Published online Dec 15, 2021. doi: 10.4251/wjgo.v13.i12.2180
Peer-review started: July 5, 2021
First decision: July 29, 2021
Revised: August 10, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: December 15, 2021
Processing time: 162 Days and 14.6 Hours
The diagnosis of both cancer and intracerebral hemorrhage (ICH) in the same patient is not uncommon, but the clinical features and pathogenesis of patients with colorectal cancer (CRC) and ICH are still not well known.
To investigate the clinical features and underlying pathogenesis of ICH in patients with CRC.
A retrospective review of CRC patients complicated with ICH from three centers between January 2014 and December 2020 was performed. Clinical data such as laboratory examinations, imaging features, prognosis, and underlying patho
Of 16673 identified CRC patients, 20 (0.12%) suffered from ICH. There were 13 males and 7 females, with an average age (mean ± SD) of 68.45 ± 10.66 years. Fourteen patients (70%) had distant metastases and most patients (85%) showed an elevation of one or more cancer biomarkers. The hemorrhagic lesions in 13 patients (65%) were in the intracerebral lobe. Four patients were completely dependent and 4 died within 30 days after hemorrhage. Intratumoral hemorrhage (50%) and coagulopathy (50%) accounted for the majority of hemorrhages.
Patients with ICH and CRC often have clinical features with lobar hemorrhage, distant metastases and poor prognosis. Intratumoral hemorrhage and coagu
Core Tip: The association between cancer and intracerebral hemorrhage (ICH) has long been studied, however little attention has been paid to the hemorrhagic cerebrovascular events in patients with colorectal cancer (CRC). CRC has been reported to increase the risk of ICH. The present study retrospectively analyzed the clinical data of patients with CRC and ICH, and indicated that intratumoral hemorrhage and coagulopathy were the main causes of ICH in CRC patients.
- Citation: Deng XH, Li J, Chen SJ, Xie YJ, Zhang J, Cen GY, Song YT, Liang ZJ. Clinical features of intracerebral hemorrhage in patients with colorectal cancer and its underlying pathogenesis. World J Gastrointest Oncol 2021; 13(12): 2180-2189
- URL: https://www.wjgnet.com/1948-5204/full/v13/i12/2180.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i12.2180
Intracerebral hemorrhage (ICH) and cancer are both common disorders, and are significant causes of morbidity and mortality worldwide in the elderly. It is reported that ICH accounts for almost half of cerebrovascular events in cancer patients[1,2] and significantly aggravates their condition and prognosis[3,4]. Several population-based studies have demonstrated that multiple cancers are associated with an increased risk of ICH[5,6]. Despite the fact that traditional vascular risk factors for ICH are com
Colorectal cancer (CRC) is the third most frequently diagnosed cancer in the world[9,10]. It has been estimated that CRC burden rose to approximately 1.9 million new cases and 0.9 million deaths worldwide in 2020[10]. However, there are few detailed reports in the literature that focus on the hemorrhagic cerebrovascular events in CRC patients. The purpose of this study was to investigate the clinical features and underlying pathogenesis of ICH in patients with CRC.
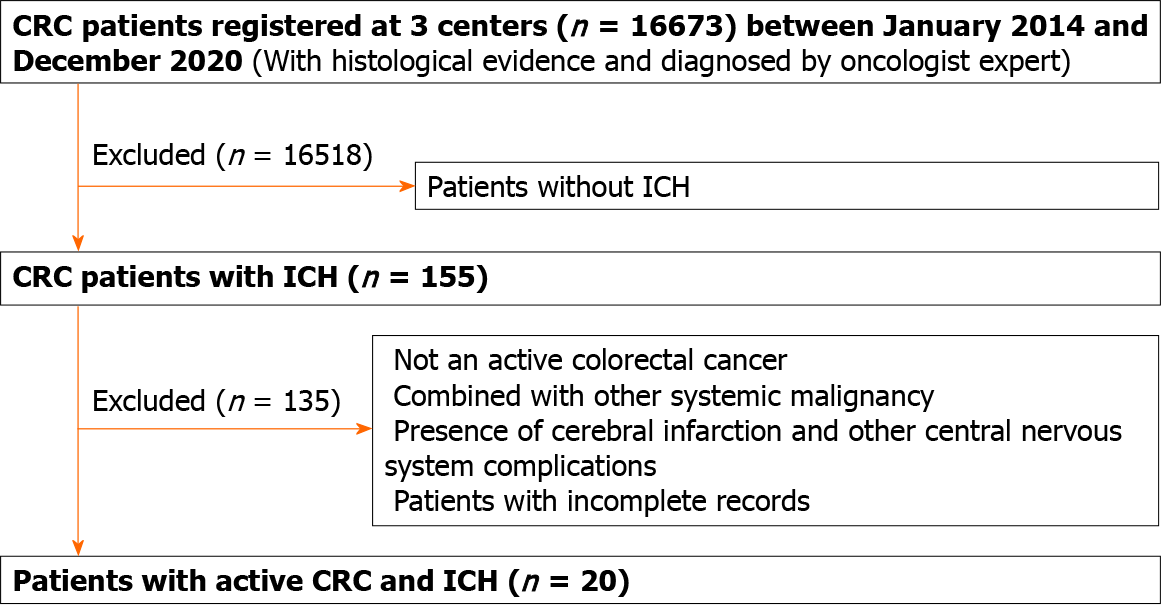
The present study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. CRC patients with ICH were recruited from the First Affiliated Hospital and the Affiliated Cancer Center of Guangxi Medical University, and the Affiliated Yuebei People’s Hospital of Shantou University Medical College between January 2014 and December 2020. The diagnostic criteria for ICH were based on the 2015 Guidelines for the Diagnosis and Treatment from the American Heart Association[11]. The diagnosis of active CRC followed the definition of active cancer in the study by Lee et al[12]. Patients who met the following criteria were included: (1) Diagnosis of CRC within 6 mo before enrollment, any treatment for CRC within the previous 6 mo, or recurrent or metastatic CRC; (2) Presence of clinical symptoms, such as sudden onset of unconsciousness, headache, hemiplegic paralysis, slurred speech, or other focal neurological deficits; and (3) The presence of ICH on intracranial computed tomography (CT) or magnetic resonance imaging (MRI) scan and susceptibility-weighted imaging, which could explain the symptoms. The exclusion criteria were as follows: (1) Combined with other systemic malignancy; (2) Presence of cerebral infarction and other central nervous system complications; (3) CRC diagnosed > 5 years ago, with no evidence of recurrence or metastasis; and (4) Patients with incomplete records (Figure 1). The selected cases were reviewed and determined by a panel which consisted of an oncologist, a neurologist and a neuroradiologist who were all blind to the study.
General demographic data such as age and gender were obtained. Vascular risk factors for ICH including hypertension, diabetes mellitus, hypercholesterolemia, tobacco and alcohol consumption, aneurysm, arteriovenous malformation and stroke history were also documented. Moreover, data on CRC, such as pathological types of cancer cells, metastasis, prior and current treatment and information related to acute ICH, including onset form, cardinal symptoms and signs, and hemorrhagic locations were recorded. In addition, routine blood examination, blood biochemistry, coagulation indices, plasma D-dimer, cancer biomarkers, electrocardiography, Doppler echocardiography, cervical vascular Doppler, head and neck CT angiography, head CT, head MRI and magnetic resonance angiography were also carried out. The National Institute of Health Stroke Scale (NIHSS) was used to evaluate the severity of focal neurological deficits. To minimize the effects of CRC progression on physical activities, patients’ functional prognosis on the 30th day after hemorrhage was measured using the modified Rankin Scale (mRS), and a mRS score > 3 was regarded as a poor prognosis[13]. According to a research study by Navi and colleagues on ICH[2], coagulopathy was identified if any of the following parameters were fulfilled: platelets < 100 × 109/L, international normalized ratio (INR) > 1.5, activated partial thromboplastin time (APTT) > 45 s, prothrombin time (PT) > 15 s, and disseminated intravascular coagulation (DIC) (fibrinogen < 200 mg/dL and D-dimer > 290 ng/dL).
All statistical analyses were undertaken using SPSS 20.0 software (IBM, Inc., Armonk, NY, United States). Quantitative data were shown as mean ± SD and qualitative data were expressed as frequency and percentages.
A total of 16673 patients with CRC were identified, and 20 patients (0.12%) with ICH met the inclusion criteria. Of these 20 patients, 13 were male (65%) and 7 were female (35%). The average age (mean ± SD) was 68.45 ± 10.66 years (range 47-82). All patients were pathologically confirmed to have adenocarcinoma. Ten patients had vascular risk factors, including tobacco use (30%), hypertension (30%), diabetes mellitus (10%), alcohol abuse (5%), hypercholesterolemia (15%), stroke history (10%) and coronary or kidney disease (5%). No aneurysms or arteriovenous malformations were identified in these patients. None of the patients were on therapeutic anticoagulation or antiplatelet agents at the time of hemorrhage. Coagulopathy was observed in 10 patients. Thrombocytopenia was present in 6 patients (30%). Six patients (30%) had a prolonged PT value, 2 (10%) had a prolonged APPT value, and 3 (15%) displayed INR values greater than 1.5. DIC was documented in 1 patient. Most patients (17/20, 85%) had at least one elevated cancer biomarker. Six patients (30%) showed hepatic dysfunction, while 14 patients (70%) did not. Fourteen patients (70%) exhibited distant metastases, intracranial metastasis occurred in 10 patients (50%) and hepatic/osseous metastases occurred in 8 patients (40%), when ICH developed. Prior to hemorrhage, 13 patients (65%) had received oncological therapy while 7 patients (35%) had not (Table 1).
| Characteristics | mean ± SD/n (%) |
| Age, yr | 68.45 ± 10.66 |
| Gender | |
| Male | 13 (65) |
| Female | 7 (35) |
| Vascular risk factors | 10 (50) |
| Tobacco | 6 (30) |
| Hypertension | 6 (30) |
| Diabetes mellitus | 2 (10) |
| Hypercholesterolemia | 3 (15) |
| Alcohol abuse | 1 (5) |
| Stroke history | 2 (10) |
| Coronary or kidney disease | 1 (5) |
| Coagulopathy | 10 (50) |
| PLT < 100 × 109/L | 6 (30) |
| PT > 15 s | 6 (30) |
| APTT > 45 s | 2 (10) |
| INR > 1.5 | 3 (15) |
| DIC | 1 (5) |
| Elevated cancer biomarkers | 17 (85) |
| CEA > 5 ng/mL | 16 (80) |
| CA125 > 35 U/mL | 4 (20) |
| CA153 > 31.3 U/mL | 2 (10) |
| CA199 > 37 U/mL | 9 (45) |
| Hepatic dysfunction | |
| Yes | 6 (30) |
| No | 14 (70) |
| Distant metastasis | |
| Intracranial metastasis | 10 (50) |
| Hepatic/osseous metastasis | 8 (40) |
| Metastasis of other organs | 7 (35) |
| None | 6 (30) |
| Cancer treatment before hemorrhage | |
| Surgery alone | 2 (10) |
| Chemotherapy | 3 (15) |
| Both of the above | 8 (40) |
| No treatment | 7 (35) |
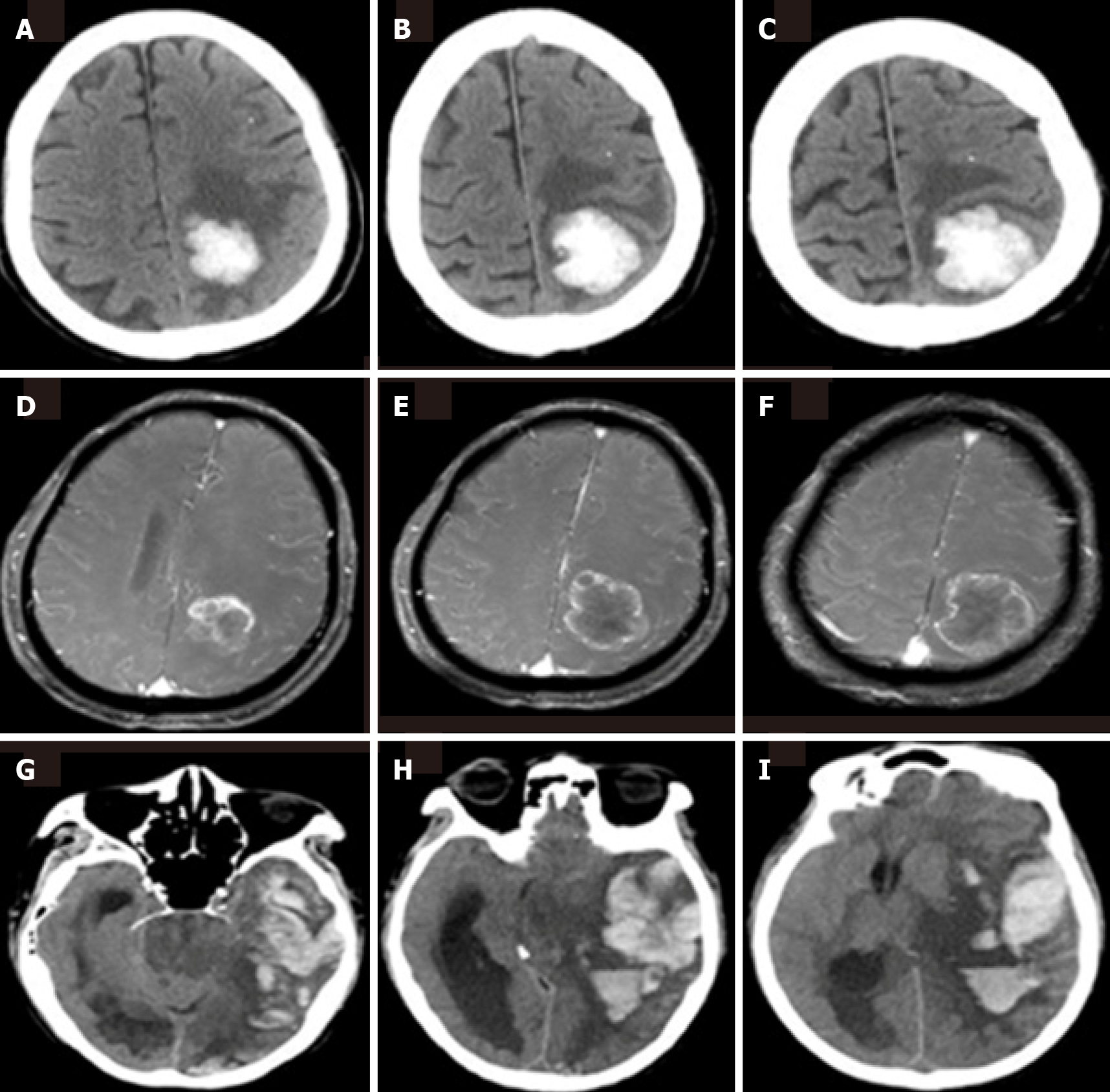
ICH occurred in 2 patients (10%) before cancer diagnosis and 18 patients (90%) after cancer diagnosis. The most frequent hemorrhagic lesion was in the cerebral lobe, occurring in 13 patients (65%). Fewer lesions were found in the basal ganglia (4/20, 20%), cerebellum (2/20, 10%), and brainstem (1/20, 5%). The etiologies of hemorrhage were ascribed to intratumoral hemorrhage (10/20, 50%), coagulopathy (10/20, 50%), both intratumoral hemorrhage and coagulopathy (5/20, 25%), hypertension (4/20, 20%), and trauma (1/20, 5%). Fifteen patients (75%) received hemorrhage targeted treatment and 5 (25%) withdrew treatment. The mean NIHSS score was 10.25 ± 7.59 (range 2-25) on the day of hemorrhage onset. On the 30th day after hemorrhage, 7 patients (35%) were completely independent, 5 (25%) were partially independent, 4 (20%) were completely dependent, and 4 (20%) died (Table 2). Among the 4 patients who died, the cause of death was fatal ICH induced by DIC in one patient, brain metastasis and intratumoral hemorrhage in one patient, and aspiration pneumonia in the other two patients (Figure 2).
| Characteristics | n (%) |
| Location of hemorrhage | |
| Lobe | 13 (65) |
| Basal ganglia | 4 (20) |
| Brainstem | 1 (5) |
| Cerebellum | 2 (10) |
| Intratumoral hemorrhage | 10 (50) |
| Treatment of hemorrhage | |
| Conservative treatment | 14 (70) |
| Surgery | 1 (5) |
| Withdrawal of treatment | 5 (25) |
| NIHSS score on the day of hemorrhage onset | |
| NIHSS (0-5) | 7 (35) |
| NIHSS (6-10) | 5 (25) |
| NIHSS (11-20) | 3 (15) |
| NIHSS (> 20) | 5 (25) |
| mRS score 30 d after hemorrhage | |
| Completely independent (mRS = 0-1) | 7 (35) |
| Partially independent (mRS = 2-3) | 5 (25) |
| Completely dependent (mRS = 4-5) | 4 (20) |
| Death (mRS = 6) | 4 (20) |
| Etiology | |
| Intratumoral hemorrhage | 10 (50) |
| Coagulopathy | 10 (50) |
| Both of the above | 5 (25) |
| Hypertension | 4 (20) |
| Trauma | 1 (5) |
| Time interval between CRC diagnosis and hemorrhage | |
| ICH onset before cancer diagnosis | 2 (10) |
| ICH onset after cancer diagnosis | |
| < 6 mo | 8 (40) |
| 6-12 mo | 5 (25) |
| 1-5 yr | 4 (20) |
| 5-10 yr | 1 (5) |
ICH is a well-known potential complication of cancer[1,2]. A nationwide follow-up study from Sweden reported that compared with a non-cancer population, the overall risk of hemorrhage after diagnosis of cancer within 6 mo, 6 mo to 1 year and 1 year to 5 years was 2.2, 1.4 and 1.3, respectively[6], indicating that cancer could increase the incidence of ICH and that cancer-related ICH theoretically exists. Patients with CRC also had a significantly increased risk of hemorrhage[6]. CRC-related ICH may also theoretically exist. In this study, although only 0.12% of CRC patients developed ICH, the incidence of hemorrhage was still much higher than that in the general population. Traditional pathogenesis were observed in the etiology of ICH in CRC patients, but more attention should be paid to the direct or indirect role of CRC itself.
In a retrospective review, cancer patients with ICH were more likely to present with coagulopathy, a lobar hemorrhage location, and poor short-term prognosis when compared with non-cancer patients[14]. In the present study, hemorrhagic lesions were more frequent in the cerebral lobe, occurring in 13 of 20 patients, and the short-term prognosis was poor, as disability and death occurred in 8 of these 20 patients within 30 d after hemorrhage onset. Coagulopathy was identified in half of the patients. In some respects, the results of this study were consistent with the research stated above. In addition, most patients developed distant metastases and had significantly elevated cancer biomarkers when ICH occurred, which was another characteristic in CRC patients with ICH.
Although ICH is a late complication in most cancer patients, it may precede cancer diagnosis. It is worth noting that 2 patients were newly diagnosed with CRC during hospitalization for ICH, suggesting that ICH might be the first manifestation of CRC. Cancers with ICH as the initial symptom include choriocarcinoma, leukocythemia, and medulloblastoma[15-17]. How to quickly identify insidious cancer in patients with ICH is still a challenge. Therefore, it is necessary to conduct cancer associated examinations, including CRC associated examinations, in patients with unexplained hemo
ICH in patients with cancer often arises from unique mechanisms which are uncommon in the general population. A clinical series of 208 cancer patients with intracranial hemorrhage found that intratumoral hemorrhage and coagulopathy were the main causes, and that hypertension, the most common cause of ICH in the population, accounted for only a small proportion[2]. In the present study, ICH was caused by hypertension in a small number of patients but was mostly caused by intratumoral hemorrhage and coagulopathy, which was similar to the above results, indicating that intratumoral hemorrhage and coagulopathy might be the main pathogenesis of ICH in patients with CRC.
Pathophysiologically, factors favoring intratumoral hemorrhage include tumor necrosis, aberrant neovascularization, vascular infiltration with rupture, imbalances in the fibrinolytic cascade, and overexpression of vascular endothelial growth factor (VEGF) and metalloproteinases[1-2,18,19]. Tumor cells can directly invade blood vessels and destroy their integrity, resulting in vascular rupture. Overexpression of VEGF in tumor cells can stimulate neovascularization and increase microvessel density. However, the fine structure of newly formed blood vessels induced by VEGF tends to have high permeability and fragility leading to hemorrhagic events[19]. Abnormal high expression of metalloproteinases can degrade extracellular matrix proteins which maintain basement membrane structural/functional integrity, and cause considerable damage to capillary integrity leading to hemorrhage[19]. Overexpression of VEGF in CRC cells has been confirmed, and anti-VEGF therapies have been proved to be effective for metastatic CRC[20,21]. The expression and activity of metalloproteinases are high in CRC cells[22], and matrix metalloproteinase inhibitors might be a new way of treating metastatic CRC.
Coagulopathy is typically related to a complex interplay of multiple mechanisms, including thrombocytopenia, coagulation factor abnormalities or both. The etiology of thrombocytopenia can be diverse and multifactorial in cancer patients. Systemic chemotherapy is the most common cause of bone marrow suppression and thrombocytopenia[23]. In patients with solid tumors, thrombocytopenia is potentially asso
CA125, CA153, CA199 and CEA are widely used as common cancer biomarkers in the clinic, and are well known carcinoma mucins. Previous studies revealed that carcinoma mucins were overexpressed by malignant cells and were shown to play a multifaceted role in the initiation, progression, metastasis and subsequent colonization of multiple malignancies[27,28]. In a clinical study of lung cancer-related ICH, elevated plasma CEA and CA199 Levels were independent risk factors for ICH in patients with active lung cancer[13]. The researchers hypothesized that elevated cancer markers could activate platelets and lead to increased platelets, hypercoagulability, and eventually thrombotic events in the early stage of cancer; however, in the later stage, elevated cancer markers could lead to decreased platelets due to consumption, coagulopathy, and finally hemorrhagic events. Significantly elevated cancer bio
The pathogenesis of ICH induced by CRC is complex and has not been fully elucidated. To date, there is no feasible method to effectively prevent and treat ICH in patients with CRC. In view of the pathophysiological changes such as thrombocytopenia and abnormalities of coagulation factors, it is possible to take active measures, including increasing nutrition supply, and supplementing platelets and fresh plasma, to improve the coagulation state in the early stage. These measures may delay or reduce the occurrence of ICH in patients with CRC, but should be confirmed in future studies.
One of the limitations of this study is its retrospective nature; thus, information bias may exist. Although clinical data were collected from three centers, the total number of selected cases was relatively small. Prospective population-based cohort studies are warranted to better elucidate the clinical characteristics and pathogenesis of CRC patients with ICH.
Patients with ICH and CRC often have clinical features with lobar hemorrhage, distant metastases and poor prognosis. Intratumoral hemorrhage and coagulopathy are the main causes of ICH in patients with CRC. More clinical trials are needed to validate these findings in the future.
Many studies have confirmed that cancer can increase the risk of intracerebral hemorrhage (ICH). However, most previous studies were conducted on multiple cancers, and few focused on a specific cancer. The clinical characteristics and mechanisms of ICH in colorectal cancer (CRC) patients have not been fully elucidated.
There are few reports on hemorrhagic cerebrovascular events in patients with CRC.
This retrospective study aimed to investigate the clinical features and underlying pathogenesis of ICH in patients with CRC.
A retrospective review of 20 patients (13 males and 7 females) with CRC and ICH from three centers between January 2014 and December 2020 was conducted. The clinical data of the patients such as vascular risk factors, laboratory results, neuroimaging and underlying pathogenesis were analyzed.
The average age (mean ± SD) of the patients was 68.45 ± 10.66 years. Fourteen patients (70%) had distant metastases and most patients (85%) had an elevation of one or more cancer biomarkers. The hemorrhagic lesions in 13 patients (65%) were in the intracerebral lobe. Four patients were completely dependent and 4 died within 30 days after hemorrhage. Intratumoral hemorrhage (50%) and coagulopathy (50%) accounted for the majority of hemorrhages.
Patients with ICH and CRC often have clinical features with lobar hemorrhage, distant metastases and poor prognosis. Intratumoral hemorrhage and coagulopathy are the main causes of ICH in patients with CRC.
The detailed mechanism of ICH in CRC patients requires further elucidation. Prospective population-based studies are needed to confirm these findings in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emran TB S-Editor: Yan JP L-Editor: A P-Editor: Yuan YY
| 1. | Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep. 2012;14:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Navi BB, Reichman JS, Berlin D, Reiner AS, Panageas KS, Segal AZ, DeAngelis LM. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology. 2010;74:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Gon Y, Todo K, Mochizuki H, Sakaguchi M. Cancer is an independent predictor of poor outcomes in patients following intracerebral hemorrhage. Eur J Neurol. 2018;25:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Murthy SB, Shastri A, Merkler AE, Hanley DF, Ziai WC, Fink ME, Iadecola C, Kamel H, Navi BB. Intracerebral Hemorrhage Outcomes in Patients with Systemic Cancer. J Stroke Cerebrovasc Dis. 2016;25:2918-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MS, Panageas KS, DeAngelis LM. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 6. | Zöller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Rogers LR. Cerebrovascular complications in patients with cancer. Semin Neurol. 2010;30:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Nakahara T, Owaki Y, Kosaka T, Fukada J, Ichimura A, Jinzaki M. Fatal Intracranial Hemorrhage Due to Thrombocytopenia in a Patient With Castration-Resistant Prostate Cancer Showing Extensive Bone Uptake of Injected 223Ra Dichloride. Clin Nucl Med. 2018;43:546-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Johdi NA, Sukor NF. Colorectal Cancer Immunotherapy: Options and Strategies. Front Immunol. 2020;11:1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 10. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1344] [Article Influence: 336.0] [Reference Citation Analysis (5)] |
| 11. | Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1852] [Cited by in RCA: 2132] [Article Influence: 213.2] [Reference Citation Analysis (0)] |
| 12. | Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1831] [Cited by in RCA: 1753] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 13. | Qin K, Chen Y, Long H, Chen J, Wang D, Chen L, Liang Z. The biomarkers and potential pathogenesis of lung cancer related cerebral hemorrhage. Medicine (Baltimore). 2019;98:e15693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Zhang YY, Chan DK, Cordato D, Shen Q, Sheng AZ. Stroke risk factor, pattern and outcome in patients with cancer. Acta Neurol Scand. 2006;114:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Menekse G, Gezercan Y, Demirturk P, Uysal I, Okten AI. Fatal cerebellar hemorrhage as an initial presentation of medulloblastoma in a child. J Pediatr Neurosci. 2015;10:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Zhou T, Zhang S, Qin Y, Zhu W. Intracerebral hemorrhage as initial presentation of metastatic choriocarcinoma: A case report. Radiol Case Rep. 2020;15:2335-2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Wang H, Cao F, Li J, Sun K, Jin J, Wang M. Intracerebral Hemorrhage as the Initial Presentation of Chronic Myeloid Leukemia: A Case Report and Review of the Literature. Front Neurol. 2020;11:571576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Katz JM, Segal AZ. Incidence and etiology of cerebrovascular disease in patients with malignancy. Curr Atheroscler Rep. 2005;7:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Jung S, Moon KS, Jung TY, Kim IY, Lee YH, Rhu HH, Sun HS, Jeong YI, Kim KK, Kang SS. Possible pathophysiological role of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in metastatic brain tumor-associated intracerebral hemorrhage. J Neurooncol. 2006;76:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 21. | Karpuz T, Araz M, Korkmaz L, Kılınc I, Findik S, Karaagaç M, Eryilmaz MK, Artac M. The Prognostic Value of Serum Semaphorin3A and VEGF Levels in Patients with Metastatic Colorectal Cancer. J Gastrointest Cancer. 2020;51:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Morini SR, Denadai MV, Waisberg J, Lopes Filho GJ, Matos D, Saad SS. Metalloproteinases and colorectal cancer. Correlation of gene expression and clinical-pathological parameters. Acta Cir Bras. 2020;35:e202000707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Liebman HA. Thrombocytopenia in cancer patients. Thromb Res. 2014;133 Suppl 2:S63-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Eklund EA. Thrombocytopenia and cancer. Cancer Treat Res. 2009;148:279-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Desikan SP, Mclaughlin N, McClain C, Desikan R. Recurrent Colon Cancer: Presentation With Disseminated Intravascular Coagulation From Disseminated Carcinomatosis of the Bone Marrow. J Investig Med High Impact Case Rep. 2021;9:23247096211012224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Takeyama H, Sakiyama T, Wakasa T, Kitani K, Inoue K, Kato H, Ueda S, Tsujie M, Fujiwara Y, Yukawa M, Ohta Y, Inoue M. Disseminated carcinomatosis of the bone marrow with disseminated intravascular coagulation as the first symptom of recurrent rectal cancer successfully treated with chemotherapy: A case report and review of the literature. Oncol Lett. 2017;13:4290-4294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Ganguly K, Rauth S, Marimuthu S, Kumar S, Batra SK. Unraveling mucin domains in cancer and metastasis: when protectors become predators. Cancer Metastasis Rev. 2020;39:647-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Pothuraju R, Krishn SR, Gautam SK, Pai P, Ganguly K, Chaudhary S, Rachagani S, Kaur S, Batra SK. Mechanistic and Functional Shades of Mucins and Associated Glycans in Colon Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |