-Free Survival in Patients With Stage III Colon Cancer: A Post Hoc Analysis of the IDEA France Phase III Trial (PRODIGE-GERCOR). J Clin Oncol. 2020;38:1702-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 5. | Frankel WL, Jin M. Serosal surfaces, mucin pools, and deposits, oh my: challenges in staging colorectal carcinoma. Mod Pathol. 2015;28 Suppl 1:S95-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4401] [Article Influence: 550.1] [Reference Citation Analysis (4)] |
| 7. | Lord A, Brown G, Abulafi M, Bateman A, Frankel W, Goldin R, Gopal P, Kirsch R, Loughrey MB, Märkl B, Moran B, Puppa G, Rasheed S, Shimada Y, Snaebjornsson P, Svrcek M, Washington K, West N, Wong N, Nagtegaal I. Histopathological diagnosis of tumour deposits in colorectal cancer: a Delphi consensus study. Histopathology. 2021;79:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Lord AC, D'Souza N, Pucher PH, Moran BJ, Abulafi AM, Wotherspoon A, Rasheed S, Brown G. Significance of extranodal tumour deposits in colorectal cancer: A systematic review and meta-analysis. Eur J Cancer. 2017;82:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 2388] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 10. | Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T, Ueno H, Quirke P. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol. 2017;35:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 11. | Brouwer NPM, Nagtegaal ID. Tumor deposits improve staging in colon cancer: what are the next steps? Ann Oncol. 2021;32:1209-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Goldstein NS, Turner JR. Pericolonic tumor deposits in patients with T3N+MO colon adenocarcinomas: markers of reduced disease free survival and intra-abdominal metastases and their implications for TNM classification. Cancer. 2000;88:2228-2238. [PubMed] |
| 13. | Wünsch K, Müller J, Jähnig H, Herrmann RA, Arnholdt HM, Märkl B. Shape is not associated with the origin of pericolonic tumor deposits. Am J Clin Pathol. 2010;133:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Maguire A, Sheahan K. Controversies in the pathological assessment of colorectal cancer. World J Gastroenterol. 2014;20:9850-9861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Al Sahaf O, Myers E, Jawad M, Browne TJ, Winter DC, Redmond HP. The prognostic significance of extramural deposits and extracapsular lymph node invasion in colon cancer. Dis Colon Rectum. 2011;54:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Kim CW, Kim J, Yeom SS, Lee JL, Yoon YS, Park IJ, Lim SB, Baek S, Yu CS, Kim JC. Extranodal extension status is a powerful prognostic factor in stage III colorectal cancer. Oncotarget. 2017;8:61393-61403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Veronese N, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, Sergi G, Manzato E, Fassan M, Wood LD, Scarpa A, Luchini C. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol. 2016;27:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Chen H, Tang Z, Liu F. Tumor deposit vs extra nodal extension: a differential evaluation of prognostic relevance. Eur J Cancer. 2018;105:127-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Puppa G, Maisonneuve P, Sonzogni A, Masullo M, Capelli P, Chilosi M, Menestrina F, Viale G, Pelosi G. Pathological assessment of pericolonic tumor deposits in advanced colonic carcinoma: relevance to prognosis and tumor staging. Mod Pathol. 2007;20:843-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Nagtegaal ID, Quirke P. Colorectal tumour deposits in the mesorectum and pericolon; a critical review. Histopathology. 2007;51:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | De Smedt L, Palmans S, Andel D, Govaere O, Boeckx B, Smeets D, Galle E, Wouters J, Barras D, Suffiotti M, Dekervel J, Tousseyn T, De Hertogh G, Prenen H, Tejpar S, Lambrechts D, Sagaert X. Expression profiling of budding cells in colorectal cancer reveals an EMT-like phenotype and molecular subtype switching. Br J Cancer. 2017;116:58-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Song JS, Chang HJ, Kim DY, Kim SY, Baek JY, Park JW, Park SC, Choi HS, Oh JH. Is the N1c category of the new American Joint Committee on cancer staging system applicable to patients with rectal cancer who receive preoperative chemoradiotherapy? Cancer. 2011;117:3917-3924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |