Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1741
Peer-review started: June 3, 2021
First decision: June 30, 2021
Revised: July 10, 2021
Accepted: August 24, 2021
Article in press: August 24, 2021
Published online: November 15, 2021
Processing time: 162 Days and 3.6 Hours
Spasmolytic polypeptide-expressing metaplasia (SPEM) is a potential preneoplastic lesion.
To elucidate the microRNA (miR)-7-mediated preventive and inhibitive effects of Yiwei Xiaoyu granules (YWXY) in SPEM lesions.
Gastric mucosa biopsies were collected from chronic atrophic gastritis patients and healthy people with signed informed consent. YWXY was administered to the mice with induced SPEM by tamoxifen, and the gastric mucosa was harvested on the tenth day of the experiment. Then immunohistochemistry and immunofluorescence were performed to validate the SPEM, lesions and the potential mecha
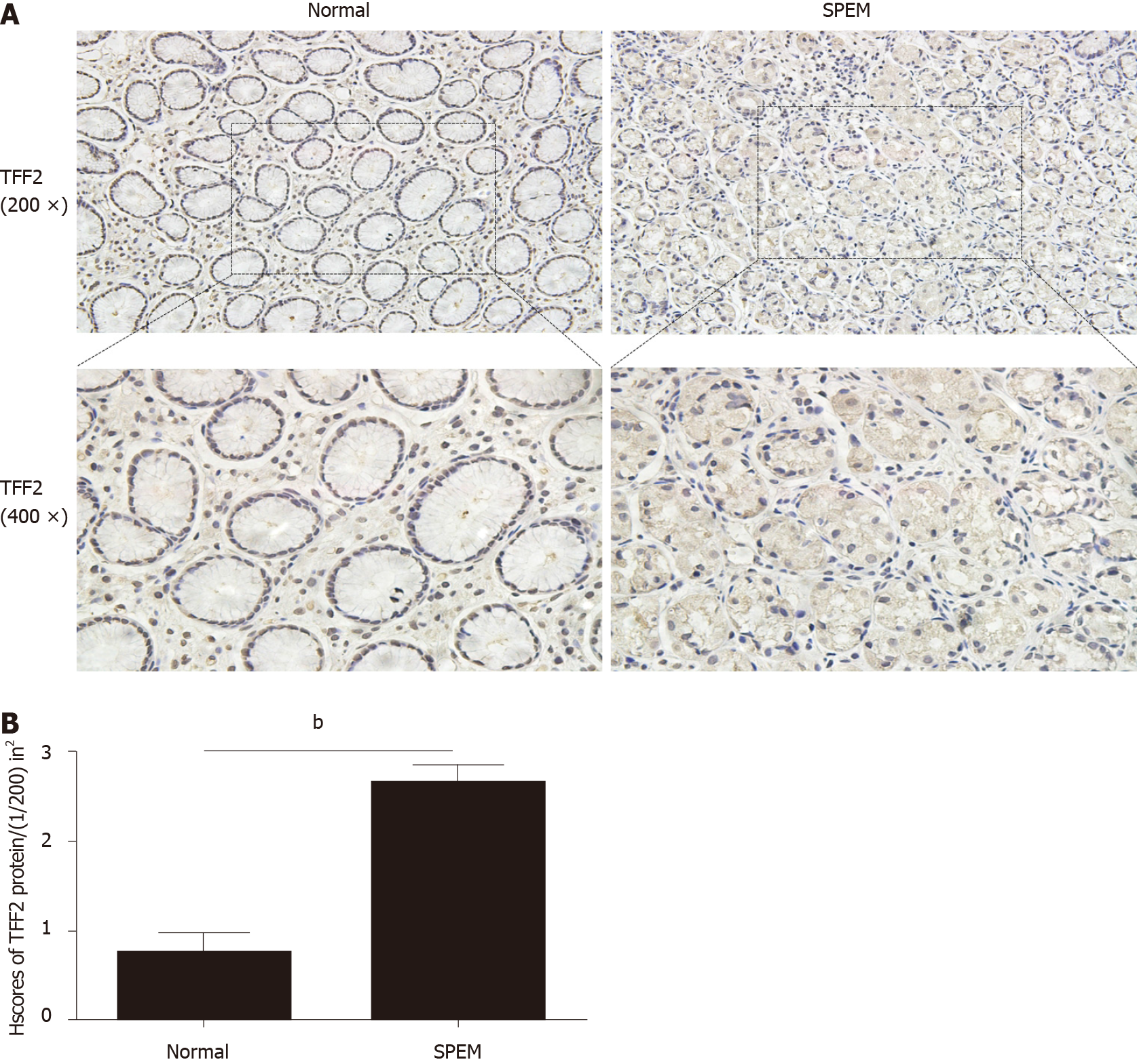
The expression of miR-7 was downregulated in the SPEM lesions, and expression of trefoil factor 2 (TFF2) and clusterin was high in the human gastric mucosa. In vivo experiments showed that YWXY could inhibit the cell proliferation in the tamoxifen-induced SPEM lesions by regulating Ki67. Simultaneously, YWXY could restore the expression of miR-7 by regulating TFF2 by detection with immunofluorescence but not with reverse transcription-quantitative polymerase chain reaction, indicating its potential mechanism of targeting miR-7 by mediating TFF2. The expression of vascular endothelial growth factor-β and gastric intrinsic factor was restored within 3 d of YWXY administration for the SPEM lesions, speculating that the possible mechanism of YWXY is to inhibit the development and progression of SPEM by regulating vascular endothelial growth factor-β and gastric intrinsic factor.
miR-7 downregulation is an early event in SPEM through regulation of TFF2 in human gastric mucosa. YWXY is able to inhibit the cell proliferation and restore the expression of miR-7 by mediating TFF2 in the SPEM mouse model.
Core Tip: We showed evidence that microRNA-7 downregulation is an early event in the cascade from metaplasia to gastric cancer and that it contributes to the establishment of an intestinal expression profile through regulation of trefoil factor 2 in both human gastric mucosa and in vivo experiments. To the best of our knowledge, we used the spasmolytic polypeptide-expressing metaplasia mouse model for the first time to reveal the effectiveness and the potential mechanism of Chinese medicine Yiwei Xiaoyu granules for the precursor of gastric adenocarcinoma.
- Citation: Chen WQ, Tian FL, Zhang JW, Yang XJ, Li YP. Preventive and inhibitive effects of Yiwei Xiaoyu granules on the development and progression of spasmolytic polypeptide-expressing metaplasia lesions. World J Gastrointest Oncol 2021; 13(11): 1741-1754
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1741.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1741
Gastric cancer is one of the most frequent and deadly cancers worldwide[1]. The Correa pathway from gastritis to gastric cancer including the oxyntic atrophy (loss of acid-secreting parietal cells) and the development of spasmolytic polypeptide-expressing metaplasia (SPEM) has been well described. SPEM and intestinal metaplasia (IM) have been considered as preneoplastic lesions, whereas SPEM is the first metaplastic lesion to evolve and probably progresses to IM[2-4]. Therefore, it is important to clarify the cause of parietal cell atrophy and the regulatory mechanisms for the chief cell transdifferentiation to explore novel treatments for the gastric precancerous lesions and eventually prevent the occurrence of gastric cancer.
The international consensus has recommended follow-up or endoscopic resection of the precancerous lesions of the stomach; however, it has been reported that gastric IM still persisted even after successful eradication of low-grade dysplasia with radiofrequency ablation[5,6]. In the previous work, our group proved that Yiwei Xiaoyu granules (YWXY) could improve the mucosa atrophy, IM and dysplasia of chronic gastric gastritis (CAG) in the clinic trial[7]. Then we optimized water reflux extraction technology and established the quality standard of YWXY[8,9]. In addition, we explored the mechanism of how YWXY inhibits atrophy and IM of the stomach using a rat model[10,11]. However, the specific mechanism of YWXY still remains largely unknown.
In our previous work, we found that targeting some microRNAs (miRNAs) could prevent or retard the occurrence and development of gastric cancer and its precancerous lesions, such as miR-7 and let-7[12-14]. miR-7a-5p and miR-7a-3p strand consist of a short duplex mature miRNA, and miR-7a-5p has been the focus of the majority of studies and is commonly referred to as “miR-7”[15]. miR-7 has been proved to be a novel prognostic biomarker and potential therapeutic target by mediating p65 and activating NF-κB[16]. Based on our prior investigation, the expression of miR-7 in gastric cancer was decreased compared with the matched normal tissues and adjacent tissues[14]. Therefore, we hypothesized that miR-7 might serve as a suppressor gene in the process of SPEM progression into gastric cancer. Furthermore, in the light of these facts that YWXY could relieve or even reverse the atrophy and IM of rat mucosa by inhibiting NF-κB[10], we assumed that YWXY could inhibit the progression of gastric mucosa metaplasia even in the earlier phase such as SPEM.
Here, we detected the expression of miR-7 in SPEM with CAG biopsy samples and demonstrated the therapeutic effect of YWXY for the SPEM model induced by tamoxifen, in order to illustrate the possible mechanism.
Animal experiments and human sample management were performed in accordance with protocols approved by the Ethics Committee of Chongqing Hospital of Traditional Chinese Medicine. All participants signed informed consent forms. From October 2019 to June 2020, 35 pairs of gastric endoscopic biopsy samples, including 30 CAG, and 5 healthy volunteers, were collected from the Department of Gastroenterology, Chongqing Hospital of Traditional Chinese Medicine. The diagnostic standards of CAG followed the Consensus on Chronic Gastritis[17], while the diagnostic standards of normal gastric mucosa with healthy volunteers followed the suggestion from Watanabe et al[18].
A total of 24 male wild-type mice were purchased from the Institute of Chinese Medicine in Chongqing. They were divided into four groups randomly, including normal group, model group, low-dose group of YWXY and high-dose group of YWXY, and 6 mice were in each group. From the first to the tenth day of the experiment, the mice in low-and high-dose group of YWXY were gavaged with YWXY (15 g/kg daily and 20 g/kg daily, respectively). To establish the model of SPEM, tamoxifen was intraperitoneally injected (3 mg/20 g mouse body weight) from the eighth day of the experiment for 3 consecutive days[19]. Mice in the normal group were gavaged with saline for 10 consecutive days. On the eleventh day of the experiment, after euthanasia, stomachs were immediately excised, and the gastric body was cut into three parts and fixed with 4% paraformaldehyde. One part was stored at -20 ℃, and the other two parts were stored with paraffin embedding.
YWXY components were obtained from the pharmacy department of Chongqing Hospital of Traditional Chinese Medicine and identified by two pharmacological experts, and were prepared as described previously[10,11]. Standard extraction technology for YWXY was used[8,9].
After deparaffinization and hydration, slides underwent antigen retrieval via cooking in saline sodium citrate (pH 6.0). Slides were blocked with 3% H2O2 at room temperature for 15 min and flushed with distilled water three times (5 min/time). Primary antibodies, including anti-intrinsic factor antibody (1:20, ab171418, Abcam, Cambridge, United Kingdom), anti-trefoil factor 2 (TFF2) antibody (1:200, ab203237, Abcam), vascular endothelial growth factor-β (VEGF-B) antibody (1:200, AF7019, Affinity Biosciences, Cincinnati, OH, United States) and Ki67 antibody (1:200, AF1738, Beyotime, Beijing, China) were incubated overnight at 4 °C, washed three times (5 min/time) with PBS , and then incubated with DAKO REALTM EnVisionTM/HRP, Rabbit/Mouse (EVN) (K5007, Glostrup, Denmark) at 25-27 °C for 30 min, flushed with PBS three times (5 min/time) according to the manufactures’ instructions, developed, dehydrated, cleared, mounted and examined.
The deparaffinized mouse stomach tissue sections underwent antigen retrieval with 10 mmol/L sodium citrate (pH 6.0), washed three times with PBS for 5 min each time, then with blocked with 10% normal goat serum at room temperature for 1 h, followed by overnight incubation with primary antibodies, such as anti-intrinsic factor antibody (1:10, ab171418, Abcam), anti-TFF2 antibody (1:100, ab203237, Abcam) and VEGF-B antibody (1:50, sc-101582, Santa Cruz Biotechnology, Santa Cruz, CA, United States) at 4 °C , then washed three times with PBS for 5 min each time. Goat anti-rabbit IgG (1:100) was added to incubate at room temperature for 1 h and washed three times with PBS for 5 min each time. SABC-DyLight 488 (1:200) was added to incubate at room temperature for 1 h, and PBS washed for 5 min. The diluted DAPI (1:1000) was added to make nuclear condensation at room temperature for 3 min, PBS washed four times, 5 min each time, stained with fluorescence decay resistant medium, mounted and photographed.
Total RNA was extracted according to the manufacturer’s instructions (LS1040, Promega Corporation, Shanghai, China). The PCR primers were as follows: TFF2 forward: 5’-CCTTGGTGTTTCCACCCACT-3’ and reverse, 5’-CCCACAATTCTTGCGAGCTG-3’; GAPDH forward: 5’-ATGGTGAAGGTCGGTGTGAAC-3’ and reverse 5’-AATCTCCACTTTGCCACTGC-3’. Reverse transcription was performed using the reverse transcription (K1622, Thermo Fisher Scientific, Waltham, MA, United States) and the MaximaTMSYBRGreen/ROXqPCRMasterMi (2X) (K0221, Thermo Fisher Scientific) kits. The thermocycling conditions were as follows: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min on an ABI Step One QPCR System (Applied Biosystems, Waltham, MA, United States). GAPDH was used as an endogenous control, and the ΔΔCt method was used for TFF2 quantification.
Fluorescence in situ hybridization (FISH) process was performed according to the manufacturer’s protocol (MK1030; Boster Biological Technology, Beijing, China). Ten-micrometer paraffin-embedded sections were deparaffinized and rehydrated, then incubated with proteinase and 1 mL 3% citric acid for 30 min at 37 °C, washed with PBS three times (5 min/time) and flushed with distilled water once for 5 min. Then, 20 μL prehybridization solution was added on each slide. To retain moisture, 20% glycerin was put into the dry hybridization chamber and incubated for 2 h at 37 °C . After the prehybridization, the excess liquid was absorbed. The locked nucleic acid probe (mmu-miR-7a-5p FISH probe, 5’FAM-ACAACAAAATCACTAGTCTTCCA-FAM3’) was dissoluted with 47.5 μL nuclease-free water and diluted (1:100), added with 20 μL hybridization solution with oligonucleotide probe, incubated at 42 °C overnight, washed, nuclear stained with DAPI (1:1000), mounted and observed under a fluorescence microscope (MF31, MSHOT, Guangzhou, China).
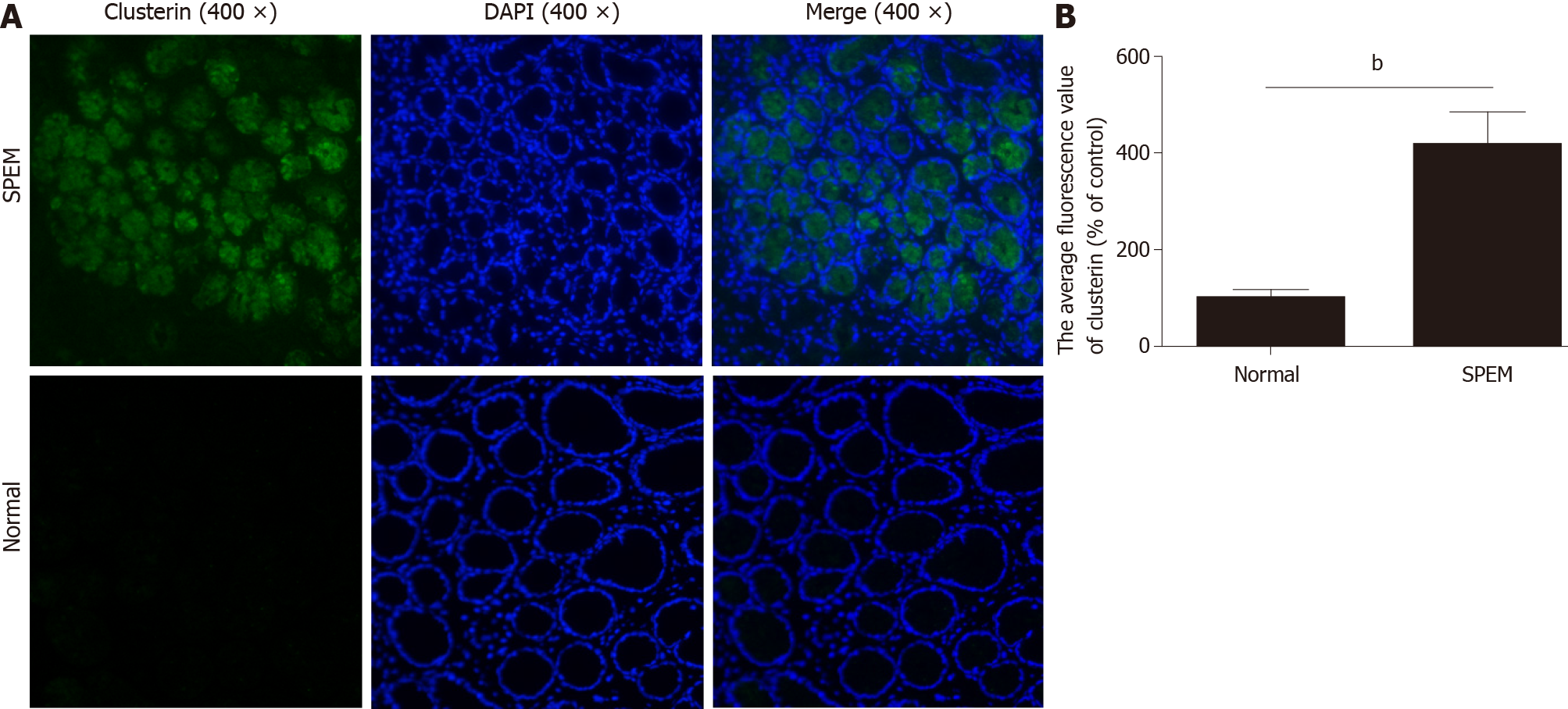
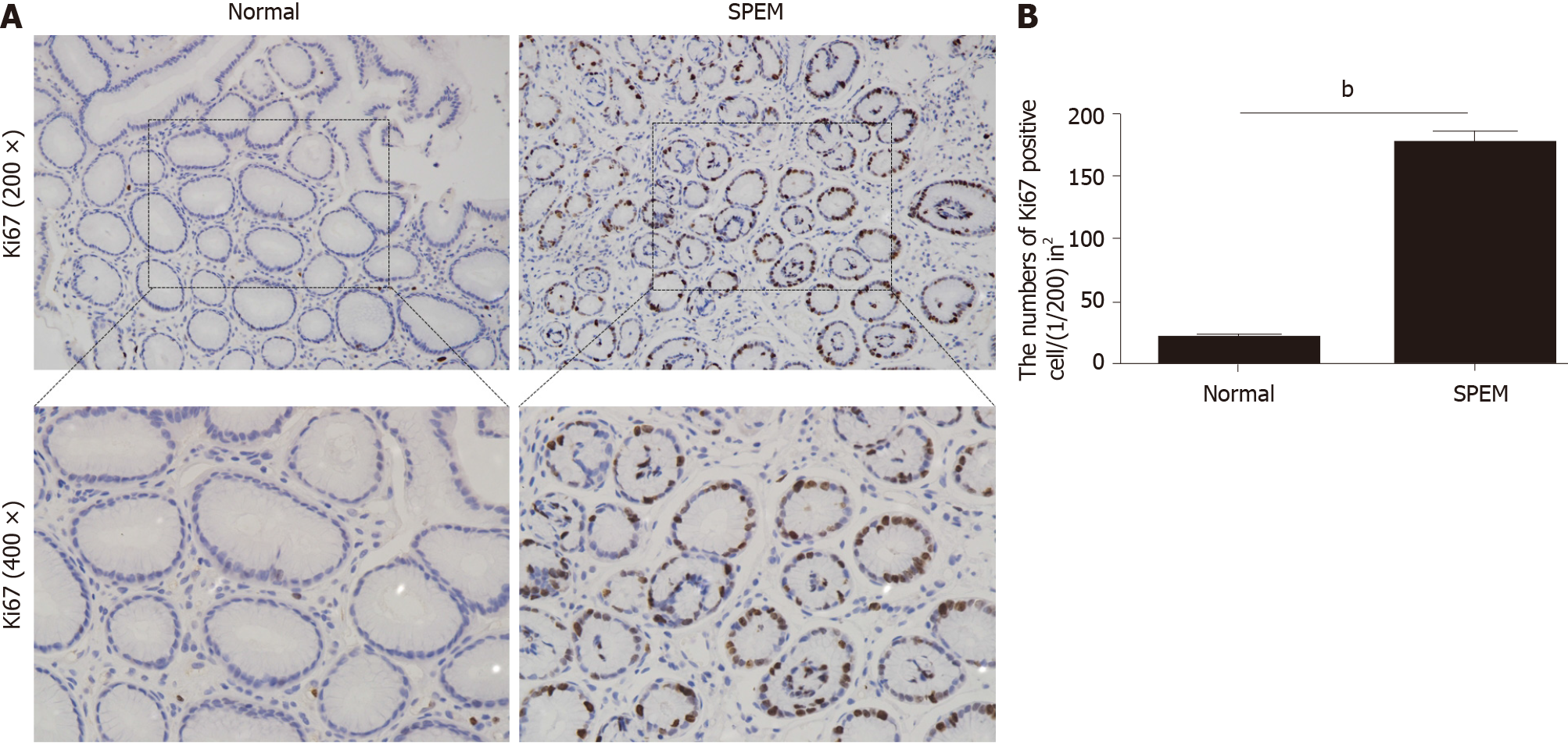
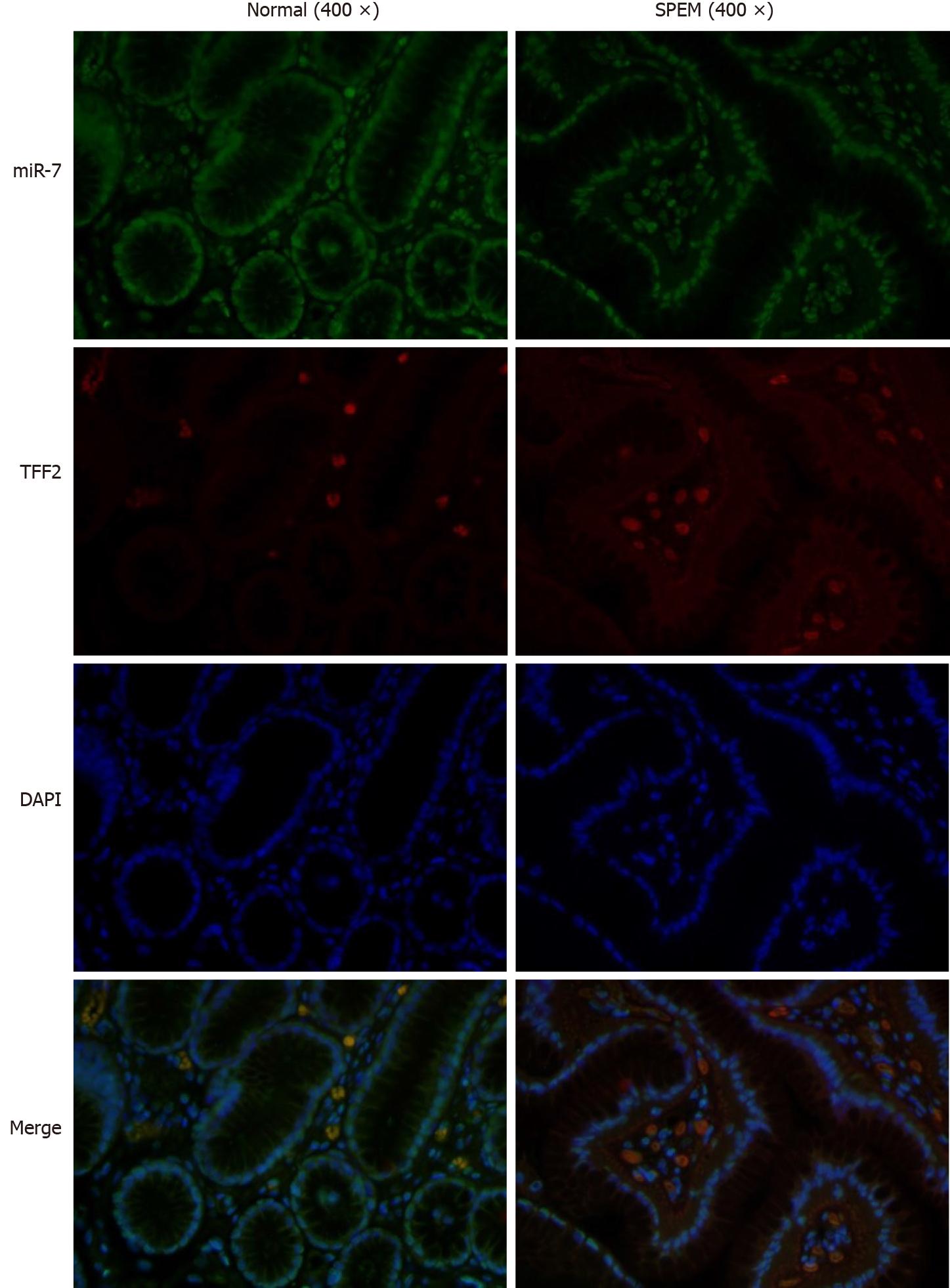
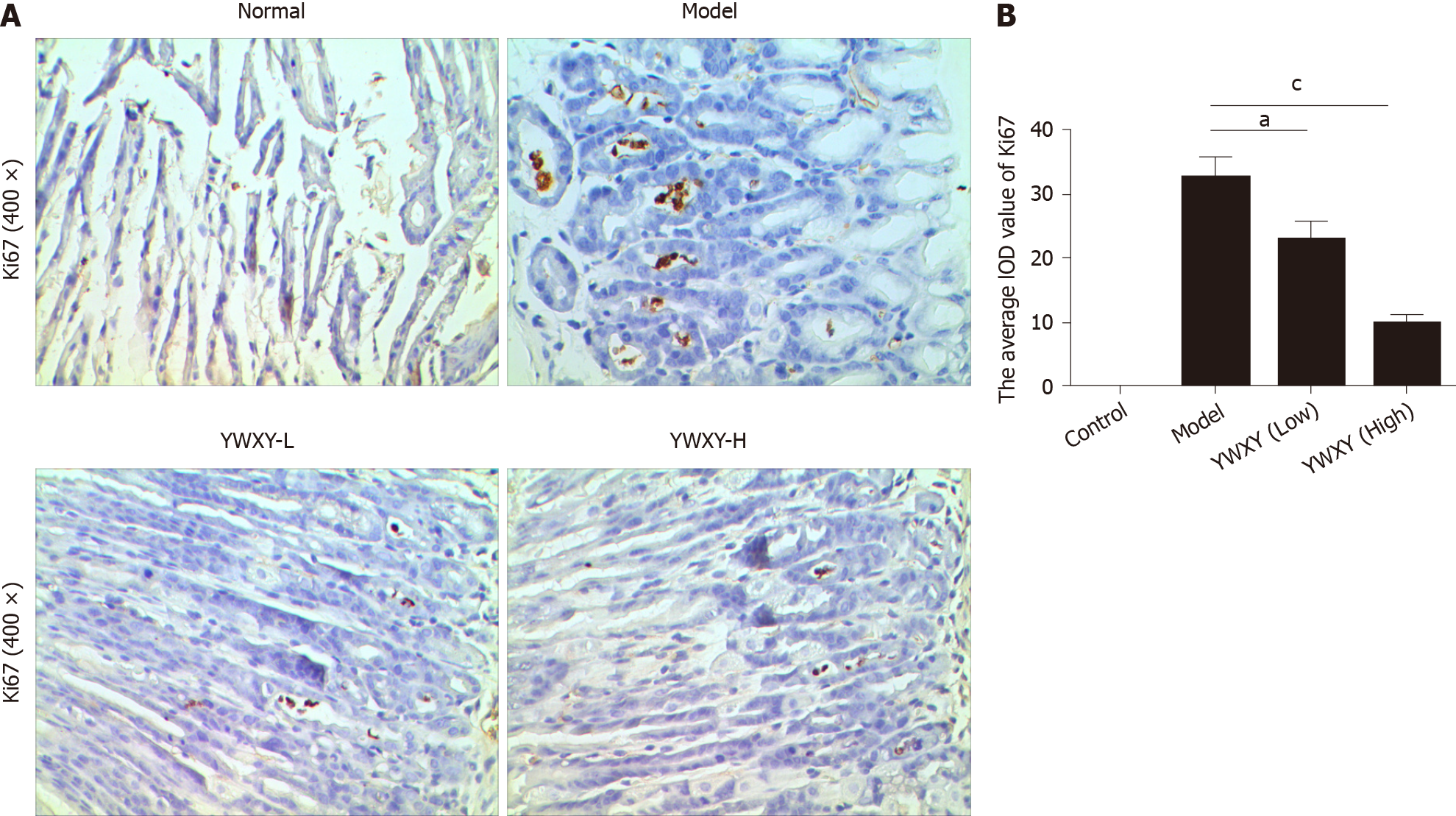
To determine if miR-7 is inhibited in the specimens of CAG, we first examined the potential SPEM tissue with hematoxylin and eosin staining[3]. Ten slides were selected and examined with immunohistochemistry (IHC). As it is reported, the clusterin-positive intestinal metaplasia does not express TFF2 in the gastric cancer tissue, whereas SPEM is a metaplasia mucous cell lineage with strong expression of TFF2 and clusterin[4,20]. As a result, the expression of TFF2 and clusterin in SPEM are upregulated compared to the normal stomach tissue with IHC and immunofluorescence (Figures 1 and 2). Also, the expression of Ki67 protein in the SPEM was significantly higher than that in the normal stomach tissue (P < 0.001) (Figure 3). It is consistent with the results in the previous animal experiments[23]. It implies that SPEM is actually the precancerous lesion with high proliferative activity.
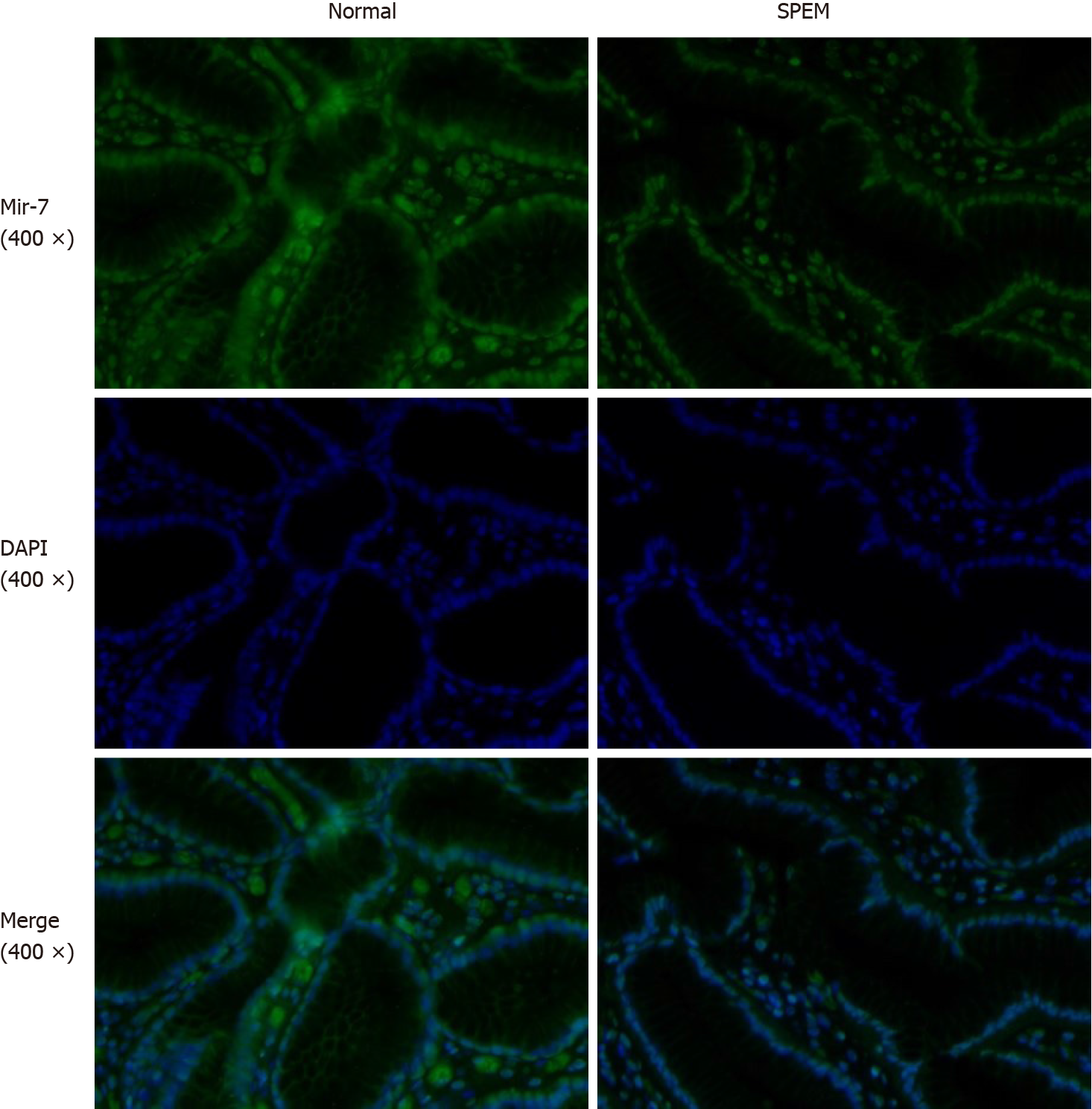
In our previous study, the expression of miR-7 was significantly downregulated in gastric cancer tissue compared with the normal and adjacent tissue samples, which demonstrated that it was a tumor suppressor of gastric cancer[17]. Here, we sought to identify if miR-7 was dysregulated in the SPEM tissue. By FISH, the results showed that the expression of miR-7 in SPEM was lower than that in normal tissue (Figure 4). Furthermore, to confirm the relationship of miR-7 and TFF2, FISH was performed. The data showed that with the decreased expression of miR-7, the expression of TFF2 was upregulated in the tissue of SPEM (Figure 5). Our results may have major implications for understanding the occurrence and development of SPEM.
We tested, for the first time, the hypothesis that YWXY acts on miR-7 regulating TFF2 to inhibit SPEM. We induced SPEM in the mouse stomach by intraperitoneal administration of tamoxifen. The expression of Ki67 was higher in SPEM models than that in normal controls (Figure 6). Obviously, both YWXY decreased the expression of Ki67 compared to the model group (P < 0.05). These results imply that YWXY prevents the progression of precancerous lesions.
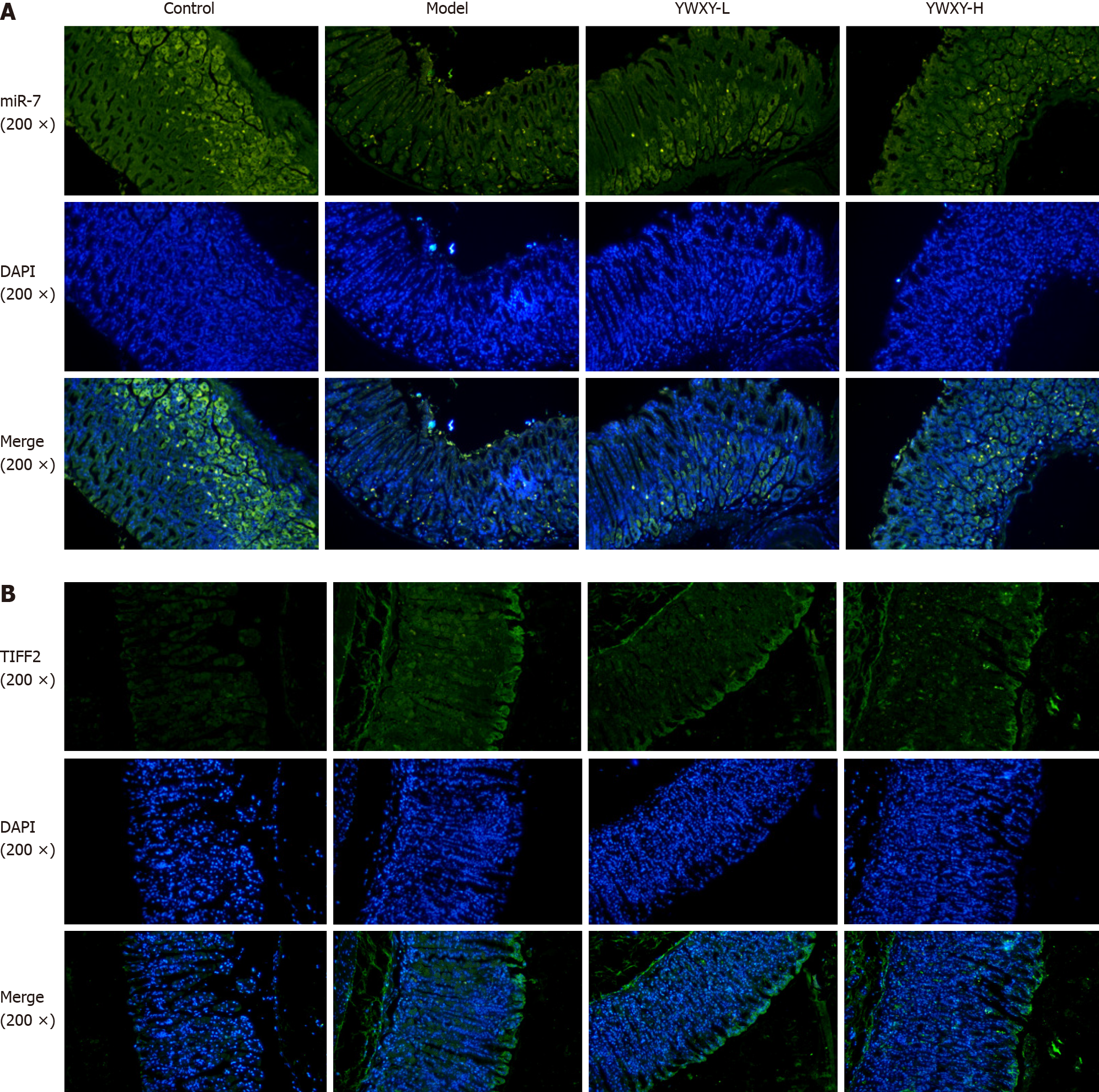
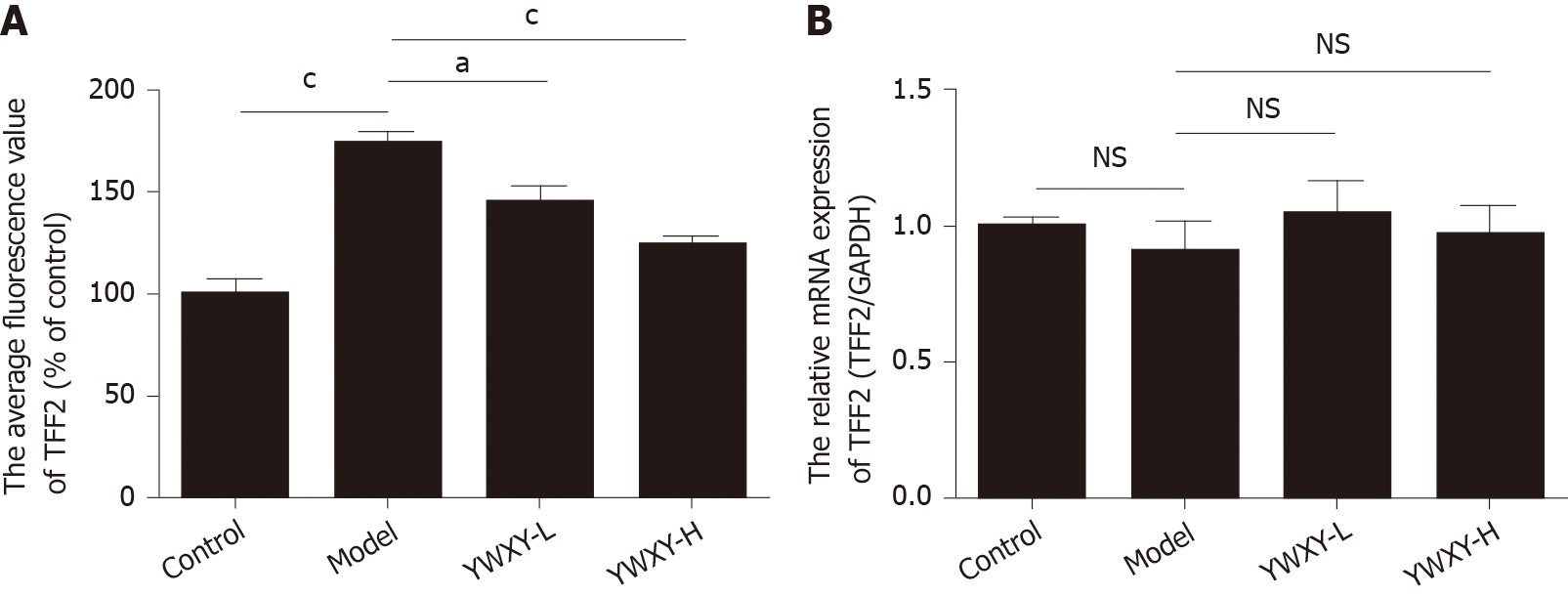
To address the hypothesis of the mechanism of YWXY inhibiting the progression of SPEM, we examined the expression of miR-7 with FISH and TFF2 with immunofluorescence. Our findings suggest that YWXY administration could restore the expression of miR-7. In addition, with high dosage of YWXY, there is an upward trend of miR-7 upregulation (Figure 7A). On the contrary, the expression of TFF2 was downregulated with YWXY administration compared to the healthy controls as measured by immunofluorescence (Figure 7B). The results show that the Chinese medicine YWXY has the ability to inhibit the development of SPEM, the mechanism of which might target miR-7 by mediating TFF2. In order to prove it, we compared the average fluorescence value of TFF2. Clearly, the expression of TFF2 in the model group was much higher than that in the control group (P < 0.001). Furthermore, intervening with YWXY could decrease the expression of TFF2 compared to the model (P < 0.001 in YWXY-H, P < 0.05 in YWXY-L) (Figure 8A). However, by reverse transcription-quantitative polymerase chain reaction, we did not find any difference in the relative mRNA expression of TFF2 between the different groups (Figure 8B).
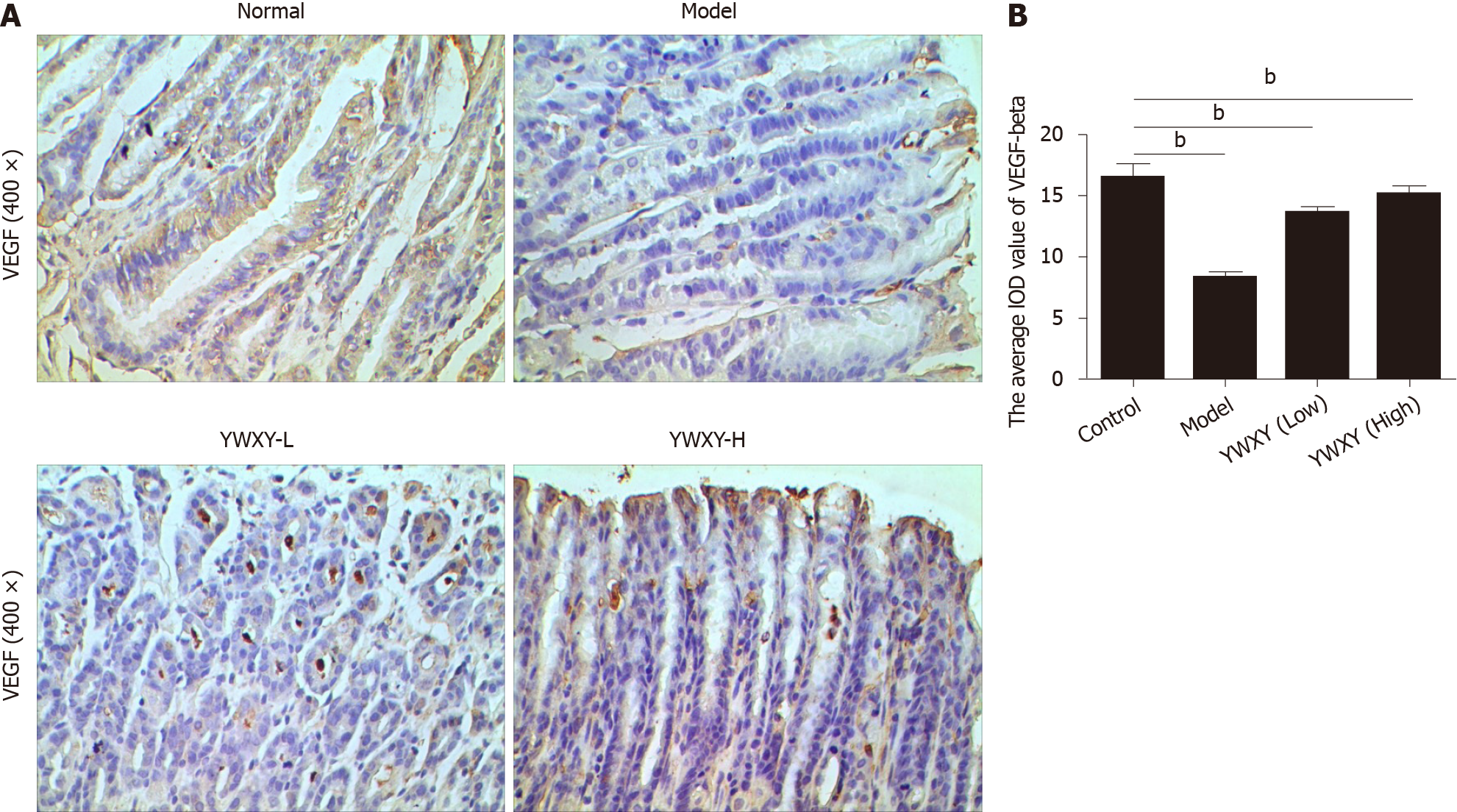
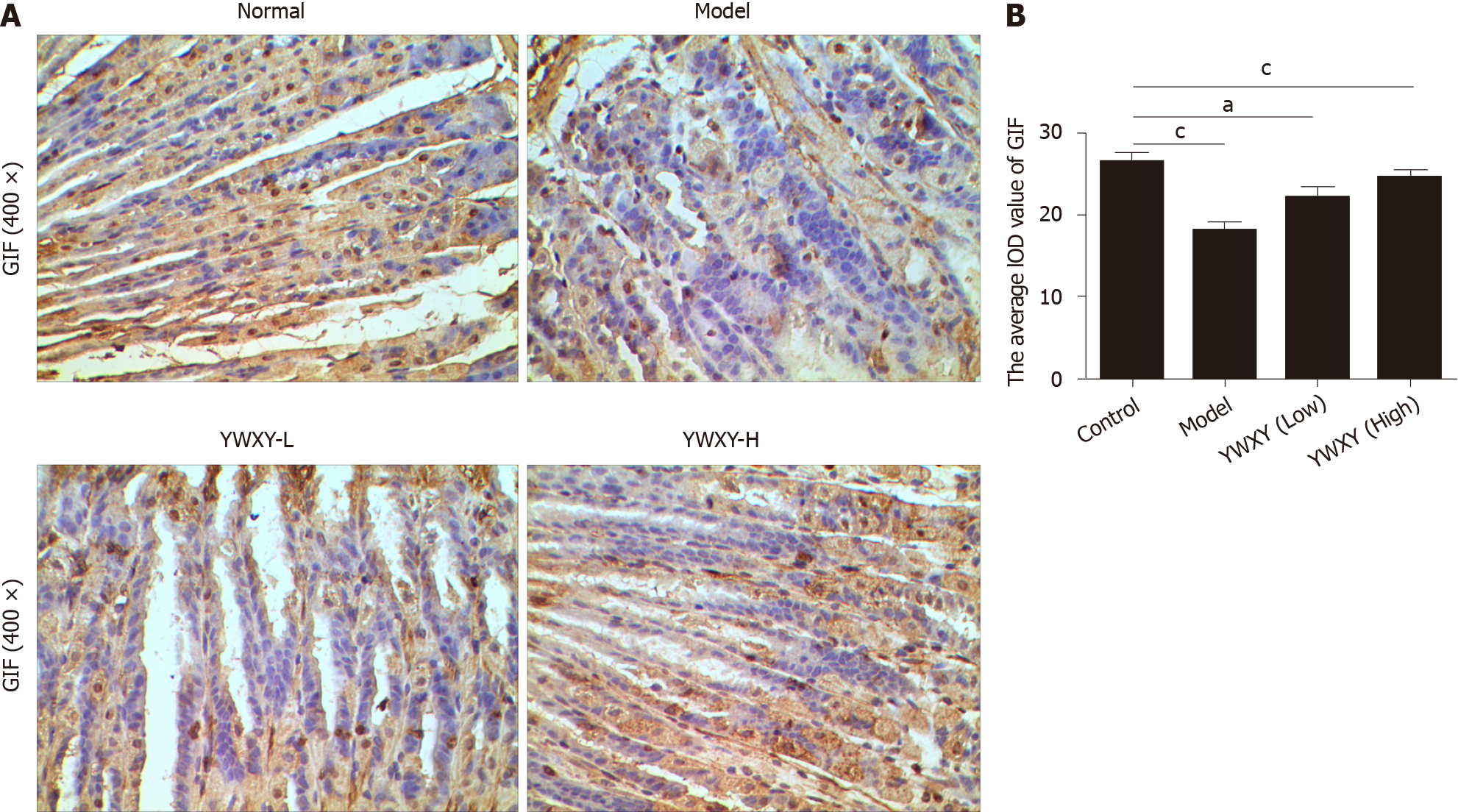
SPEM is a specific preneoplastic lesion, and cancer related pathways should be analyzed. Therefore, we detected the expression of VEGF-β with IHC, and the results showed that YWXY could restore the expression of VEGF-β, while statistical differences existed between the control and the model groups (P < 0.05) (Figure 9). Simultaneously, the cell proliferation was also detected with IHC. Unsurprisingly, the expression of gastric intrinsic factor (GIF) in the model group was scanty compared with the control. With the increased dosage of YWXY, the expression of GIF was restored (Figure 10).
Our research focused on the therapeutic effect of YWXY, and its mechanism of action for atrophy and IM have been interpreted[10,11]. However, it is conceivable that YWXY may play a role even in the prior neoplastic precursor, SPEM. Therefore, it was given to the tamoxifen-induced SPEM mice, and the therapeutic effect and underlying mechanisms were investigated.
It has been proven that two different SPEM mouse models induced by drug or chronic inflammation were identical. We chose a tamoxifen-induced reversible SPEM mouse model[22]. SPEM was confirmed by the coexpression of TFF2, Mucin 6 and GIF[23]. Clusterin was detected in all SPEM lineages, and it represented a specific marker of SPEM induction in the gastric oxyntic mucosa, whereas clusterin-positive IM cells do not express TFF2. Therefore, SPEM was identified with the positive coexpression of clusterin and TFF2 in our study[4,20].
In order to test the cell proliferation activity, Ki67 was detected. Interestingly, the cell proliferation at the interface between SPEM and intestinal metaplasia was more active than that in normal gastric mucosa, which was consistent with the results by Goldenring et al[20], implicating some evidence for the existence of IM emanating from SPEM. Recent studies have also highlighted the existence of SPEM and IM as useful biomarker for gastric cancer risk, and miR-7 has been identified as a tumor suppressor of gastric cancer[12,14]. Therefore, miR-7 was compared between the normal and SPEM gastric mucosa. It is particularly exciting to implicate that the expression of miR-7 in SPEM was inhibited obviously compared with that in normal gastric mucosa of CAG patients, supporting the hypothesis that miR-7 has the potential to represent earlier regulatory events in the cascade to gastric cancer.
Recently, several investigations have focused on the role of miRNAs in the development of stomach metaplasia[24-27]. The novelty of this study is the use of gastric specimens derived from CAG patients and healthy people. To our best knowledge, it is the first study to detect the expression of miR-7 and elucidate the potential mechanism of Chinese medicine mediated by microRNAs in SPEM. To test the idea that the profile of YWXY inhibiting activity from precursor to gastric malignancy was mediated by miR-7, the expression of Ki67 was discerned with a tamoxifen-induced SPEM mouse model. We showed evidence that the expression of Ki67 was inhibited with YWXY compared to the control group. YWXY may inhibit cell proliferation to induce epigenetic modification of gastric mucosal genes. The expression of miR-7 was restored in the group with YWXY intragastric administration compared with the model group by FISH. On the other hand, downregulation of TFF2 was speculated in the YWXY group compared with the model group by immunofluorescence, whereas TFF2 mRNA levels were not significantly changed by reverse transcription-quantitative polymerase chain reaction detection.
Studies revealed that TFF2 was a protective rapid response peptide coping with mucosal damage because of its motogenic effects in vitro and protective or healing effects in vivo[28,29]. Moreover, TFF2 was also considered to have protection against the progression of premalignant lesions in Helicobacter pylori-infected mice[30]. On the contrary, for those people who were Helicobacter pylori-infected gastric cancer relatives, there was no relation with the serum TFF2 levels and the presence of either IM or SPEM[31]. In our study, high levels of TFF2 were detected in the SPEM lesions of CAG patients and the mucosa of the SPEM mouse model. miR-7 might regulate the expression of TFF2 at the protein level but not the mRNA level.
VEGF-β as a parietal cell marker was illustrated to be downregulated in a tamoxifen-induced SPEM model at 3 d, whereas it recovered itself at 10 d and 21 d because of the rapid and reversible characteristics of the model[32,33]. Also, previous studies have reported that aging impairs angiogenesis and reduces expression of VEGF, which could illustrate the development of SPEM responsible for wound healing after ulcer injury[34-36]. In our study, with the YWXY intragastric administration for 10 d, the expression of VEGF-β was restored. Based on the acknowledgement of SPEM as a neoplastic precursor, it is conceivable that YWXY may inhibit the development and progression of SPEM by regulating VEGF-β.
Similarly, GIF was reported to locate in zymogenic chief cells at the base of control mice. The expression was downregulated significantly in the tamoxifen-induced mouse model[32,37]. In our study, scant GIF was discerned in the model group. However, with YWXY intragastric administration, the expression of GIF was restored. Thus, we speculated that SPEM lesion as a precursor to intestinal metaplasia and gastric adenocarcinoma could be treated by YWXY in gastric gland bases.
We showed evidence that miR-7 downregulation is an early event in the cascade from metaplasia to gastric cancer and that it contributes to the establishment of an intestinal expression profile through regulation of TFF2 both in human gastric mucosa and an in vivo model.
To the best of our knowledge, it is the first study to use the SPEM mouse model to uncover the effectiveness and the potential mechanism of Chinese medicine for the precursor of gastric adenocarcinoma. We performed a preliminary experiment to validate that YWXY had the ability to inhibit cell proliferation and restore the expression of miR-7 by mediating TFF2 in SPEM lesions. Nevertheless, the detailed mechanism of YWXY to prevent and inhibit the development and progression of SPEM lesions should be examined carefully in our future experiments.
Spasmolytic polypeptide-expressing metaplasia (SPEM) is the first metaplastic lesion to evolve and probably progresses to intestinal metaplasia.
Our group proved that Yiwei Xiaoyu granules (YWXY) could improve the mucosa atrophy, intestinal metaplasia and dysplasia of chronic gastric gastritis in a clinical trial, while the specific mechanism of YWXY still remains largely unknown.
To elucidate microRNA-7-mediated preventive and inhibitive effects of YWXY in SPEM lesions.
Gastric mucosa biopsies were collected both in human and in a tamoxifen-induced SPEM mouse model. Then immunohistochemistry and immunofluorescence were performed to validate the SPEM lesions, and the potential mechanism was investigated. RNA transcripts were detected with reverse transcription-quantitative polymerase chain reaction.
We showed evidence that microRNA-7 downregulation was an early event in the cascade from metaplasia to gastric cancer and that it contributed to the establishment of an intestinal expression profile through regulation of trefoil factor 2 both in human gastric mucosa and an in vivo model. We validated that YWXY had the ability of inhibiting the cell proliferation and restoring the expression of microRNA-7 by mediating trefoil factor 2 in SPEM lesions.
To the best of our knowledge, it is the first time to use the SPEM mouse model to uncover the effectiveness and potential mechanism of Chinese medicine for the precursor of gastric adenocarcinoma.
Nevertheless, the detailed mechanism of YWXY to prevent and inhibit the development and progression of SPEM lesions should be examined carefully in our next experiment. We believe it shows great potential for drug development to prevent and treat precancerous lesions.
We wish to thank Xiao-Jing Cao, pathologist of our hospital, for assistance with pathological examination of our biopsy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Mario F S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Liu JH
| 1. | Mattiuzzi C, Lippi G. Current Cancer Epidemiology. J Epidemiol Glob Health. 2019;9:217-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 847] [Article Influence: 169.4] [Reference Citation Analysis (1)] |
| 2. | Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM Jr, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028-2037.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin North Am. 2013;42:261-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Weis VG, Sousa JF, LaFleur BJ, Nam KT, Weis JA, Finke PE, Ameen NA, Fox JG, Goldenring JR. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2013;62:1270-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 498] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 6. | Leung WK, Tong DK, Leung SY, Chan FS, Tong TS, Ho RS, Chu KM, Law SY. Treatment of Gastric Metaplasia or Dysplasia by Endoscopic Radiofrequency Ablation: A Pilot Study. Hepatogastroenterology. 2015;62:748-751. [PubMed] |
| 7. | Wu L, Xie H, Tian F, Li Y. Zhang C. 30 cases of chronic atrophic gastritis treatment with Yiwei Xiaoyu granules. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2007;01:57-58. |
| 8. | Liu X, Xu C, Leng J, Guo X, Tian F. Optimization of the water extraction process of Yiwei Xiaoyu Granules by orthogonal test. Zhonghua Zhongyiyao Zazhi. 2018;33:1605-1607. |
| 9. | Liu X, Xu C, Wu W, Guo X, Sun Q, Tian F. Study on Quality Standard of Yiwei Xiaoyu Granules. Zhonghua Zhongyiyao Xinxi Zazhi. 2018;25:77-80. |
| 10. | Tian F, Li Y, Yang X, Liu Y, Chen W. Improvement effect of Yiwei Xiaoyu granules on gastric mucosa intestinal metaplasia of rats. Chongqing Yixue. 2018;47:4102-4106+4111. |
| 11. | Yang X, Tian F, Liu Y, Li Y. Effects of yiwei xiaoyu granules on intestinal metaplasia of gastric mucosa and the related inflammatory mediators in the rats. Shijie Zhongxiyi Jiehe Zazhi. 2019;14:198-202. |
| 12. | Chen WQ, Hu L, Chen GX, Deng HX. Role of microRNA-7 in digestive system malignancy. World J Gastrointest Oncol. 2016;8:121-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Deng HX, Yu YY, Zhou AQ, Zhu JL, Luo LN, Chen WQ, Hu L, Chen GX. Yangzheng Sanjie decoction regulates proliferation and apoptosis of gastric cancer cells by enhancing let-7a expression. World J Gastroenterol. 2017;23:5538-5548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Chen W, Yu Y, Yang N, Zhu J, Li K, Li R, Su W, Luo L, Hu L, Chen G, Deng H. Effects of Yangzheng Sanjie Decoction-containing serum mediated by microRNA-7 on cell proliferation and apoptosis in gastric cancer. Oncol Lett. 2018;15:3621-3629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Horsham JL, Kalinowski FC, Epis MR, Ganda C, Brown RA, Leedman PJ. Clinical Potential of microRNA-7 in Cancer. J Clin Med. 2015;4:1668-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Ye T, Yang M, Huang D, Wang X, Xue B, Tian N, Xu X, Bao L, Hu H, Lv T, Huang Y. MicroRNA-7 as a potential therapeutic target for aberrant NF-κB-driven distant metastasis of gastric cancer. J Exp Clin Cancer Res. 2019;38:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Rugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L, De Pretis G, Graham DY. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 18. | Watanabe K, Nagata N, Nakashima R, Furuhata E, Shimbo T, Kobayakawa M, Sakurai T, Imbe K, Niikura R, Yokoi C, Akiyama J, Uemura N. Predictive findings for Helicobacter pylori-uninfected, -infected and -eradicated gastric mucosa: validation study. World J Gastroenterol. 2013;19:4374-4379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Saenz JB, Burclaff J, Mills JC. Modeling Murine Gastric Metaplasia Through Tamoxifen-Induced Acute Parietal Cell Loss. Methods Mol Biol. 2016;1422:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207-2210, 2210.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Nam KT, O'Neal RL, Coffey RJ, Finke PE, Barker N, Goldenring JR. Spasmolytic polypeptide-expressing metaplasia (SPEM) in the gastric oxyntic mucosa does not arise from Lgr5-expressing cells. Gut. 2012;61:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21-24.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Bockerstett KA, Lewis SA, Wolf KJ, Noto CN, Jackson NM, Ford EL, Ahn TH, DiPaolo RJ. Single-cell transcriptional analyses of spasmolytic polypeptide-expressing metaplasia arising from acute drug injury and chronic inflammation in the stomach. Gut. 2020;69:1027-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Sousa JF, Nam KT, Petersen CP, Lee HJ, Yang HK, Kim WH, Goldenring JR. miR-30-HNF4γ and miR-194-NR2F2 regulatory networks contribute to the upregulation of metaplasia markers in the stomach. Gut. 2016;65:914-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Kuo HY, Chang WL, Yeh YC, Cheng HC, Tsai YC, Wu CT, Lin SH, Yang HB, Lu CC, Sheu BS. Spasmolytic polypeptide-expressing metaplasia associated with higher expressions of miR-21, 155, and 223 can be regressed by Helicobacter pylori eradication in the gastric cancer familial relatives. Helicobacter. 2019;24:e12578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Shimizu T, Sohn Y, Choi E, Petersen CP, Prasad N, Goldenring JR. Decrease in MiR-148a Expression During Initiation of Chief Cell Transdifferentiation. Cell Mol Gastroenterol Hepatol. 2020;9:61-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Ding L, Li Q, Chakrabarti J, Munoz A, Faure-Kumar E, Ocadiz-Ruiz R, Razumilava N, Zhang G, Hayes MH, Sontz RA, Mendoza ZE, Mahurkar S, Greenson JK, Perez-Perez G, Hanh NTH, Zavros Y, Samuelson LC, Iliopoulos D, Merchant JL. MiR130b from Schlafen4+ MDSCs stimulates epithelial proliferation and correlates with preneoplastic changes prior to gastric cancer. Gut. 2020;69:1750-1761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Playford RJ. Peptides and gastrointestinal mucosal integrity. Gut. 1995;37:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Wong WM, Playford RJ, Wright NA. Peptide gene expression in gastrointestinal mucosal ulceration: ordered sequence or redundancy? Gut. 2000;46:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Fox JG, Rogers AB, Whary MT, Ge Z, Ohtani M, Jones EK, Wang TC. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2 -/- C57BL6 x Sv129 Helicobacter pylori-infected mice. Am J Pathol. 2007;171:1520-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Kuo HY, Chang WL, Yeh YC, Tsai YC, Wu CT, Cheng HC, Yang HB, Lu CC, Sheu BS. Serum Level of Trefoil Factor 2 can Predict the Extent of Gastric Spasmolytic Polypeptide-Expressing Metaplasia in the H. pylori-Infected Gastric Cancer Relatives. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Burclaff J, Osaki LH, Liu D, Goldenring JR, Mills JC. Targeted Apoptosis of Parietal Cells Is Insufficient to Induce Metaplasia in Stomach. Gastroenterology. 2017;152:762-766.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Lee C, Lee H, Hwang SY, Moon CM, Hong SN. IL-10 Plays a Pivotal Role in Tamoxifen-Induced Spasmolytic Polypeptide-Expressing Metaplasia in Gastric Mucosa. Gut Liver. 2017;11:789-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Ahluwalia A, Jones MK, Deng X, Sandor Z, Szabo S, Tarnawski AS. An imbalance between VEGF and endostatin underlies impaired angiogenesis in gastric mucosa of aging rats. Am J Physiol Gastrointest Liver Physiol. 2013;305:G325-G332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Ahluwalia A, Jones MK, Szabo S, Tarnawski AS. Aging impairs transcriptional regulation of vascular endothelial growth factor in human microvascular endothelial cells: implications for angiogenesis and cell survival. J Physiol Pharmacol. 2014;65:209-215. [PubMed] |
| 36. | Engevik AC, Feng R, Choi E, White S, Bertaux-Skeirik N, Li J, Mahe MM, Aihara E, Yang L, DiPasquale B, Oh S, Engevik KA, Giraud AS, Montrose MH, Medvedovic M, Helmrath MA, Goldenring JR, Zavros Y. The Development of Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) During Gastric Repair Is Absent in the Aged Stomach. Cell Mol Gastroenterol Hepatol. 2016;2:605-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Radyk MD, Burclaff J, Willet SG, Mills JC. Metaplastic Cells in the Stomach Arise, Independently of Stem Cells, via Dedifferentiation or Transdifferentiation of Chief Cells. Gastroenterology. 2018;154:839-843.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |