INTRODUCTION
Gastric cancer is the third deadliest cancer in the world and ranks second in the incidence and mortality of cancers in China[1,2]. Despite advances in prevention, diagnosis, and therapy, the absolute number of cases is increasing every year due to aging and the growth of high-risk populations, and gastric cancer is still a leading cause of cancer-related death[1,3]. Gastric cancer is a consequence of the complex interaction of microbial agents, with environmental and host factors, resulting in the dysregulation of multiple oncogenic and tumor-suppressing signaling pathways[1]. Global efforts have been made to investigate in detail the genomic and epigenomic heterogeneity of this disease, resulting in the identification of new specific and sensitive predictive and prognostic biomarkers[4]. Trastuzumab, a monoclonal antibody against the HER2 receptor, is approved in the first-line treatment of patients with HER2+ tumors, which accounts for 13%-23% of the gastric cancer population[5]. Ramucirumab, a monoclonal antibody against VEGFR2, is currently recommended in patients progressing after first-line treatment[6]. Several clinical trials have also tested novel agents, such as anti-EGFR and anti-MET monoclonal antibodies, to treat advanced gastric cancer but mostly with disappointing results[3]. Therefore, it is still of great significance to screen specific molecular targets for gastric cancer and drugs directed against the molecular targets.
Several epidemiological studies have found that diabetes is linked with an increased risk of gastric cancer and can increase the risk of death in patients with gastric cancer, resulting in a worse prognosis[7,8]. Recently, many studies have focused on diabetes and its causal relation with neoplasms[9]. The emergence of cancer-related properties was likely a change in biological characteristics caused by the hyperglycemic environment created by diabetes, which provides the opportunity to trigger abnormal expression of the healthy genome and promote changes in biological characteristics[10]. It was demonstrated that glucose increased ATP levels to inhibit AMPK and induce the increased expression of hepatocyte nuclear factor 4α (HNF4α)[11]. Hepatocyte nuclear factor 4α is a member of the nuclear receptor transcription factor family, which has a conserved DNA-binding domain and a ligand-binding domain[12]. HNF4α is at the center of a complex transcriptional regulatory network and regulates the biological effects of different pathways via transcriptional regulation of differential target genes[13]. HNF4α is involved in a variety of human diseases. HNF4α plays an important role in the development and treatment of diabetes. Neonatal patients with diabetes or young adults with diabetes with mutations in the HNF4α gene usually respond better to oral treatment with sulfonylureas[14]. Furthermore, many studies also suggest that HNF4α might be related to the occurrence and development of tumors. HNF4α was found to be highly expressed in various tumor tissues, such as lung cancers and liver cancers[15,16]. Compared with normal gastric mucosa, the protein and transcript levels of HNF4α in gastric cancer tissues and gastric cancer cell lines are also at higher levels[17]. The role of HNF4α in gastric cancer has gradually received attention. HNF4α can promote the intestinal metaplasia of the gastric mucosa[18]. HNF4α can also be used as a gold standard marker to distinguish gastric metastasis from primary gastric cancer and breast cancer. Studies showed that HNF4α was positively expressed in all patients diagnosed with primary gastric adenocarcinoma and negatively expressed in all cases of primary breast cancer[19,20].
The hyperglycemic environment created by diabetes provides the opportunity to trigger abnormal expression of the healthy genome and promote changes in biological characteristics[10]. The hyperglycemic state observed in the diabetic milieu is predicted to enhance the gastric cancer risk in prediabetic and diabetic individuals[9]. Studies have shown that some diabetes treatment drugs can effectively reduce the risk of cancer and improve prognosis, which is of great significance for understanding the relationship between diabetes and tumorigenesis and provides a new idea for the research of cancer prevention and treatment[21]. Berberine, an extract from the Chinese herbal medicine Coptis, has been widely used in clinical treatment and has shown excellent efficacy in the treatment of intestinal infections. Multiple studies have displayed the potential therapeutic effect of berberine on diabetes[22]. Berberine can target HNF4α to inhibit gluconeogenesis in the treatment of type 2 diabetes and its complications[23]. Berberine improvs insulin resistance associated not only with gut microbiota alteration in branched-chain amino acid biosynthesis, but also with branched-chain amino acid catabolism in liver and adipose tissues[24]. Berberine alleviates tau hyperphosphorylation and axonopathy-associated with diabetic encephalopathy by restoring the PI3K/Akt/GSK3β pathway[25]. Meanwhile, several studies have also showned the potential therapeutic effects of berberine against tumor growth[26-28]. Berberine exerts an inhibitory effect on cell growth and induces apoptosis by inhibiting the activation of EFGR signaling[27]. Berberine can also suppress the growth and induce apoptosis of colorectal cancer cell lines through regulation of the Long Non-Coding RNA (lncRNA) Cancer Susceptibility Candidate 2 (CASC2)/AU-Binding Factor 1 (AUF1)/B-Cell CLL/Lymphoma 2 (Bcl-2) Axis[28]. The structural interaction of berberine with Aldo-keto reductase family 1 member C3 is attributed to the suppression of Aldo-keto reductase family 1 member C3 enzyme activity and the inhibition of 22Rv1 prostate cancer cell growth by decreasing the intracellular androgen synthesis[29]. Berberine could effectively affect both tumor outgrowth and spontaneous metastasis in triple-negative breast cancer, which was associated with the inhibition of NLRP3 inflammasome pathway[30].
Whether HNF4α, which plays an important role in regulating the progression of diabetes and tumors, is just the right target for berberine to exert anti-gastric cancer effects remains to be elucidated. Our previous experiments demonstrated that berberine could induce cycle arrest of gastric cancer cells by targeting AMPK/HNF4α/WNT5a pathways in vitro[31]. In this study, berberine was administrated intragastrically to MGC803 and SGC7901 subcutaneous gastric cancer xenograft models. We investigated the effects of berberine against tumor growth and the role of HNF4α-WNT5a/β-catenin pathways involved in the antitumor effects of berberine.
MATERIALS AND METHODS
Chemicals and materials
Berberine chloride with a purity of 98% was purchased from the Sigma-Aldrich, United States. Antibodies against β-catenin were purchased from Cell Signaling Technologies (Boston, MA, United States), WNT5a was purchased from Abgent and HNF4a was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States).
Cell lines and cell culture
The gastric cancer cell lines MGC803 and SGC7901 were supplied by Hubei Biossci Biological Co., Ltd. Both cell lines were routinely cultured in RPMI1640(HyClone, China), supplemented with 10% fetal bovine serum (FBS) (SiJiQing, China), and 1% penicillin-streptomycin solution at 37 ℃ in a 5% CO2 humidified atmosphere. All experiments were performed when cells were in a logarithmic phase.
In vivo tumor xenograft model
Female BALB/c nude mice approximately 4 weeks old (13 g-17 g) were purchased from Hunan Slake Jingda Experimental Co., Ltd. (Hunan, China). Upon arrival, these animals were maintained under specific-pathogen-free conditions (23 ± 2 ℃ and 55 ± 5% humidity) with an automatically controlled 12h light/dark cycle and with a free access to sterilized flood and autoclaved water. After mice were acclimatized for about seven day, MGC803 or SGC7901 gastric cancer cells (107 cells per mouse) were subcutaneously injected into the right flank of mice. Animals bearing tumors were randomly divided into two groups, and treatment was initiated after injection for 72 h when a mass of more than 6 mm in maximal diameter was identified in each mouse with daily intragastric administration of normal saline (control group) and 100 mg/kg berberine (BBR group). All animals were sacrificed on Day 18 after treatment and blood as well as tissues from all animals were collected. Tumor xenograft length (L) and width (W) were measured at two-day intervals with Vernier calipers, and tumor volume (V) was calculated using the Formula (V = W2 × L/2). Mouse body weight was monitored every two d. Animal experiments were performed according to the institutional guidelines and regulations approved by the Huazhong University of Science and Technology Institutional Animal Care and Use Committee.
Immunohistochemistry staining assay
The tumor tissues removed from mice were fixed with 4% formaldehyde and then embedded in paraffin, and then 4μm thick tissue sections were made. After heating in an oven at 60 ℃ for 1 h, the sections was dewaxed in a dewaxing agent and rehydrated by a concentration-gradient of alcohol (100%, 95%, 85%, 75%). Tissue sections were boiled in 10 mmol/L sodium citrate buffer (pH 6.0) at high temperature to recover the antigen and incubated with 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase activity. Sections were incubated with primary antibodies against HNF4a (1: 200), WNT5a (1: 80) and β-catenin (1: 100) at 4 ℃ overnight. Then the tissue was incubated with a secondary antibody and observed under a microscope.
Total RNA Extraction and real-time polymerase chain reaction
Total RNA was extracted using the TRIzol Reagent (Magent, Wuhan) according to the manufacturer’s instructions, and then cDNA was synthesized from 2 µg of total RNA using the 5X All-In-One RT MasterMix (ABP) at 25 ℃ for 10 min, 42 ℃ for 15 min and 85 ℃ for 5 min. Real-time polymerase chain reaction (PCR) was used to detect the mRNA expression levels of HNF4α, WNT5a and β-catenin. PCR reactions were performed using LightCycler 480 (Roche Applied Science) in a total volume of 20 µL, including 10 µL of 2X SYBR Green qPCR Master Mix (ABP) at 95 ℃ for 10 min, 95 ℃ for 15 s and 60 ℃ for 60 s, 40 cycles according to the manufacturer’s instructions. The relative gene expression was calculated with normalization to an endogenous reference (GAPDH).
The primers were as follows: GENE; Forward Primer (5’-3’); Reverse Primer (5’-3’); HNF4α; ATGCGACTCTCTAAAACCCTTG; ACCTTCAGATGGGGACGTGT; WNT5a; CAACTGGCAGGACTTTCTCAA; CCTTCTCCAATGTACTGCATGTG; β-catenin; ATGGAGCCGGACAGAAAAGC; TGGGAGGTGTCAACATCTTCTT; GAPDH; TGGCCTTCCGTGTTCCTAC; GAGTTGCTGTTGAAGTCGCA.
Western blotting
Total protein was extracted from the tumor tissues and liver tissues, and its concentration was determined by the BCA assay. Then, equal amounts of proteins (30ug/Lane) were loaded for electrophoresis, seperated by SDS-polyacrylamide electrophoresis gel (SDS–PAGE) and subsequently transferred to nitrocellulose membranes (NC membrane) ( Millipore, United States). After blocking with 5% nonfat milk at room teamperature for 1h, the NC membranes were incubated with the primary antibodies overnight with gentle agitation at 4 ℃. After washing with TBST for three times, the NC membranes were incubated with the secondary antibodies for 1h at room temperature, followed by visualization with a near-infrared double color laser imaging system (Odyssey, Lincoln, NE, United States).
Statistical analysis
All experiments were repeated at least three times. All data are expressed as the mean ± standard deviation. All data were plotted by GraphPad Prism 6 software. Statistical analysis was performed using SPSS (version 20.0) software. A t test was also performed to determine differences between groups. P < 0.05 was considered statistically significant.
RESULTS
Berberine inhibited the growth of MGC803 and SGC7901 gastric cancer xenograft tumors
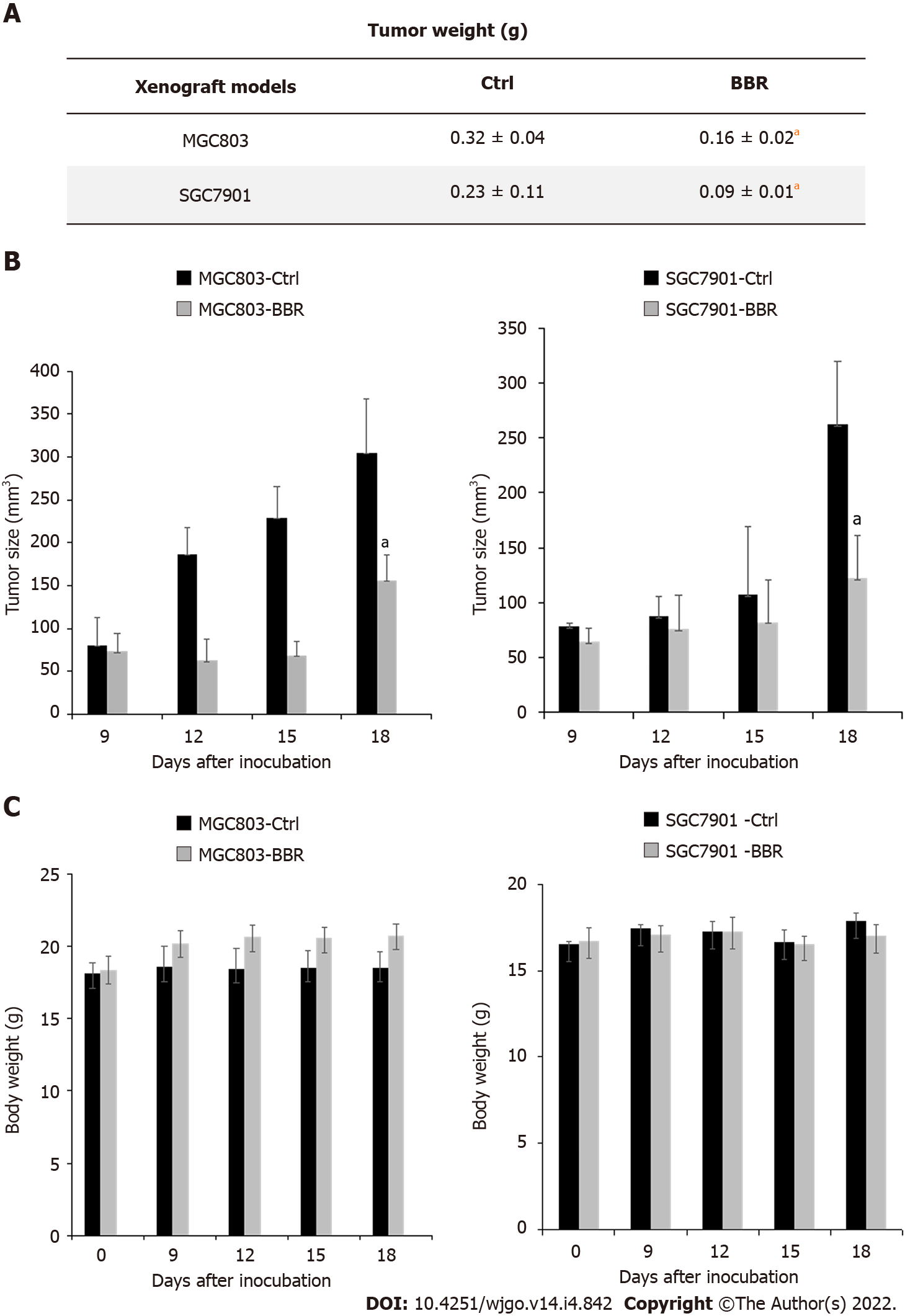
To explore the effect of berberine on gastric cancer xenograft models, we established MGC803 and SGC7901 xenograft tumor models. As shown in Figure 1A, intragastric administration of berberine significantly reduced the tumor weight of MGC803 and SGC7901 subcutaneously transplanted tumors, and the reduction rates were approximately 50.0% and 60.9% of the control group respectively (P < 0.05). In addition, as shown in Figure 1B, berberine led to 48.6% inhibition of tumor size in MGC803 xenograft models and 51.3% inhibition of tumor volume in SGC7901 xenograft models when compared to the control group. Berberine significantly inhibited the growth rate of MGC803 and SGC7901 xenograft tumors. Moreover, berberine did not affect the body weight of MGC803 and SGC7901 xenograft models in this experiment (Figure 1C, P > 0.05). These results indicated that berberine could significantly retard the growth of MGC803 and SGC7901 xenograft tumors, which was coordinated with the experimental results of Yi et al[32].
Figure 1 Berberine inhibited the growth of MGC803 and SGC7901 xenograft tumors.
A: After 18 d of drug treatment, tumors were removed from the mice and weighed; B: Changes in tumor size of mice during the experiment; C: Changes in body weight of mice during the experiment. aP < 0.05. Ctrl: The control group; BBR: The berberine group.
Berberine inhibited HNF4α expression in tumor and liver tissues in both MGC803 and SGC7901 xenograft tumor models.
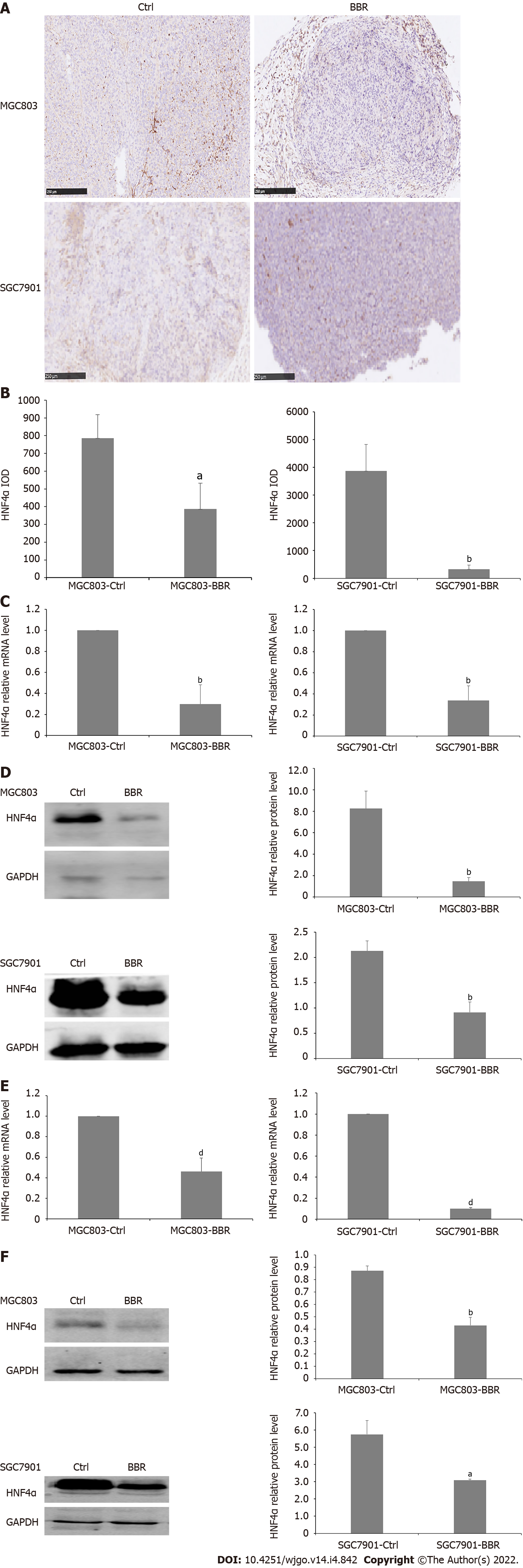
HNF4α is at the center of a complex transcriptional regulatory network and is implicated in the initiation and progression of gastric cancer. To investigate the role of HNF4α in the growth of gastric cancer and the underlying mechanism, we examined the effect of berberine on the expression of HNF4α in tumor tissues and liver tissues of MGC803 and SGC7901 xenograft tumor models. Immunohistochemical staining showed that berberine downregulated the expression of HNF4α in tumor tissues from MGC803 and SGC7901 xenograft tumor models. Besides, in the tumor tissues from the MGC803 and SGC7901 xenograft tumor models, berberine significantly reduced both the transcriptional expression and protein expression of HNF4α compared with the control group. Moreover, berberine inhibited the mRNA level of HNF4α in the liver tissues from the MGC803 and SGC7901 xenograft models. Furthermore, berberine also reduced the protein expression of HNF4α in liver tissue from the MGC803 and SGC7901 xenograft models. Therefore, berberine inhibited the expression of HNF4α in MGC803 and SGC7901 xenograft models at both the transcriptional and posttranscriptional levels, and the reduction of HNF4α significantly inhibited the growth of tumor cells (Figure 2).
Figure 2 Berberine inhibited hepatocyte nuclear factor 4α expression in MGC803 and SGC7901 xenograft tumor models.
A: Immunohistochemistry showed that berberine reduced the expression of hepatocyte nuclear factor 4α (HNF4α) in tumor tissues of MGC803 and SGC7901 xenograft tumor models; B: The quantification of immunohistochemistry of the expression of HNF4α in tumor tissues of MGC803 and SGC7901 xenograft tumor models; C: The effect of berberine on HNF4α mRNA expression in tumor tissues of MGC803 and SGC7901 xenograft tumor models; D: The effect of berberine on HNF4α protein expression in tumor tissues of MGC803 and SGC7901 xenograft tumor models; E: The effect of berberine on HNF4α mRNA level in liver tissues of MGC803 and SGC7901 xenografts; F: The effect of berberine on HNF4α protein expression in liver tissues of MGC803 and SGC7901 xenografts. aP < 0.05, bP < 0.01, dP < 0.0001. Ctrl: The control group; BBR: The berberine group.
Berberine inhibited WNT5a expression in tumor and liver tissues in both MGC803 and SGC7901 xenograft tumor models.
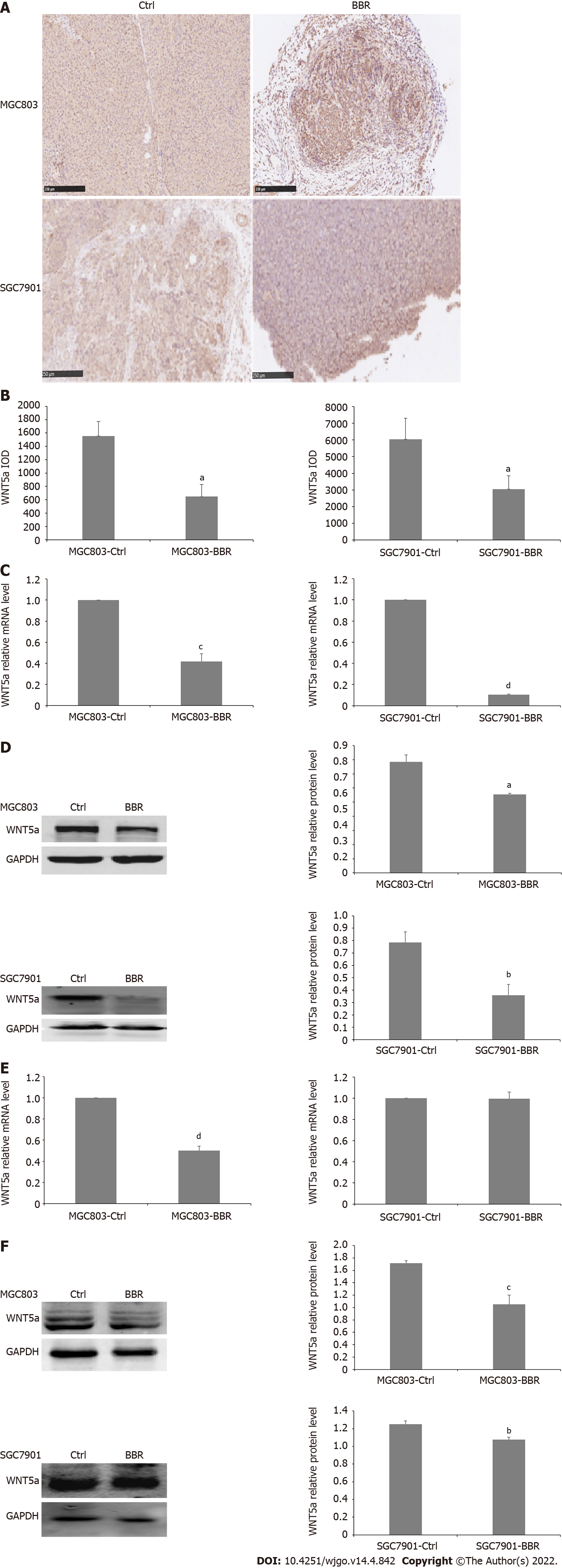
WNT5a has been proven to be involved in the development and progression of gastric cancers. WNT5a was the downstream of HNF4α and HNF4α regulated WNT signaling through its target gene WNT5a, a potential prognostic marker of diffuse-type gastric tumours[33]. Therefore, we examined the alteration of the expression levels of WNT5a in tumor tissues and liver tissues from xenograft tumor models. Immunohistochemical staining results showed that compared with the control group, the expression level of WNT5a in tumor tissues from both the MGC803 and SGC7901 xenograft tumor models in the berberine group was significantly down-regulated. Furthermore, berberine also significantly reduced the mRNA and protein levels of WNT5a in the tumor tissues from both the MGC803 and SGC7901 xenograft models compared to the control group. Whereas, in the liver tissues from xenograft models, berberine reduced the mRNA level of WNT5a in MGC803 xenograft models, but did not affect the transcription level of WNT5a in SGC7901 xenograft models. However, berberine reduced the protein expression of WNT5a in the liver tissues from MGC803 as well as SGC7901 xenograft model. Therefore, berberine inhibited WNT5a protein expression in MGC803 and SGC7901 xenograft models, and the downregulation of WNT5a protein expression was associated with the retardation of the tumor growth rate (Figure 3).
Figure 3 Berberine inhibited WNT5a expression in MGC803 and SGC7901 xenograft tumor models.
A: Immunohistochemistry showed that berberine reduced the expression of WNT5a in tumor tissues of MGC803 and SGC7901 xenograft tumor models; B: The quantification of immunohistochemistry of the expression of WNT5a in tumor tissues of MGC803 and SGC7901 xenograft tumor models; C: The effect of berberine on WNT5a mRNA level in tumor tissues of MGC803 and SGC7901 xenograft tumor models; D: The effect of berberine on WNT5a protein expression in tumor tissues of MGC803 and SGC7901 xenograft tumor models; E: The effect of berberine on WNT5a mRNA expression in liver tissues of MGC803 and SGC7901 xenografts; F: The effect of berberine on WNT5a protein expression in liver tissues of MGC803 and SGC7901 xenografts. aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001. Ctrl: The control group; BBR: The berberine group.
Berberine inhibited β-catenin expression in tumor and liver tissues in both MGC803 and SGC7901 xenograft tumor models.
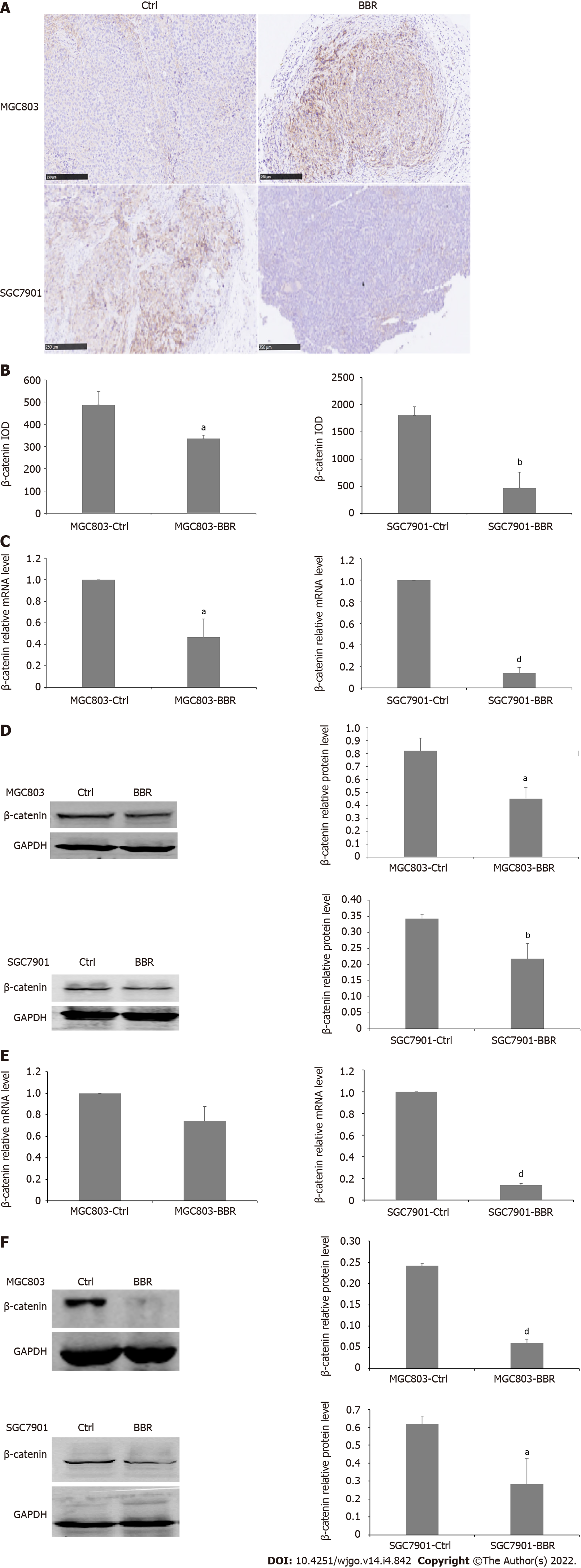
However, as an extracellular ligand, WNT5a activates different intracellular signaling cascades: β-catenin-dependent and β-catenin-independent pathways[34]. In the classical WNT pathway, β-catenin acts as a major mediator and functions as a protein organizer by interacting with numerous partners at the membrane, in the cytosol, and in the nucleus[35,36]. To further illustrate the mechanism of the antitumor effects of berberine, we also detected the expression level of β-catenin in tumor tissues and liver sections upon treatment with berberine. Immunohistochemical staining results showed that in the SGC7901 and MGC803 xenograft models, berberine significantly reduced the expression of β-catenin in tumor tissues. In addition, in both the MGC803 and SGC7901 xenograft tumor models, both the transcriptional and the protein levels of β-catenin in tumor tissue in the berberine group were significantly reduced. Besides, berberine reduced the mRNA level of β-catenin in the liver tissues from SGC7901 xenograft tumor models but did not affect the transcription level of β-catenin in MGC803 xenograft tumor models. Simultaneously, berberine also downregulated the protein expression of β-catenin in the liver tissues of MGC803 xenograft tumor models and SGC7901 xenograft tumor models. Therefore, berberine downregulated the protein expression of β-catenin in both MGC803 and SGC7901 xenograft models, and berberine targeted the WNT5a/β-catenin signaling pathways to exert its growth inhibition effects to MGC803 and SGC7901 xenograft model tumors (Figure 4).
Figure 4 Berberine inhibited β-catenin expression in MGC803 and SGC7901 xenograft tumor models.
A: Immunohistochemistry showed that berberine reduced the expression of β-catenin in tumor tissues of MGC803 and SGC7901 xenograft tumor models; B: The quantification of immunohistochemistry of the expression of β-catenin in tumor tissues of MGC803 and SGC7901 xenograft tumor models; C: The effect of berberine on β-catenin mRNA alteration in tumor tissues of MGC803 and SGC7901 xenograft tumor models; D: The effect of berberine on β-catenin protein expression in tumor tissues of MGC803 and SGC7901 xenograft tumor models; E: The effect of berberine on β-catenin mRNA in liver tissues of MGC803 and SGC7901 xenografts; F: The effect of berberine on β-catenin protein expression in liver tissues of MGC803 and SGC7901 xenografts. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001. Ctrl: The control group; BBR: The berberine group.
DISCUSSION
Berberine is a benzyl tetraisoquinoline alkaloid extracted from Chinese herbal medicines such as Coptis, Phellodendron chinense and Hydrastis canadensis. Berberine has been used clinically for many years and is effective in counteracting numerous diseases such as gastroenteritis, abdominal pain and diarrhea, with antimicrobial and anti-inflammatory properties[37]. Over the past decades, growing evidence clearly indicates that berberine can improve insulin-resistance and the levels of plasma lipids and glucose in type 2 diabetes animal models and humans[38-40]. Berberine could modulate the composition of the gut microbiome and reduce body weight, blood glucose levels, and intestinal inflammation in diabetic mice, which demonstrates its effectiveness in the reduction of diabetic complications in this model[38]. Berberine also attenuated intestinal mucosal barrier dysfunction and immune barrier damage in type 2 diabetic rats[39]. Berberine stimulated insulin secretion in impaired glucose tolerance rats[40]. Recently, berberine has shown potential therapeutic effects in treating cancers[26]. Berberine inhibited the proliferation of SGC-7901 cells and induced apoptosis[41]. Berberine also inhibited EGFR signaling and enhanced the antitumor effects of EGFR inhibitors in gastric cancer[27]. Berberine could also inactivate MAPK signaling pathways to exert growth inhibition effects on MGC803 cells[42]. In this experiment, berberine was discovered to induce a 48.6% inhibition of tumor growth in MGC803 xenograft models and 51.3% retardation of tumor growth in SGC7901 xenograft models without any influence on body weight[42]. Therefore, berberine might be an effective and safe drug candidate for treating gastric cancer. However, whether there exists a shared molecular target which is responsible for the antitumor effects and the antidiabetic effects of berberine remains unclear?
HNF4α is a transcription factor with important roles in liver and gastrointestinal tract development, hepatocyte differentiation, and lipid and glucose metabolism[43]. HNF4α is at the center of a complex transcriptional regulatory network where its disruption is directly linked to glucose metabolism and lipid metabolism[44]. The activation of HNF4α may represent a novel single agent for the treatment of insulin resistance[44]. The activation of HNF4α stimulates glucose metabolism in acute myeloid leukemia cells[45]. What’s more, the regulation of HNF4α expression and activity is highly complex, reflecting on the downstream transcriptional networks with diverse functional roles including drug metabolism, lipid homeostasis, gluconeogenesis, cell adhesion, proliferation, and apoptosis[46]. Our previous studies found that HNF4α was involved in the glucose metabolism of DM[47]. Recently, one further study illustrated that inhibition of HNF4α expression could induce cell cycle arrest and apoptosis in gastric cancer cells, thereby exerting antitumor growth effects[31], corresponding to the experiments in which inhibitory RNA and pharmacological inhibition of HNF4α demonstrated antineoplastic activity in vitro and in vivo via downregulation of cyclins, cell cycle arrest and apoptosis in gastric cancer[33]. HNF4α downregulation could promote tumor migration and invasion in renal cell carcinoma[48]. HNF4α promotes the malignant phenotype of acute myeloid leukemia cells[45]. In this study, berberine could suppressed the expression of HNF4α in tumor tissues and liver tissues from MGC803 and SGC7901 gastric cancer xenograft tumor models. More importantly, the reduced expression of HNF4α might to be associated with the growth retardation of tumors from gastric cancer xenograft models. Therefore, berberine might target HNF4α to inhibit tumor growth in MGC803 and SGC7901 xenograft models.
Studies have shown that WNT5a contains several highly conserved HNF4α binding sites in its promoter region[49]. The expression level of WNT5a decreases after HNF4α gene knockout, which further inhibits cellular glucose uptake and cell proliferation, and also induces apoptosis[33,45]. WNT5a is a member of the WNT family and can activate classical WNT (β-catenin-dependent) signaling pathways[50]. The WNT/β-catenin signaling pathway has been recognized as a potentially therapeutic target for cancers[51]. In the classical WNT pathway, β-catenin acts as a major mediator, facilitates signal transduction, and participates in embryogenesis, cell differentiation, and proliferation processes. When the WNT pathway is on, the cytoplasmic concentration of β-catenin increases, and then it translocates into the nucleus, which activates more than one hundred target genes of the WNT pathway, while when the WNT pathway is off, β-catenin is degraded by the proteasome[52]. In this experiment, we found that the protein expression of WNT5a and β-catenin was both downregulated by berberine, indicating that inactivation of the WNT/β-catenin pathway resulted from berberine. The WNT/β-catenin pathway is involved in the process of tumorigenesis, invasion and metastasis. The role of WNT5a in the occurrence and progression of gastric cancer has gradually been elucidated. It was confirmed that downregulation of WNT5a expression in gastric cancer cells could inhibit EMT onset and increased WNT5a expression could also result in poor differentiation in GC[53,54]. By activating the WNT5a signaling pathway, gastric cancer cell growth was promoted and G1-S cell cycle transition was induced[55]. Overexpression of WNT5a is also associated with enhanced tumor aggressiveness[56,57]. WNT5a was also a potential suppressor of EMT and downregulation of WNT5a mRNA and protein by EGF is necessary for EGF-induced EMT in gastric cancer SGC-7901 cells[58]. The role of WNT5a in the development of gastric cancer is controversial. Recently the role of β-catenin in promoting the migration and invasion of gastric cancer has also been elucidated. CD44, which interacts with IL-13Rα2, promotes gastric cancer development and metastasis through regulation of the β-catenin signaling pathways[59]. HOXA11-AS not only promotes gastric cancer cell migration and invasion in vitro, but also promotes gastric cancer cell metastasis in vivo, at least in part, by regulating β-catenin[60]. Studies have also shown that the berberine combined drug HMQ1611 negatively regulates the WNT5a signaling pathway by upregulating axin and inhibiting nuclear transport of β-catenin, thereby inhibiting cancer cell proliferation and metastasis[61]. This experiment demonstrated that the reduction in the expression of WNT5a/β-catenin signaling pathway is associated with tumor growth suppression in MGC803 and SGC7901 xenograft tumor models. This experiment displayed that berberine could downregulate the expression of WNT5a/β-catenin in tumor tissues and liver tissues of MGC803 and SGC7901 gastric cancer xenograft tumor models at the protein level, and thereby inhibiting the growth of MGC803 and SGC7901 gastric cancer xenograft tumors.
ARTICLE HIGHLIGHTS
Research background
Gastric cancer is the third deadliest cancer in the world and ranks second in incidence and mortality of cancers in China. Despite advances in prevention, diagnosis, and therapy, the absolute number of cases is increasing every year due to aging and the growth of high-risk populations, and gastric cancer is still a leading cause of cancer-related death. Gastric cancer is a consequence of the complex interaction of microbial agents with environmental and host factors, resulting in the dysregulation of multiple oncogenic and tumor-suppressing signaling pathways. Global efforts have been undertaken to investigate in detail the genomic and epigenomic heterogeneity of this disease, resulting in the identification of new specific and sensitive predictive and prognostic biomarkers. Trastuzumab, a monoclonal antibody against the HER2 receptor, is approved in the first-line treatment of patients with HER2+ tumors, which accounts for 13%-23% of the gastric cancer population. Ramucirumab, a monoclonal antibody against VEGFR2, is currently recommended in patients progressing after first-line treatment. Several clinical trials have also tested novel agents, such as the anti-EGFR and the anti-MET monoclonal antibodies, for advanced gastric cancer but mostly with disappointing results. Therefore, screening specific molecular targets for gastric cancer and drugs directed against the molecular targets is still urgently needed.
Research motivation
To screen specific molecular targets for gastric cancer and drugs directed against the molecular targets is still urgently needed.
Research objectives
To investigate the effect and mechanism of berberine against tumor growth in gastric cancer xenograft models and to explore the role of hepatocyte nuclear factor 4α (HNF4α)-WNT5a/β-catenin pathway played in the antitumor effects of berberine.
Research methods
MGC803 and SGC7901 subcutaneous xenograft models were established. The control group was intragastrically administrated with normal saline, and the berberine group was administrated intragastrically with 100mg/kg/d berberine. The body weight of nude mice during the experiment was measured to assess whether berberine has any adverse reaction. The volume of subcutaneous tumors during this experiment was recorded to evaluate the inhibitory effect of berberine on the growth of MGC803 and SGC7901 subcutaneous transplantation tumors. Polymerase chain reaction assays were conducted to evaluate the alteration of transcriptional expression of HNF4α, WNT5a and β-catenin in tumor tissues and liver tissues from the MGC803 and SGC7901 xenograft models. Western blotting and IHC were performed to assess the protein expression of HNF4α, WNT5a and β-catenin in tumor tissues and liver tissues from the MGC803 and SGC7901 xenograft models.
Research results
In both MGC803 and SGC7901 xenograft tumor models, berberine significantly reduced tumor volume and weight and retarded the growth rate of tumors. In the SGC7901 and MGC803 subcutaneously transplanted tumor models, berberine downregulated the expression of HNF4α, WNT5a and β-catenin in tumor tissues from both transcription and protein levels. Besides, berberine also suppressed the protein expression of HNF4α, WNT5a and β-catenin in liver tissues.
Research conclusions
Berberine retarded the growth of MGC803 and SGC7901 xenograft model tumors, and the mechanism behind this antigrowth effects might be the downregulation of the expression of HNF4α-WNT5a/β-catenin signaling pathways both in tumor tissues and liver tissues of the xenograft models.
Research perspectives
HNF4α might be a potential target through which berberine exerts effects of both improving diabetes and countering gastric cancers.