Published online Nov 15, 2022. doi: 10.4251/wjgo.v14.i11.2122
Peer-review started: July 4, 2022
First decision: August 13, 2022
Revised: August 24, 2022
Accepted: October 11, 2022
Article in press: October 11, 2022
Published online: November 15, 2022
Processing time: 133 Days and 20.7 Hours
Colorectal cancer (CRC) is one of the most common and fatal cancers worldwide. Synaptophysin-like 2 (SYPL2) is a neuroendocrine-related protein highly expressed in skeletal muscle and the tongue. The involvement of SYPL2 in CRC, including its level of expression and function, has not been evaluated.
To evaluate the correlations of SYPL2 expression with lymph node metastasis (LNM) and prognosis in patients with CRC.
The levels of expression of SYPL2 in CRC and normal colorectal tissues were analyzed in multiple public and online databases. The associations between clinical variables and SYPL2 expression were evaluated statistically, and the associations between SYPL2 expression and prognosis in patients with CRC were analyzed using the Kaplan-Meier method and univariate/multivariate Cox regression analyses. SYPL2 expression was assessed in 20 paired CRC tissue and adjacent normal colorectal tissue samples obtained from Fuyang People’s Hospital, and the associations between SYPL2 expression and the clinical characteristics of these patients were investigated. Correlations between the levels of expression of SYPL2 and key targeted genes were determined by Pearson’s correlation analysis. The distribution of immune cells in these samples was calculated using the CIBERSORT algorithm. Gene set enrichment analysis (GSEA) was performed to evaluate the biofunction and pathways of SYPL2 in CRC.
SYPL2 expression was significantly lower in CRC tissue samples than in normal colorectal tissue samples (P < 0.05). High SYPL2 levels in CRC tissues correlated significantly with LNM (P < 0.05) and a poorer patient prognosis, including significantly shorter overall survival (OS) [hazard ratio (HR) = 1.9, P < 0.05] and disease-free survival (HR = 1.6, P < 0.05). High SYPL2 expression was an independent risk factor for OS in both univariate (HR = 2.078, P = 0.014) and multivariate (HR = 1.754, P = 0.018) Cox regression analyses. In addition, SYPL2 expression correlated significantly with the expression of KDR (P < 0.0001, r = 0.47) and the BRAFV600E mutation (P < 0.05). Higher SYPL2 expression was associated with the enrichment of CD8 T-cells and M0 macrophages in the tumor microenvironment. GSEA revealed that SYPL2 was associated with the regulation of epithelial cell migration, vasculature development, pathways in cancer, and several vital tumor-related pathways.
SYPL2 expression was lower in CRC tissue than in normal colorectal tissue. Higher SYPL2 expression in CRC was significantly associated with LNM and poorer survival.
Core Tip: In this research, we reported the expression and biofunctions of synaptophysin-like 2 (SYPL2) in colorectal cancer (CRC) for the first time. SYPL2 correlated with lymph node metastasis and a poor prognosis (both overall and disease-free survival) in CRC. SYPL2 mainly influence CD8 T-cell and M0 macrophage enrichment in the tumor microenvironment. Gene set enrichment analysis indicated that SYPL2 might also influence the tumor vasculature development. In addition, we found that SYPL2 was correlated with the effect of bevacizumab therapy.
- Citation: Zhao ZX, Liu QL, Yuan Y, Wang FS. Synaptophysin-like 2 expression correlates with lymph node metastasis and poor prognosis in colorectal cancer patients. World J Gastrointest Oncol 2022; 14(11): 2122-2137
- URL: https://www.wjgnet.com/1948-5204/full/v14/i11/2122.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i11.2122
Colorectal cancer (CRC), which is responsible for an estimated 8% of new cancer diagnoses and 8% of cancer deaths annually, is the third-most common cause of cancer deaths worldwide[1]. The stage at diagnosis is the most important predictor of survival, with 5-year relative survival rates of 90% for patients diagnosed with localized disease compared to 14% for patients diagnosed with distant-stage disease[2]. Complete mesocolic excision is the cornerstone of CRC treatment, showing good pathological outcomes as well as improvements in overall survival (OS), disease-free survival (DFS) and local recurrence[3]. Lymph node metastasis (LNM) is important in CRC staging and patient prognosis[4], with regional LNM being one of the most important indications for adjuvant chemotherapy[5,6]. Risk factors for LNM include lymphovascular invasion, histological grade, submucosal invasion depth, and tumor budding[7-9]. Although LNM of CRC is usually evaluated by radiologic methods, including computed tomography, magnetic resonance imaging, and positron emission tomography/computed tomography, these imaging methods cannot accurately evaluate LNM using criteria like short-axis diameter, signal heterogeneity, shape, and boundaries[10,11]. Several key biomarkers, however, have been reported to be predictive of LNM and prognosis in patients with CRC[12,13].
Immunotherapy and targeted therapy play important roles in the management of CRC. The molecular targets of CRC identified to date include epithelial growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), human EGFR2, V-raf murine sarcoma viral oncogene homolog B1 (BRAF), Kirsten rat sarcoma (KRAS), P53 mutation, programmed cell death protein 1, and cytotoxic T-lymphocyte–associated protein 4[14,15]. Targeting these proteins in clinical practice has provided survival benefits for patients.
Synaptophysin-like 2 (SYPL2) is a neuroendocrine-related cytosolic protein enriched primarily in skeletal muscles and the tongue. The role of SYPL2 in cancer, including CRC, has not been determined. Thus, the present study comprehensively and systematically compared SYPL2 expression in CRC and normal colorectal tissues. Survival (Cox regression) analyses were also performed to assess the prognostic value of SYPL2 expression, along with other clinicopathological features. The correlation between SYPL2 expression and the expression of key targeted genes in CRC was analyzed. Moreover, gene set enrichment analysis (GSEA) was performed to evaluate the SYPL2-associated biological pathways involved in CRC pathogenesis, providing clues about the function of SYPL2.
The gene-expression profiles and associated clinicopathological data of patients with CRC were downloaded from the Cancer Genome Atlas (TCGA) Genomic Data Commons Data Portal (https:// portal.gdc.cancer.gov/repository) on March 25, 2022. RNA-sequencing gene-expression HTSeq-FPKM data for 571 CRC tissue samples and 44 normal adjacent tissue samples were collected for further analysis. The GSE87211, GSE44076, GSE60331, and GSE103479 datasets were obtained from Gene Expression Omnibus microarrays. In addition, CRC and normal adjacent tissue samples were collected from 20 patients who underwent surgery for CRC at the Fuyang People’s Hospital. Demographic and clinical characteristics of these patients, including their age, sex, cancer stage, and lymph node status, were also recorded and analyzed. All participating patients provided written informed consent, and the study protocol was approved by the ethics review committees of Fuyang People’s Hospital.
Gene Expression Profiling Interactive Analysis (GEPIA) is a newly developed, interactive web server that includes the RNA-sequencing expression data of 9736 tumors and 8587 normal samples from the TCGA and Genotype-Tissue Expression datasets, utilizing a standard processing pipeline. GEPIA offers customizable functions, such as tumor/normal differential expression analysis, profiling according to cancer type or pathological stage, patient survival analysis, detection of similar genes, correlation analysis, and dimensional reduction analysis. In the present study, GEPIA was used to perform differential expression, survival, and correlation analyses, the latter of which was performed with key targeted genes using Pearson’s test.
Total RNA extracted from cells using RNAprep Pure Tissue kits (Tiangen, Beijing, China) was reverse-transcribed to complementary DNA using the FastKing gDNA Dispelling RT SuperMix for quantitative polymerase chain reaction (qPCR) (Tiangen). The samples were subjected to quantitative real-time PCR (qRT-PCR) using 2 × SYBR Green qPCR Master Mix (Tiangen) and primers specific for SYPL2 (forward, 5’-CGCTGGTGGACTTCTGTG-3’; reverse, 5’-GCTGGATGGTCGTGTGG-3’) and GAPDH (forward, 5’-AAGGTCGGAGTCAACGGA-3’; reverse, 5’-TTAAAAGCAGCCCTGGTGA-3’), with all gene primers obtained from Aoke Dingsheng Biotechnology (Beijing, China). Thermal cycling conditions consisted of an initial denaturation at 95 ºC for 15 s, followed by 40 cycles of denaturation at 95 ºC for 15 s, annealing at 55 °C for 30 s, and extension at 72 ºC for 30 s. Relative messenger RNA expression levels were calculated using the 2-∆∆CT method, with the average level of SYPL2 expression in the 20 normal colorectal tissues defined as the reference for normalization and comparison with the 20 CRC tissues.
Univariate and multivariate Cox analyses were used to investigate the association between SYPL2 expression and other clinical characteristics, such as age, sex, cancer stage, distant metastasis status, and lymph node status. OS was assessed by univariate Cox regression analyses, with factors significantly associated with OS subsequently entered into a multivariate Cox model. In addition, survival was directly analyzed using the Kaplan-Meier method (KM plotter: http://kmplot.com). The presence of 22 types of immune cells in the CRC microenvironment was assessed using the CIBERSORT algorithm[16].
Datasets and phenotype label files from TCGA were generated and uploaded into the GSEA software program. The phenotype labels were SYPL2 high expression and SYPL2 low expression (grouped relative to the median SYPL2 expression). Gene set permutations were conducted 1000 times for each analysis. Gene sets with ES > 0.6 and FWER P < 0.05 were considered enriched.
Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. Cox regression analyses were performed using the R “survival” package. Correlation analyses were performed by determining Pearson’s correlation coefficients. Categorical variables were compared using the chi-squared and Fisher’s exact tests, parametric continuous variables were compared using Student’s t tests, and non-parametric continuous variables were compared using Mann-Whitney U tests. Statistical analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria), Bioconductor (https://www.bioconductor.org/), and GraphPad Prism 8 (GraphPad Software, San Diego, CA, United States), with P < 0.05 defined as statistically significant.
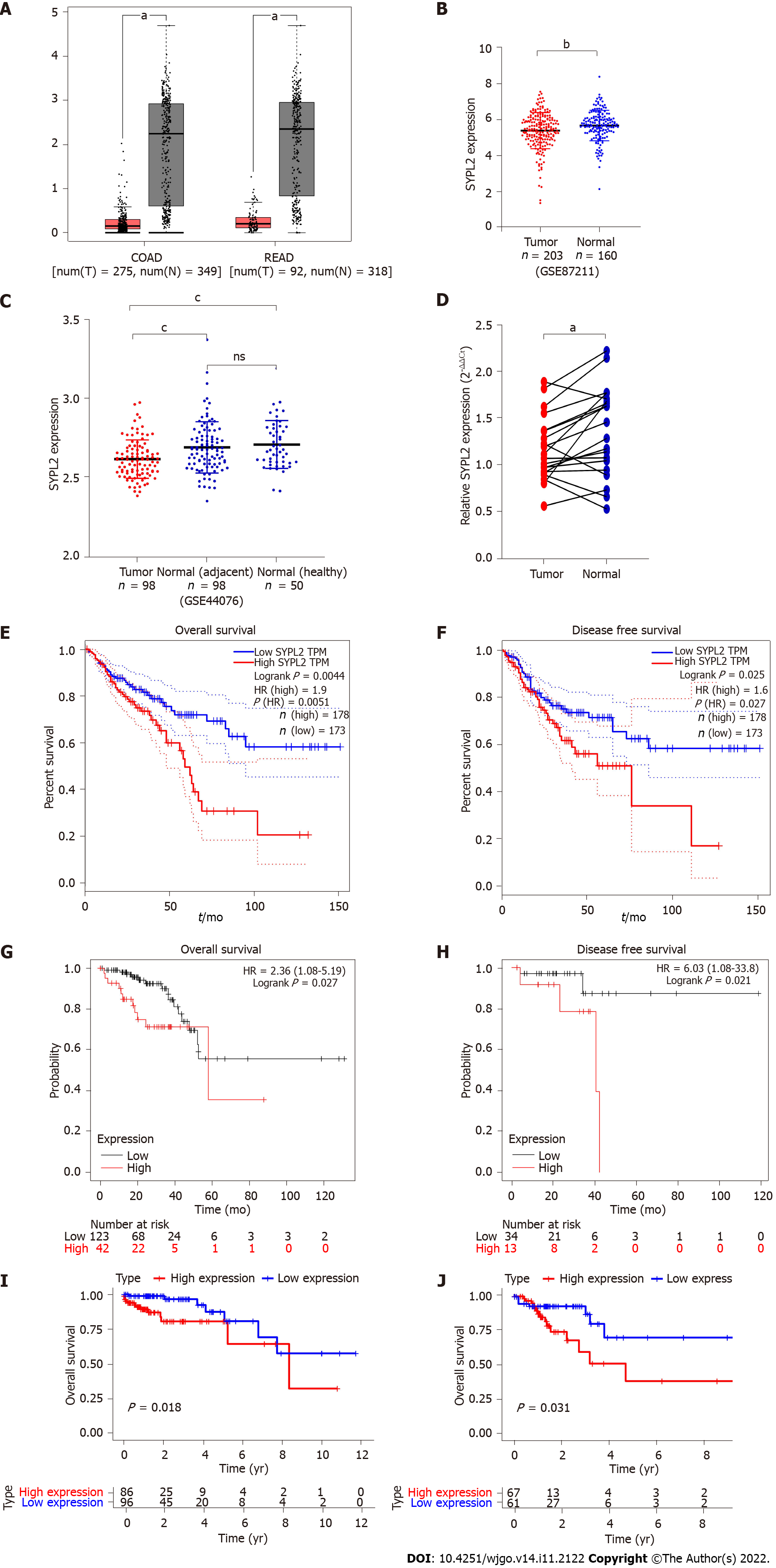
GEPIA showed that the mean expression of SYPL2 was significantly lower in 275 colon cancer tissue samples than in 349 normal colon tissue samples and was lower in 92 rectal cancer tissue samples compared to 318 normal rectal tissue samples (P < 0.05, Figure 1A). Similar findings were observed when SYPL2 expression was compared between 203 CRC and 160 normal colorectal tissue samples (GSE87211) (P < 0.001, Figure 1B) and between 98 CRC samples, 98 adjacent normal colorectal tissue samples, and 50 healthy normal tissue samples (P < 0.0001, Figure 1C). These findings were confirmed by comparing SYPL2 expression by qRT-PCR in 20 freshly obtained CRC and adjacent normal tissue samples (P < 0.05, Figure 1D). A high expression of SYPL2 was significantly associated with poorer OS and DFS (P < 0.05, Figures 1E and 1F), with these results confirmed by Kaplan-Meier analysis (P < 0.05, Figures 1G and 1H). Furthermore, high expression of SYPL2 was significantly associated with a worse OS in both stage II and III CRC (Figures 1I and 1J).
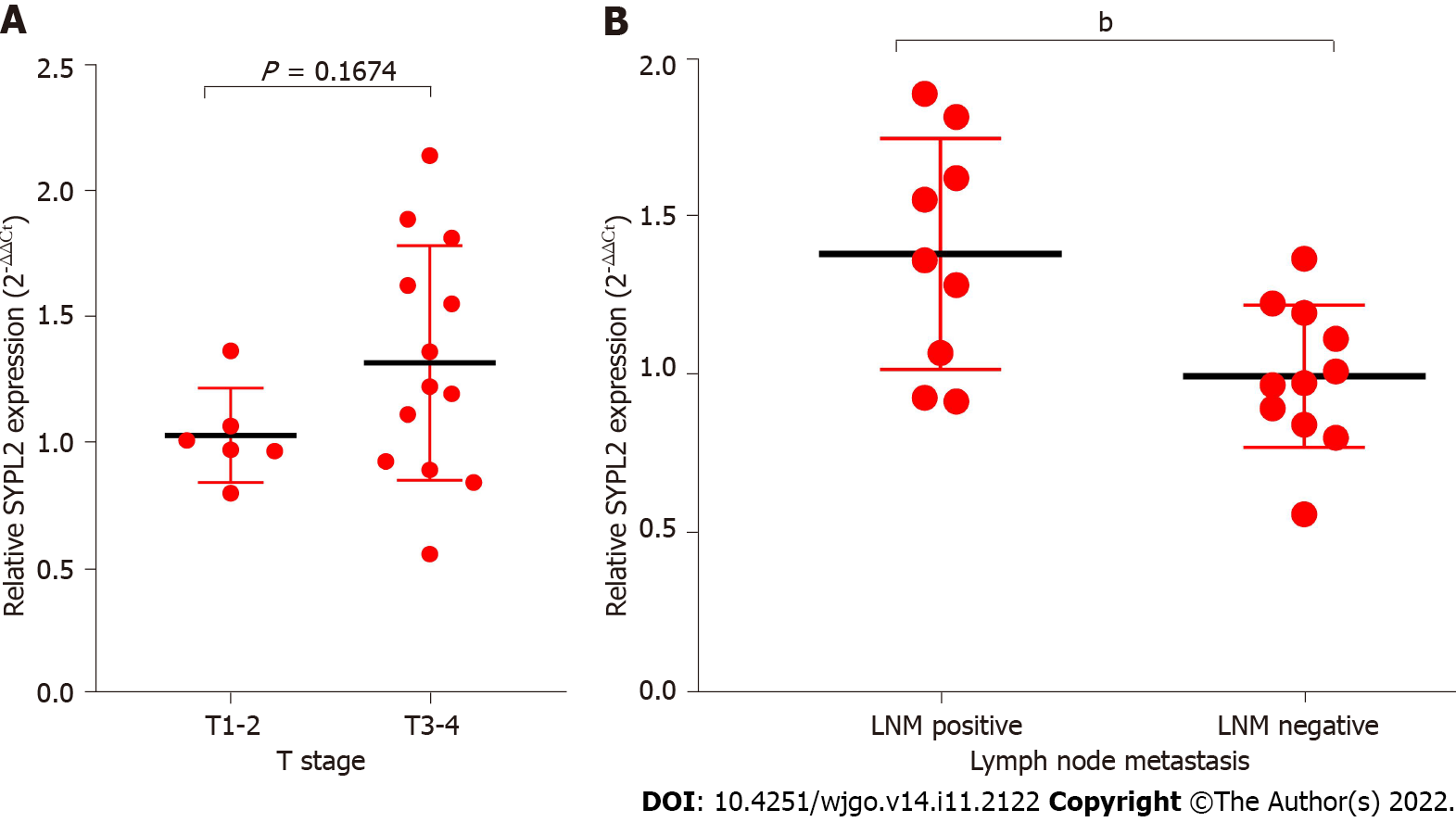
Associations between SYPL2 expression and the clinicopathological characteristics of patients with CRC were assessed by dividing CRC patients into two groups based on the median SYPL2 expression. High expression of SYPL2 was significantly associated with greater tumor depth (P = 0.0140, χ2 = 6.078), LNM (P = 0.0312, χ2 = 4.644), and greater American Joint Committee on Cancer stage (P = 0.0228, χ2 = 5.182) (Table 1). An analysis of the 20 paired CRC and normal colon tissue samples showed that SYPL2 expression was significantly correlated with LNM (P < 0.001, Figure 2B). Univariate Cox regression analysis showed that high expression of SYPL2 was associated with poorer OS [hazard ratio (HR) = 2.078; 95% confidence interval (CI): 1.162-3.716; P = 0.014], older age (P < 0.05), and higher TNM stage (P < 0.05) (Table 2), with multivariate Cox analysis revealing that high SYPL2 expression remained an independent risk factor for OS (HR = 1.754; 95%CI: 1.103-2.790; P = 0.018).
| Characteristics | SYPL2 level | P value | |
| Low (n = 285) | High (n = 286) | ||
| Age (yr) | 68.00 ± 12.14 | 65.31 ± 13.37 | 0.1148 |
| Gender | |||
| Female | 125 | 128 | 0.8273 |
| Male | 145 | 143 | |
| Unknow | 20 | 15 | |
| T | |||
| T1 + T2 | 61 | 37 | 0.0140a |
| T3 + T4 | 179 | 192 | |
| Unknow | 45 | 57 | |
| N | |||
| N0 | 153 | 123 | 0.0312a |
| N1-2 | 87 | 105 | |
| Unknow | 45 | 56 | |
| M | |||
| M0 | 185 | 164 | 0.0955 |
| M1 | 29 | 39 | |
| Unknow | 71 | 83 | |
| AJCC stage | |||
| I-II | 146 | 114 | 0.0228a |
| III-IV | 88 | 106 | |
| Unknow | 51 | 66 | |
| Characteristics | Univariate Cox | Multivariate Cox | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 1.037 | 1.015-1.059 | 0.0001a | 1.051 | 1.027-1.075 | 0.0001a |
| Gender (male) | 1.160 | 0.746-1.803 | 0.509 | 0.987 | 0.621-1.571 | 0.957 |
| T | ||||||
| T1 | 1 | 1 | ||||
| T2 | 0.819 | 0.169-3.953 | 0.804 | 0.354 | 0.069-1.805 | 0.212 |
| T3 | 1.324 | 0.322-5.456 | 0.697 | 0.424 | 0.096-1.863 | 0.256 |
| T4 | 4.661 | 1.082-20.075 | 0.039a | 0.906 | 0.189-4.348 | 0.901 |
| N | ||||||
| N0 | 1 | 1 | ||||
| N1 | 2.547 | 1.484-4.368 | 0.0001a | 1.988 | 1.048-3.770 | 0.035a |
| N2 | 4.195 | 2.479-7.099 | 0.0001a | 2.253 | 1.179-4.304 | 0.014a |
| M (M1) | 4.482 | 2.829-7.100 | 0.0001a | 2.585 | 1.468-4.660 | 0.001a |
| ACJJ | ||||||
| I | 1 | |||||
| II | 1.474 | 0.548-3.961 | 0.442 | |||
| III | 2.858 | 1.086-7.627 | 0.033a | |||
| IV | 8.096 | 3.135-20.903 | 0.0001a | |||
| SYPL2 (high) | 2.078 | 1.162-3.716 | 0.014a | 1.754 | 1.103-2.790 | 0.018a |
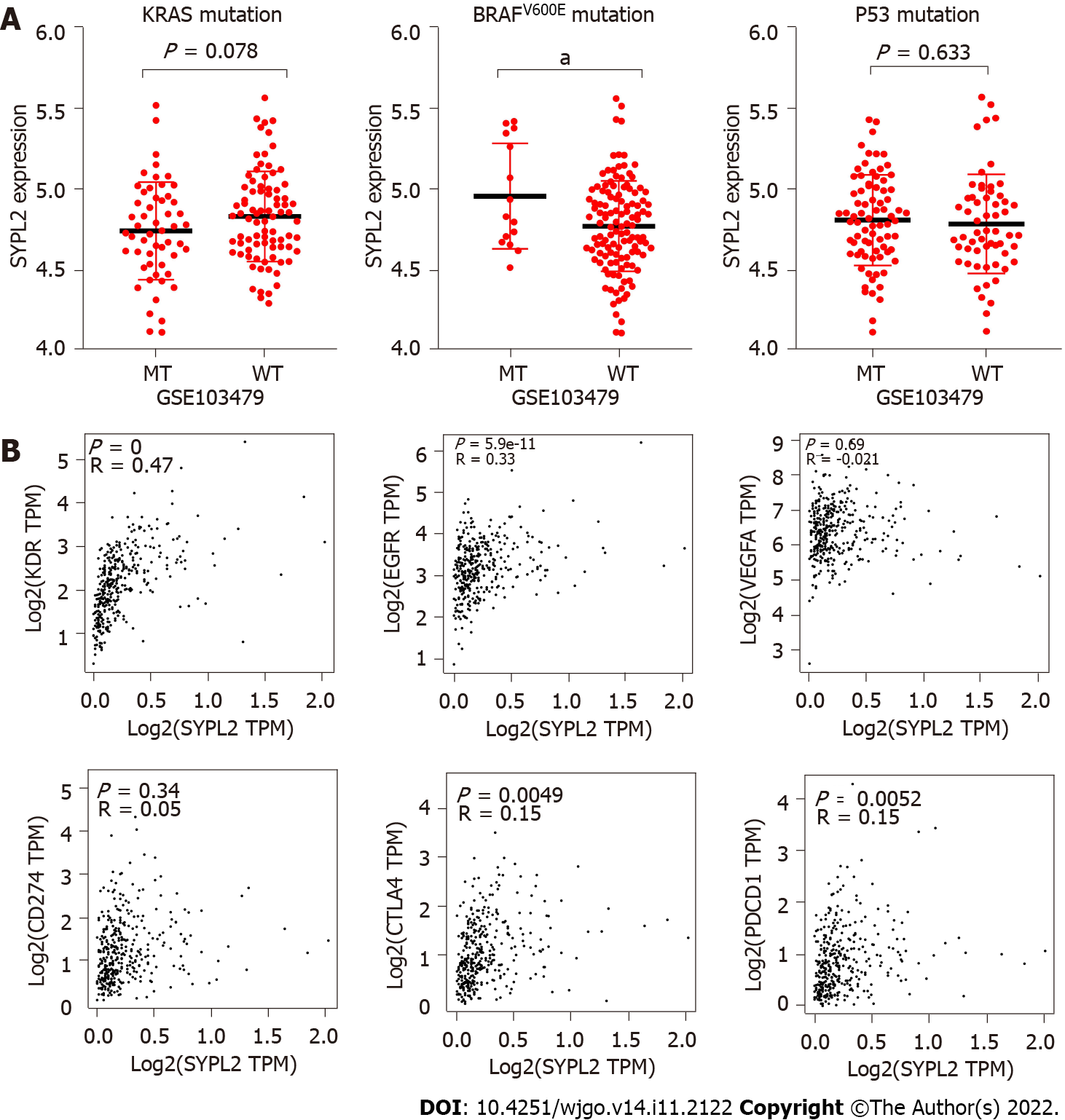
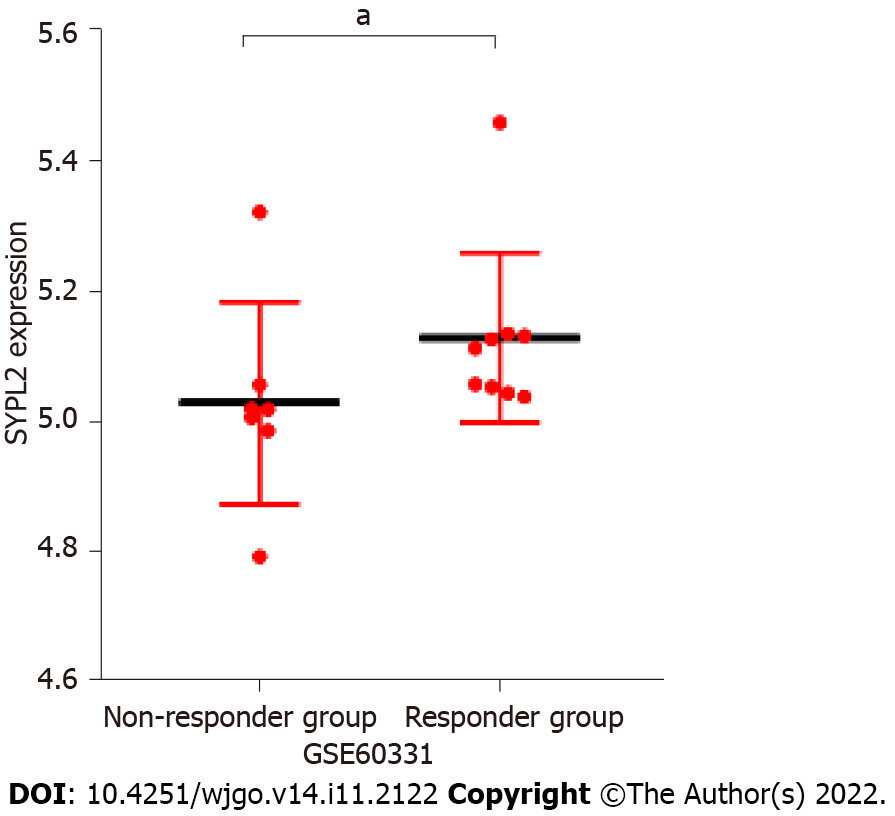
Correlation analyses were performed to determine whether SYPL2 could act as a biomarker to predict the outcomes of targeted therapy in patients with CRC. SYPL2 expression correlated significantly with the mutation of BRAFV600E (P < 0.05) (Figure 3A) and the expression levels of KDR (P < 0.0001, r = 0.47), EGFR (P < 0.0001, r = 0.33), CTLA4 (P < 0.01, r = 0.15), and PDCD1 (P < 0.01, r = 0.15) (Figure 3B). We collected a total of 16 tumor samples entered into GSE60331 prior to undergoing treatment with bevacizumab; the responder group included seven samples and the non-responder group contained nine samples. Some detailed information can be retrieved from GSE60331[17]. Moreover, high SYPL2 expression was found to correlate with the response to bevacizumab treatment (P < 0.05, Figure 4). Taken together, these findings indicate that SYPL2 may be a potential biomarker of response of CRC to targeted therapy.
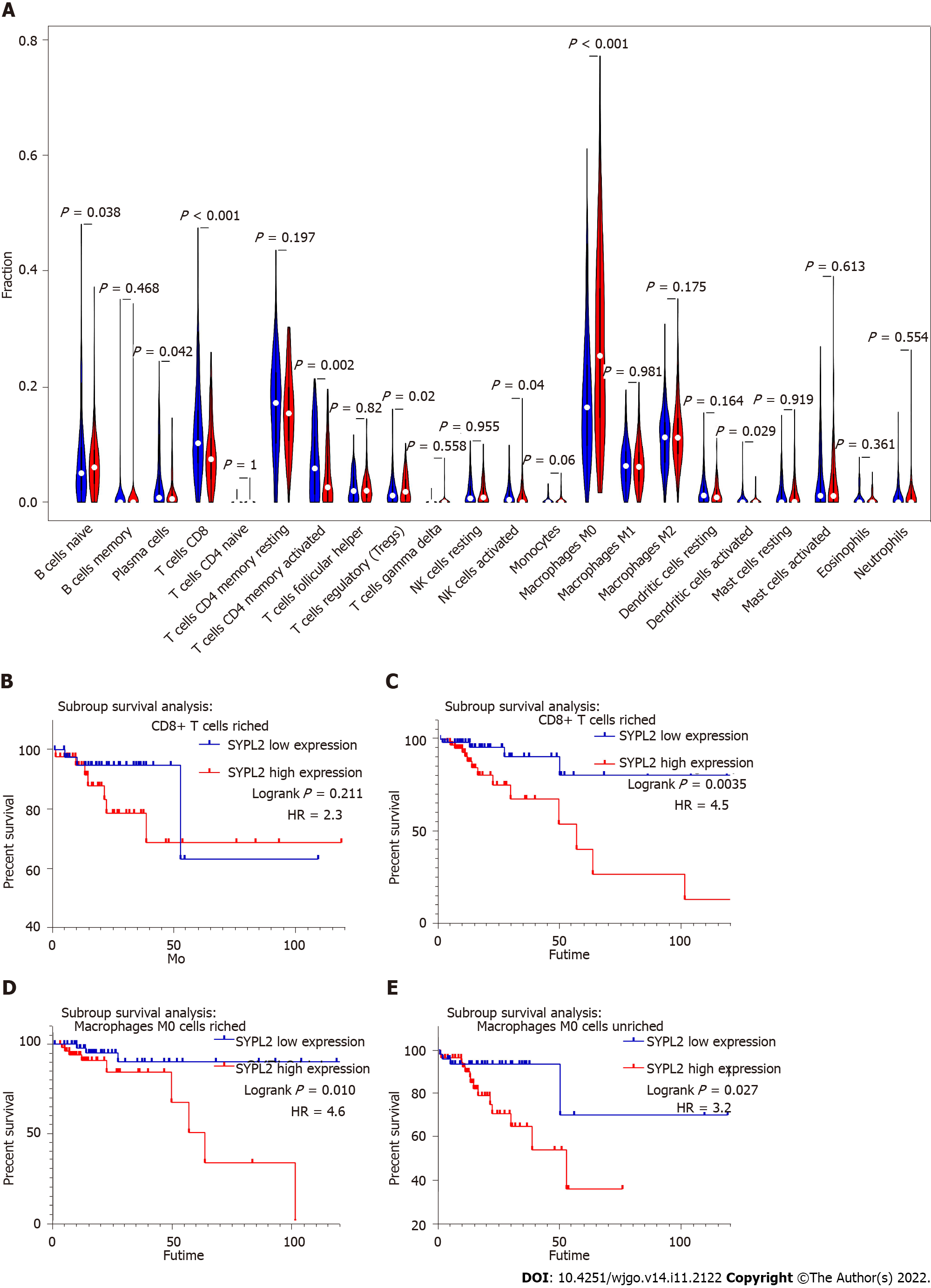
To assess the roles of SYPL2 in the tumor immune microenvironment, it was necessary to investigate the types of infiltrating immune cells in CRC patients. CIBERSORT evaluation of the relative proportions of 22 types of immune cells in all CRC specimens from TCGA showed high infiltration of regulatory T-cells and M0 macrophages in tumors with high SYPL2 expression (Figure 5A) and high infiltration of CD8 T-cells, activated CD4 memory T-cells, activated natural killer cells, and activated dendritic cells in tumors with low SYPL2 expression (Figure 5A). The level of SYPL2 expression had no effect on OS in tumors enriched with CD8 T-cells (P > 0.05, Figure 5B), whereas high expression of SYPL2 was closely associated with poorer OS in tumors unenriched with CD8 T-cells (P < 0.05, Figure 5C). High expression of SYPL2 was also associated with poorer OS in tumors both enriched (P < 0.05, Figure 5D) and unenriched (P < 0.05, Figure 5E) with M0 macrophages. Collectively, these findings show that SYPL2 expression correlates with the level of infiltration of most immune cells, possibly indicating the state of the tumor immune microenvironment and suggesting that SYPL2 might play different roles in different immune microenvironments.
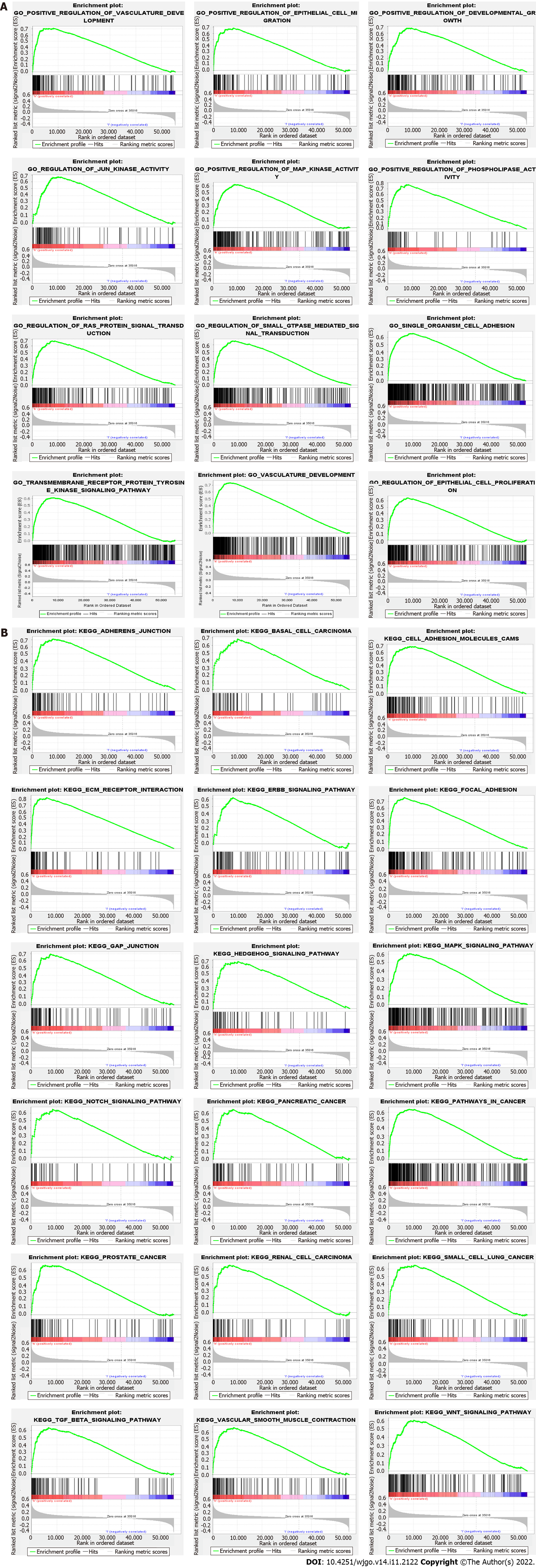
GSEA was performed to determine the biological characteristics shared by tissue samples displaying different levels of SYPL2 expression and to identify the functions and pathways in which SYPL2 may be involved. Gene Ontology (GO) enrichment analyses indicated that SYPL2 was associated with the enrichment of genes involved in the positive regulation of vasculature development, epithelial cell migration, development growth, JUN kinase activity, MAP kinase activity, phospholipase activity, and single-organism cell adhesion (Figure 6A). In addition, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis found that genes involved in basal cell carcinoma; cell-adhesion molecules; extracellular matrix receptor interactions; the epidermal growth factor receptor, hedgehog, mitogen-activated protein kinases (MAPK), NOTCH, transforming growth factor (TGF)-β, and WNT signaling pathways; GAP junctions; and vascular smooth muscle contraction were significantly enriched in CRC samples expressing high levels of SYPL2 (Figure 6B).
Members of the synaptophysin-like family, including SYPL1 and SYPL2, are synaptic vesicle membrane proteins. SYPL1 was originally considered a neuroendocrine-related protein but was found to be expressed in both neuronal and non-neuronal tissues[18]. A recent immunohistochemistry-based study showed that SYPL1 was prognostic of poor outcomes in patients with hepatocellular carcinoma and was associated with the epithelial-mesenchymal transition[19]. SYPL1 is also upregulated in pancreatic ductal adenocarcinoma, with higher SYPL1 expression being associated with tumor cell proliferation and poorer prognosis[20]. Serum SYPL1 may be a diagnostic marker for CRC, especially in patients with low serum carcinoembryonic antigen concentrations[21]. SYPL2, also called MG29, is primarily expressed in skeletal muscles and the tongue and is functionally thought to participate in cellular calcium ion homeostasis[22]. In addition, the SYPL2 gene has been associated with morbid obesity and may be involved in the development of excess body fat[23]. However, the roles and functions of SYPL2 in cancer and its related molecular mechanisms remain unknown.
The present study, using multiple public databases and donor-matched CRC and adjacent normal tissues, showed that the level of SYPL2 expression was lower in cancerous tissues than in normal tissues. An analysis of the associations between SYPL2 expression and clinical pathologic features revealed that higher SYPL2 levels in CRC patients were associated with lymph node metastases (N stage) and more advanced tumors (T stage), although qRT-PCR analysis found that higher SYPL2 expression was associated only with LNM. Univariate and multivariate Cox analyses and survival analyses indicated that SYPL2 expression level is a potential independent marker of poor prognosis in patients with CRC. Correlation analyses showed that the SYPL2 gene-expression level was significantly associated with the expression of KDR (also called VEGFR) (R > 0.4) and EGFR (R > 0.3) and with
Agents targeting VEGFR or EGFR and multiple tyrosine kinase inhibitors play an important role in CRC management[26]. The mutation of BRAFV600E residue occurs in approximately 10% of CRCs, constituting a group with a particularly poor prognosis. And our result also found that SYPL2 was higher expression in the BRAFV600E mutation group, and associated with poor prognosis. The mutation of
GO enrichment analysis of the biological functions of SYPL2 in CRC indicated that SYPL2 might regulate epithelial cell migration, vasculature development, MAPK kinase activity, and cell adhesion. Furthermore, KEGG enrichment analysis found that SYPL2 might participate in cell adhesion; several cancer-related pathways, including pathways in basal cell carcinoma, renal cancer, and small-cell lung cancer; and several vital tumor-related signaling pathways, including the hedgehog, MAPK, NOTCH, and TGF-β signaling pathways.
In this study, SYPL2 expression in tumor tissues was significantly lower than that in normal tissue. However, higher SYPL2 expression was associated with worse survival in CRC. The paradox of the opposite effect of SYPL2 expression might be due to the following reasons. First, compared to normal tissue, tumor tissue can abnormally activate a series of signaling pathways and have special tumor microenvironment[29,30]. Some genes (including SYPL2) may play roles in promoting or suppressing cancer in specific signal pathways in the tumor microenvironment. Second, SYPL2 might work as a biomarker in CRC. Higher expression of SYPL2 could be associated with some specific and powerful activated oncogenic genes and enhanced malignant behavior of tumors. Finally, tumor-infiltrating immune cells are closely related to tumorigenesis, angiogenesis, and tumor cell growth and metastasis, which may in turn regulate the quantity and differentiation of immune cells[31]. CD8+ T-cells are typically thought to be a homogenous group of cytotoxic cells that produce interferon-γ[32]. In addition, CD8+ T lymphocytes are the major anti-tumor effector cells[33]. In this study, CD8+ T-cell counts in the SYPL2 high-expression group were significantly lower than those in the SYPL2 low-expression group. Therefore, SYPL2 might contribute to the poor prognosis of CRC by affecting immune cell infiltration. However, the relevant molecular and pathway mechanisms still necessitate further experiments for verification.
The present study was designed to evaluate SYPL2 gene expression in CRC and reveal the associations of SYPL2 with pathologic features and survival outcomes. This study used only GSEA to analyze biological functions and the molecular mechanism of SYPL2 in CRC. Further studies of SYPL2 protein expression and its associations with biological functions and molecular mechanisms in CRC are warranted.
SYPL2 expression was lower in CRC than in adjacent normal tissue, suggesting that SYPL2 may be a potential diagnostic and prognostic CRC-specific molecular marker. High SYPL2 expression was significantly associated with lymph node metastases and poorer survival.
Colorectal cancer (CRC) is the third-most common cause of cancer deaths worldwide and lymph node metastasis (LNM) is important in CRC staging and patient prognosis. Risk factors for LNM include lymphovascular invasion, histological grade, submucosal invasion depth, and tumor budding. In addition, LNM of CRC is usually evaluated by radiologic methods, including computed tomography, magnetic resonance imaging etc. However, these imaging methods cannot accurately evaluate LNM. It was necessary to investigate key biomarkers to predict LNM and prognosis in patients with CRC. Moreover, synaptophysin-like 2 (SYPL2) is a neuroendocrine-related cytosolic protein enriched primarily in skeletal muscles and the tongue. The role of SYPL2 in cancer, including CRC, has not been determined.
The role of SYPL2 in CRC has not been studied. The present study comprehensively and systematically compared SYPL2 expression and potential functions. The relationship between SYPL2 expression and clinicopathological characteristics was completed. And we found that high expression of SYPL2 was significantly associated with LNM and worse prognosis. And we verified the results by experiment. In addition, we analyzed the correlation between SYPL2 expression and the expression and mutation of target genes.
This study aimed to investigate the SYPL2 expression, potential biological functions and pathways, correlation clinicopathological characteristics and prognosis in CRC.
The gene expression profiles and associated clinicopathological data of patients with CRC were downloaded from multiple public and online databases {The Cancer Genome Atlas, GEO, Gene Expression Profiling Interactive Analysis [gene set enrichment analysis (GSEA)]}. The associations between clinical variables, prognosis and SYPL2 expression were analyzed statistically using the Kaplan-Meier method, univariate/multivariate Cox regression analyses, chi-squared and Fisher’s exact tests. In addition, we collected 20 paired CRC tissue and adjacent normal colorectal tissue samples for validation by quantitative real-time polymerase chain reaction (qRT-PCR). GSEA was performed to evaluate the biofunction and pathways of SYPL2 in CRC.
SYPL2 expression was significantly lower in CRC tissue samples than in normal colorectal tissue samples. High SYPL2 levels in CRC tissues correlated significantly with LNM and worse prognosis. High SYPL2 expression was an independent risk factor for overall survival in both univariate and multivariate Cox regression analyses. SYPL2 expression correlated significantly with the expression of KDR and high SYPL2 expression was correlate with the response to bevacizumab treatment. Higher SYPL2 expression was associated with the enrichment of CD8 T-cells and M0 macrophages. GSEA revealed that SYPL2 was associated with the regulation of epithelial cell migration, vasculature development, pathways in cancer, and several vital tumor-related pathways.
SYPL2 expression was lower in CRC than in adjacent normal tissue. However, high SYPL2 expression was significantly associated with lymph node metastases and poorer survival.
The SYPL2 gene expression and the correlations between clinical variables, prognosis were analyzed by multiple public and online databases. Furthermore, we collected 20 paired CRC tissue and adjacent normal colorectal tissue samples for validation by qRT-PCR.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hamaya Y, Japan; Herold M, Hungary S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11440] [Article Influence: 3813.3] [Reference Citation Analysis (4)] |
| 2. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3272] [Article Influence: 654.4] [Reference Citation Analysis (2)] |
| 3. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2294] [Article Influence: 208.5] [Reference Citation Analysis (1)] |
| 4. | Provenzale D, Ness RM, Llor X, Weiss JM, Abbadessa B, Cooper G, Early DS, Friedman M, Giardiello FM, Glaser K, Gurudu S, Halverson AL, Issaka R, Jain R, Kanth P, Kidambi T, Lazenby AJ, Maguire L, Markowitz AJ, May FP, Mayer RJ, Mehta S, Patel S, Peter S, Stanich PP, Terdiman J, Keller J, Dwyer MA, Ogba N. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 2.2020. J Natl Compr Canc Netw. 2020;18:1312-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 5. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hoffe S, Hubbard J, Hunt S, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gurski LA. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020;18:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 6. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 960] [Article Influence: 240.0] [Reference Citation Analysis (16)] |
| 7. | Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D; ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 810] [Article Influence: 162.0] [Reference Citation Analysis (0)] |
| 8. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1199] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 9. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1313] [Article Influence: 262.6] [Reference Citation Analysis (1)] |
| 10. | Li M, Zhang J, Dan Y, Yao Y, Dai W, Cai G, Yang G, Tong T. A clinical-radiomics nomogram for the preoperative prediction of lymph node metastasis in colorectal cancer. J Transl Med. 2020;18:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Yang L, Liu D, Fang X, Wang Z, Xing Y, Ma L, Wu B. Rectal cancer: can T2WI histogram of the primary tumor help predict the existence of lymph node metastasis? Eur Radiol. 2019;29:6469-6476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Huang D, Sun W, Zhou Y, Li P, Chen F, Chen H, Xia D, Xu E, Lai M, Wu Y, Zhang H. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 2018;37:173-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 13. | Yang C, Cao F, Huang S, Zheng Y. Follistatin-Like 3 Correlates With Lymph Node Metastasis and Serves as a Biomarker of Extracellular Matrix Remodeling in Colorectal Cancer. Front Immunol. 2021;12:717505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1445] [Article Influence: 361.3] [Reference Citation Analysis (0)] |
| 15. | Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 2019;125:4139-4147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 16. | Ciavarella S, Vegliante MC, Fabbri M, De Summa S, Melle F, Motta G, De Iuliis V, Opinto G, Enjuanes A, Rega S, Gulino A, Agostinelli C, Scattone A, Tommasi S, Mangia A, Mele F, Simone G, Zito AF, Ingravallo G, Vitolo U, Chiappella A, Tarella C, Gianni AM, Rambaldi A, Zinzani PL, Casadei B, Derenzini E, Loseto G, Pileri A, Tabanelli V, Fiori S, Rivas-Delgado A, López-Guillermo A, Venesio T, Sapino A, Campo E, Tripodo C, Guarini A, Pileri SA. Dissection of DLBCL microenvironment provides a gene expression-based predictor of survival applicable to formalin-fixed paraffin-embedded tissue. Ann Oncol. 2018;29:2363-2370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 17. | Verstraete M, Debucquoy A, Dekervel J, van Pelt J, Verslype C, Devos E, Chiritescu G, Dumon K, D'Hoore A, Gevaert O, Sagaert X, Van Cutsem E, Haustermans K. Combining bevacizumab and chemoradiation in rectal cancer. Translational results of the AXEBeam trial. Br J Cancer. 2015;112:1314-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Windoffer R, Borchert-Stuhlträger M, Haass NK, Thomas S, Hergt M, Bulitta CJ, Leube RE. Tissue expression of the vesicle protein pantophysin. Cell Tissue Res. 1999;296:499-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Chen DH, Wu QW, Li XD, Wang SJ, Zhang ZM. SYPL1 overexpression predicts poor prognosis of hepatocellular carcinoma and associates with epithelial-mesenchymal transition. Oncol Rep. 2017;38:1533-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Song Y, Sun X, Duan F, He C, Wu J, Huang X, Xing K, Sun S, Wang R, Xie F, Mao Y, Wang J, Li S. SYPL1 Inhibits Apoptosis in Pancreatic Ductal Adenocarcinoma via Suppression of ROS-Induced ERK Activation. Front Oncol. 2020;10:1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Liu L, He Q, Li Y, Zhang B, Sun X, Shan J, Pan B, Zhang T, Zhao Z, Song X, Guo Y. Serum SYPL1 is a promising diagnostic biomarker for colorectal cancer. Clin Chim Acta. 2020;509:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Kurebayashi N, Takeshima H, Nishi M, Murayama T, Suzuki E, Ogawa Y. Changes in Ca2+ handling in adult MG29-deficient skeletal muscle. Biochem Biophys Res Commun. 2003;310:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol. 2002;4:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Carvalho B, Lopes JM, Silva R, Peixoto J, Leitão D, Soares P, Fernandes AC, Linhares P, Vaz R, Lima J. The role of c-Met and VEGFR2 in glioblastoma resistance to bevacizumab. Sci Rep. 2021;11:6067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Szablewski V, Solassol J, Poizat F, Larrieux M, Crampette L, Mange A, Bascoul-Mollevi C, Costes V. EGFR Expression and KRAS and BRAF Mutational Status in Intestinal-Type Sinonasal Adenocarcinoma. Int J Mol Sci. 2013;14:5170-5181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Khotskaya YB, Holla VR, Farago AF, Mills Shaw KR, Meric-Bernstam F, Hong DS. Targeting TRK family proteins in cancer. Pharmacol Ther. 2017;173:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 27. | Grothey A, Fakih M, Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol. 2021;32:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 28. | Wang C, Li Y, Jia L, Kim JK, Li J, Deng P, Zhang W, Krebsbach PH, Wang CY. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell. 2021;28:1597-1613.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 202] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 29. | Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18:9-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 596] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 30. | Hernández Borrero LJ, El-Deiry WS. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer. 2021;1876:188556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 352] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 31. | Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, Signoretti S, Liu JS, Liu XS. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1262] [Cited by in RCA: 1658] [Article Influence: 184.2] [Reference Citation Analysis (0)] |
| 32. | St Paul M, Ohashi PS. The Roles of CD8+ T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020;30:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 383] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 33. | Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C, Tihy I, Tartour E. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. 2018;6:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |