Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1213
Peer-review started: March 22, 2021
First decision: June 14, 2021
Revised: June 28, 2021
Accepted: August 12, 2021
Article in press: August 12, 2021
Published online: October 15, 2021
Processing time: 205 Days and 6.7 Hours
The incidence and mortality of hepatocellular carcinoma have continued to increase over the last few years, and the medicine-based outlook of patients is poor. Given great ideas from the development of nanotechnology in medicine, especially the advantages in the treatments of liver cancer. Some engineering nanoparticles with active targeting, ligand modification, and passive targeting capacity achieve efficient drug delivery to tumor cells. In addition, the behavior of drug release is also applied to the drug loading nanosystem based on the tumor microenvironment. Considering clinical use of local treatment of liver cancer, in situ drug delivery of nanogels is also fully studied in orthotopic chemotherapy, radiotherapy, and ablation therapy. Furthermore, novel therapies including gene therapy, phototherapy, and immunotherapy are also applied as combined therapy for liver cancer. Engineering nonviral polymers to function as gene delivery vectors with increased efficiency and specificity, and strategies of co-delivery of therapeutic genes and drugs show great therapeutic effect against liver tumors, including drug-resistant tumors. Phototherapy is also applied in surgical procedures, chemotherapy, and immunotherapy. Combination strategies significantly enhance therapeutic effects and decrease side effects. Overall, the application of nanotechnology could bring a revolutionary change to the current treatment of liver cancer.
Core Tip: With the development of nanotheranostic strategies for liver cancer treatment, the efficacy of drug delivery is improved by smart nanoparticles with excellent targeting capacity. To overcome the complex tumor microenvironment, nanosystems with combined strategies of curative or palliative treatments have significant synergistic therapeutic effect against unfavorable clinical obstacles in the treatment of liver cancer.
- Citation: Cao L, Zhu YQ, Wu ZX, Wang GX, Cheng HW. Engineering nanotheranostic strategies for liver cancer. World J Gastrointest Oncol 2021; 13(10): 1213-1228
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1213.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1213
According to newly reported cancer statistics, the liver cancer death rate is much higher than the incidence, suggesting that the clinical treatments for liver cancer are unsatisfactory[1]. With the development of nanotechnology in medicine, especially in the improvement of combined treatments, many studies have attempted to improve the therapeutic effect and decrease the side effects affecting normal organs[2,3]. To fully understand the engineering nanotheranostic strategies for liver cancer, the dilemma of clinical liver cancer treatments should be described. Poor diagnosis and ineffective treatments are the general shortcomings of the clinical management of liver cancer. To overcome the poor therapeutic effect, this frontier demonstrates the potential engineering nanosystems based on curative or palliative treatments. Firstly, the expanding intersection of curative treatments and nanotheranostic strategy emphasizes surgical resection and tumor ablation, which improve the efficacy of therapeutic solutions to prevent tumor occurrence and metastasis. Moreover, improved nanotheranostic strategies are also presented based on palliative treatments, including chemotherapy, radiotherapy, embolization therapy, and novel therapies, such as gene therapy, phototherapy, and immunotherapy. Many smart nanomaterials are designed to act as effective drug delivery platforms to enhance the therapeutic effect against tumor cells, especially to combat drug resistance[4-6]. In addition, strategies that combine chemotherapy, phototherapy, gene therapy, and immunotherapy increase the sensitivity tumor cells to treatment[7-10]. To sum up, the engineering nanotheranostic strategy could revolutionize the current treatment of liver cancer and have great transformative value to ameliorate the prognosis of liver cancer patients.
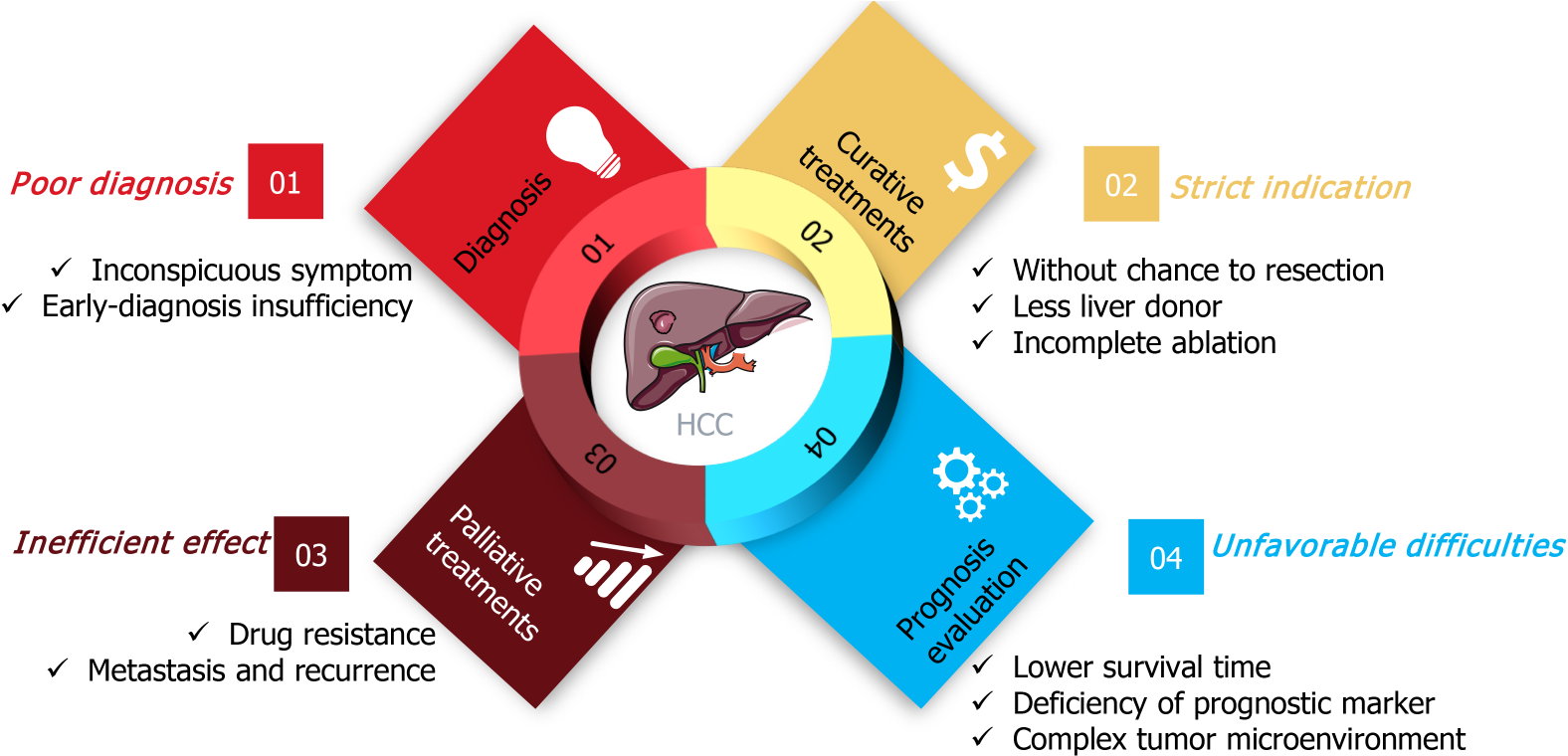
According to worldwide cancer statistics, the estimated death rate of liver cancer is significantly higher than its estimated incidence[1,11,12], suggesting that the clinical practice for liver cancer is unsatisfactory. Many complex factors contribute to this dilemma. As Figure 1 shows, clinical shortcomings for liver cancer include four areas, diagnosis, curative treatments, palliative treatments, and prognostic evaluation[13,14].
The clinical diagnosis of liver cancer is poor, the early symptoms of liver cancer are inconspicuous, and most patients are diagnosed in the intermediate or advanced stages, and without a chance to employ curative treatments including tumor resection, liver transplantation, and ablation[15]. Therefore, discovery of effective biomarkers has been extensively studied in recent years, with the expectation to overcome the shortcomings of low specificity and sensitivity of existing clinical markers, such as alpha-fetoprotein (AFP) and glypican 3 (GPC3)[15].
Curative treatments for liver cancer are applicable to patients with very early stage or early stage disease, thus many patients cannot benefit from these strategies because of their strict indications[16,17]. For tumor resection, the disease stages and complex physiological factors, including liver function, cirrhosis and surgery tolerance, limit its application for liver cancer patients[18,19]. In recent years, advances in liver transplantation have resulted in promising prospects in improving the prognosis of liver cancer patients with early stage disease[20-22]. However, because of the limitation of liver donors, few patients benefit from that treatment. Tumor ablation, including radiofrequency ablation (RFA), NanoKnife, and microwave ablation are available, but incomplete ablation is the main constraint in clinical practice[23-25].
Palliative treatments are available for most liver cancer patients, including chemotherapy, transarterial embolization, radiotherapy and immunotherapy. However, drug resistance is an important factor that deserves particular attention, because it significantly impairs treatment outcome. Immunotherapy is a novel, promising strategy in with potential benefits in some cancers. However, clinical trials of programmed death-1 (PD-1) monoclonal antibody therapy in patients with advanced hepatocellular carcinoma did not improve overall survival or progression-free survival of patients[26,27]. Incomplete transarterial embolization leads to tumor metastasis and recurrence and limits its clinical outcome. Consequently, the improvement of palliative treatments is urgently needed to reduce the mortality of liver cancer.
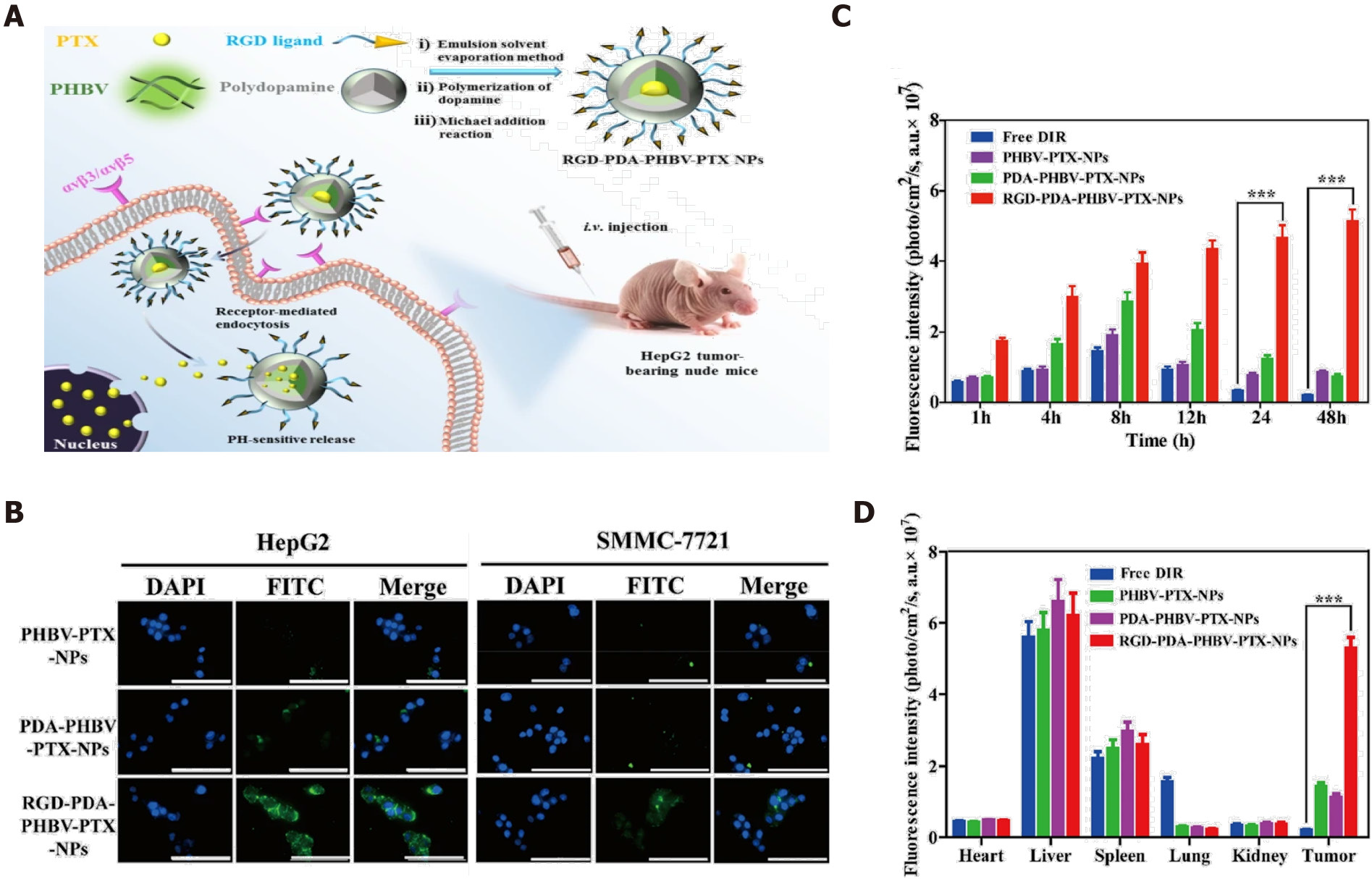
Chemotherapy plays an indispensable role in the liver cancer clinic, but the outcome is unsatisfactory[28]. Considering the underlying reasons, minimal drug bioavailability and side effects are the two main problems[29]. The development of nanotechnology allows for the improvement of effectiveness of chemotherapy. First, the nanostructure design enhances the solubility of chemotherapeutics like paclitaxel (PTX) and sorafenib, which are poorly soluble[30]. Improved biocompatibility can enhance the drug bioavailability. Second, the involvement of nanomaterials allows modification of drug behavior, and binding to the targeted ligand is an excellent solution to improve drug delivery and enhance the therapeutic effect of tumor inhibition. For example, Wu et al[31] described PTX-loaded poly (3-hydroxybutyrate-co-3-hydroxyvalerate) PHBV nanoparticles coated with polydopamine (PDA-PHBV-PTX-NPs) and modified by hepatocellular carcinoma (HCC)-targeted arginine-glycine-aspartic acid (RGD-peptide). As shown in Figure 2A, integrin αvβ3 or αvβ5 are overexpressed in liver cancer cells, and RGD is a specific ligand of integrin αvβ3/αvβ5. The PTX-loaded nanoparticles actively target and enter liver tumor cells. Figure 2B shows the cellular uptake of fluorescently labeled RGD-modified nanoparticles was remarkably higher than other groups (Figure 2B), and in vivo results of fluorescence imaging confirmed that RGD active targeting nanoparticles significantly increased the drug concentration in tumor tissues (Figure 2C and D). The evidence suggests that active targeting of nanomaterials has remarkable advantages in the efficacy of drug delivery. In recent years, with advances in biomarker discovery for liver cancer, many nanomaterials with active targeting ability have improved drug delivery, especially of insoluble drugs, with targeted peptides or antibodies used to confer the active targeting ability and thus improve drug bioavailability and therapeutic effects[4,5,32].
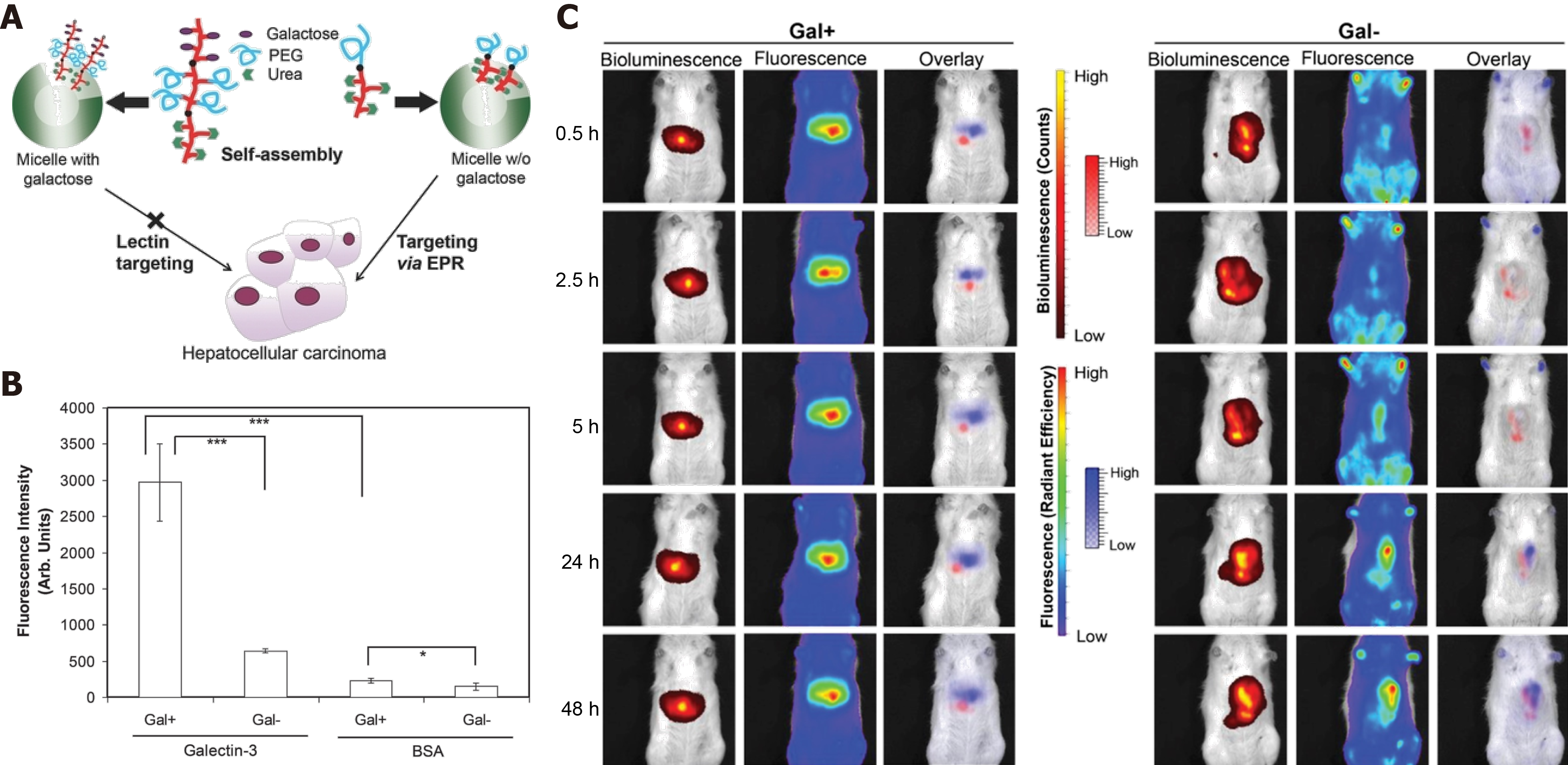
In recent studies, nanomaterials used as a drug delivery platform have made significant progress in cancer treatment. Active targeting medications are “the eyes” of nanomedicine, but the unique passive targeting capability of nanostructured materials is also a widely recognized uptake mechanism via enhanced permeability and retention (EPR)[33]. In a previous study, Ebrahim et al[34] designed galactose-conjugated nanoparticles to study the difference between ligand-active targeting and passive EPR targeting in liver cancer therapy. As in previous reports, collecting-3 was highly expressed in the liver tumor vessel endothelial cells, and galactose as the ligand of galectin-3 has been used as a functional fragment for targeted drug delivery to liver cancer. Furthermore, asialoglycoprotein with exposure to galactose residues can bind to asialoglycoprotein receptors (ASGPR), which are expressed in liver cancer cells. Interestingly, the expression of ASGPR in higher in normal hepatocytes cells than in liver hepatoma cells. Thus this novel nanoparticle strategy can help us understand the difference between active targeting and passive targeting in liver cancer drug delivery (Figure 3A). The result of the binding activity of galactose-conjugated nanoparticles for galectin-3 (Figure 3B) was higher than nanoparticles without galactose modification, and the specific HCC targeting capability was also evaluated in an orthotopic HCC tumor model. Bioluminescence shows the location of liver cancer cells, and the fluorescence shows the location of DiR-loaded nanoparticles with galactose or not, and the surprising results showed that galactose-conjugated nanoparticles were accumulated mostly in the hepatocytes, but not in liver tumors. However, the nanoparticles without galactose surface expression only depended on EPR passive-targeting capability, showing better accumulation in the liver tumors (Figure 3C). The dual strategy not only shows the advantage of ligand-active targeting in nanomedicine, but also confirms the indispensable role of EPR passive targeting in drug delivery. Even the efficiency of EPR-mediated passive targeting to cancer cells is still unclear, and particle size control is essential to the EPR effect of nanoparticles on tumor targeting, which provides a theoretical reference for the design of nanomedicines[35,36].
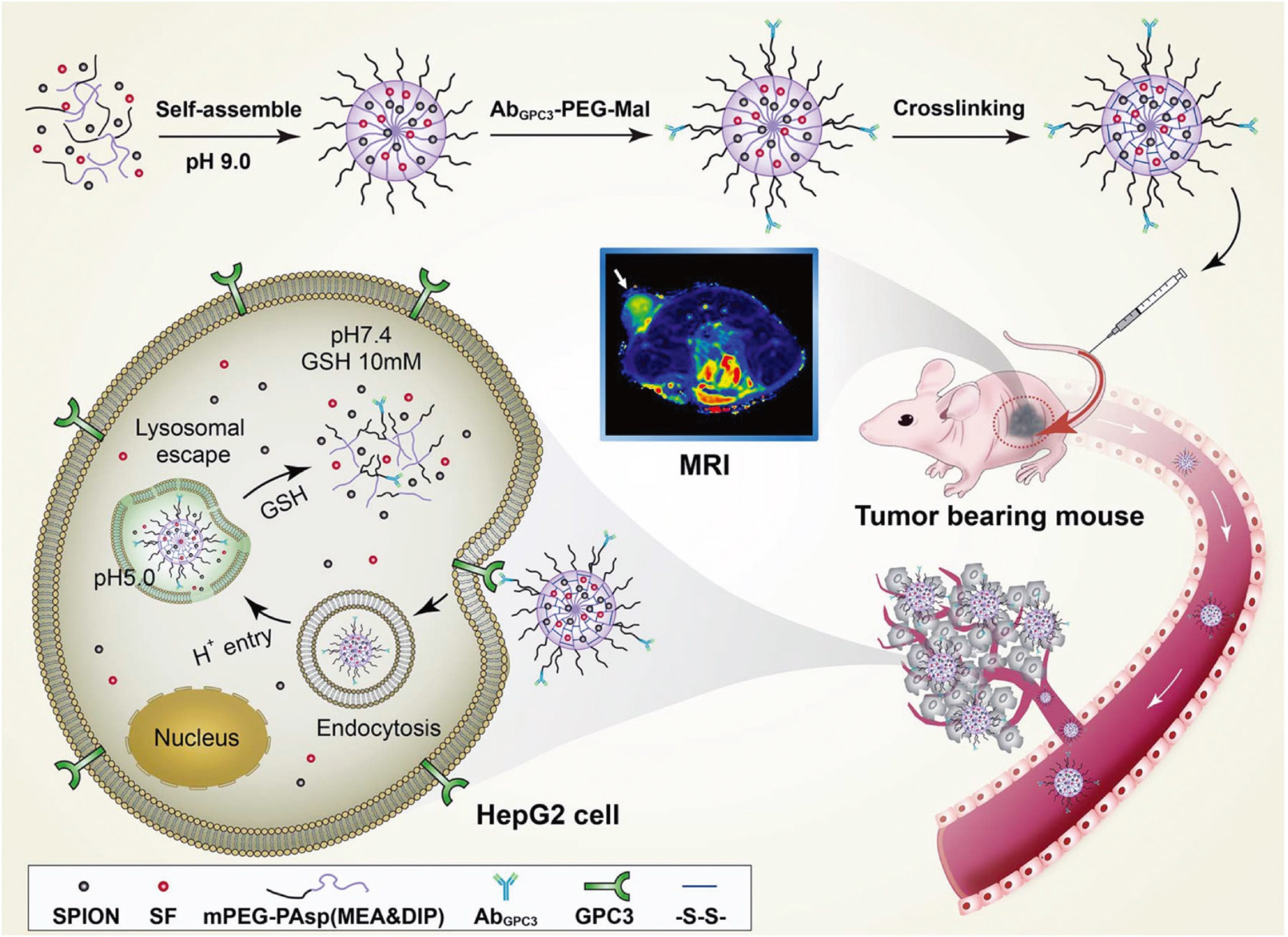
Based on the design of ligand-active or EPR passive targeting, nanomaterials have substantial advance progress in drug delivery. The detailed drug release behavior of nanomedicine has also been reported in recent years, especially in the exploitation of the tumor microenvironment (e.g., hypoxia, low pH, high glutathione, immune-suppressive microenvironment, and so on[37,38]. Therefore, while achieving higher drug delivery efficiency, the design of controllable drug release based on the tumor microenvironment has significant strengths in improving drug bioavailability, reducing toxic side effects, and improving therapeutic effects. Zhu et al[39] reported a nanodrug with reduced side effects and pH-sensitive drug release behavior applied as targeted HCC therapy. A copolymer of monomethoxyl polyethylene glycol and poly N-(2-aminoethanethiol-co-2-aminoethyldiisopropylamine) aspartamide (mPEG-PAsp) or MEA & DIP was self-assembled with sorafenib and super-paramagnetic iron oxide nanoparticles (SPIONs) into nanoparticles, and then modified with anti-GPC3 antibody (AbGPC3) to construct a nanodrug system with active targeting of liver cancer cells (Figure 4). SPIONs provided magnetic resonance imaging (MRI) capability to monitor the delivery behavior of the nanodrug. Increased glutathione and the low pH of the liver tumor microenvironment promote dissociation of the sorafenib-loaded nanodrug with sorafenib release on demand. Precise drug release significantly increases drug concentration in the tumor cells that guarantees therapeutic dosage and prevents drug resistance[40]. Furthermore, tumor microenvironment-responsive drug release decreases the toxicity to normal cells by drug blockade and nonspecific uptake by normal cells[3].
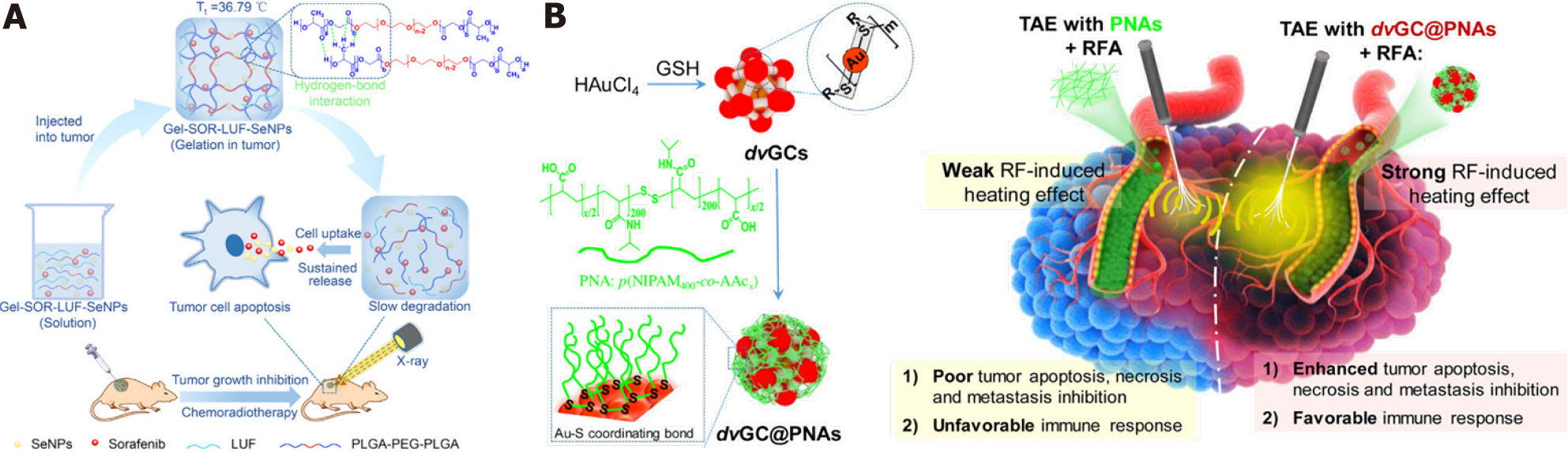
Topical therapy is an important strategy in cancer treatment, and ablation and transcatheter artery embolization (TAE) are common topical treatments in the liver cancer clinic[41]. Common embolism reagents contain lipiodol and microspheres. The advantage of liquid lipiodol is to embolize peripheral blood vessels of tumors, but it has little effect on the embolization of large blood vessels, which contributes to multiple procedures to prevent recanalization of blood vessels[2,42,43]. Incomplete peripheral blood vessel embolization also limits the therapeutic effects of microspheres, and the higher cost also impairs patient willingness to accept treatment[44]. Therefore, the construction of novel embolic reagents might be a promising solution to the obstacles to HCC artery embolization. Hydrogels are novel embolic reagents for HCC therapy. Hydrogels have the advantages of good biocompatibility, sustained drug release, high drug loading, and so on[45-47]. The three-dimensional structure can improve the loading of insoluble drugs, which is essential for improved drug bioavailability[48]. Zheng et al[7] described a thermosensitive poly (D, L-lactic acid-co-glycolic acid)-b-poly (ethylene glycol) -b-poly (D,L-lactic acid-co-glycolic acid) (PLGA-PEG-PLGA)-based hydrogel nanosystem, composed of sorafenib, lipiodol and selenium nanoparticles (SeNPs). The hydrogel was injected into liver tumors and had long-term local anticancer effects. Combination with X-ray radiotherapy further enhanced the therapeutic effects (Figure 5). More important, the thermosensitive hydrogel system takes advantage of lipiodol in the embolization of peripheral blood vessels. The dual-functional design warrants future investigation of embolic materials.
Another critical aspect of local therapy is tumor ablation. Conventional ablation is temperature- or chemical-based. Temperature-based methods include radiofrequency ablation (RFA), microwave ablation, laser ablation, high-intensity focus ultrasound, cryoablation, and nonreversible electroporation (IRE, or NanoKnife)[49,50]. Ethanol and ethanoic acid are the two main reagents used for chemical ablation. However, the evidence from clinical liver cancer practice indicates that incomplete ablation is an urgent problem that deserves more attention[51]. To overcome these flaws, nanomaterial-enhanced strategies are widely studied. Considering the superior embolic effect of thermogels and the good heat ablation effect of gold nanoclusters, Yang et al[8] described a composite system composed of poly (N-isopropylamide-co-acrylic acid) (PNAs) and dual-valent gold nanoclusters (dvGC) with a core-shell nanostructure. The incorporation of gold nanoclusters enhanced the heating effect of radiofrequency ablation and improved tumor inhibition[8]. The application of nanomaterials in topical HCC therapy achieves a better therapeutic effect over systemic treatments, and the patients can benefit from nanomaterial application in embolization and ablation-combined treatment. With the development of interventional technology, the future of tumor regional therapy is encouraging.
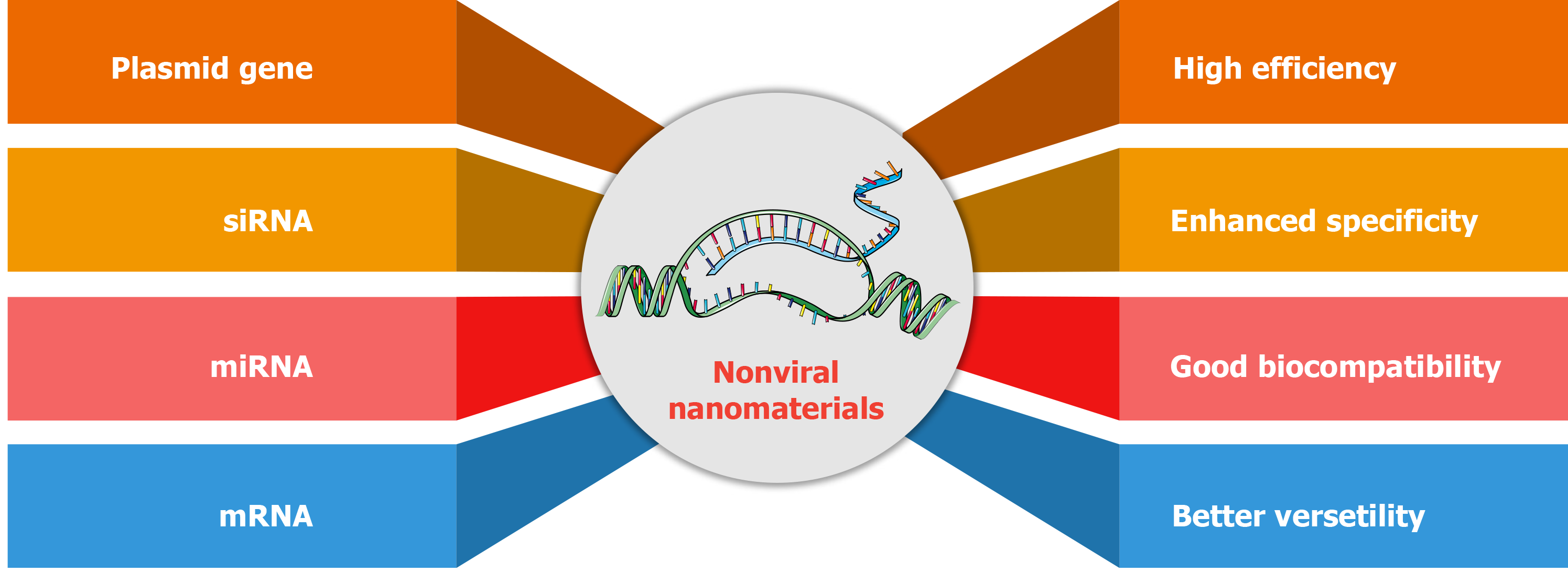
Gene therapy opens up prospects of innovative therapeutic schedules in HCC, and some therapeutic genes have been used to correct the genetic alterations. Genetic therapy includes the use of plasmid genes, small interfering RNA (siRNA), micro RNA (miRNA), or messenger RNA (mRNA)[52]. In recent years, some plasmids coding tumor suppressor genes were delivered into tumor cells to prevent tumor progression. Some siRNAs or miRNAs against oncogenic genes have been used to prevent pro-oncogenic signaling pathways. Of note, mRNA delivery is also promising in antitumor immunity[53,54]. At present, gene therapy-based chimeric antigen receptor T (CAR-T) therapy for HCC has been investigated in clinical trials. Introduction of CAR genes introduction is accomplished by viral gene delivery to guarantee efficient gene transfection ex vivo[55,56]. Reviewing the potential risks of viral vectors in vivo, nonviral gene delivery vector has developed rapidly, especially in the development of nanostructured materials[57]. Compared with viral vector gene delivery, nonviral delivery of nanomaterials has superior efficiency, specificity, biosafety, and multifunction design[58-60] (Figure 6). Common nonviral gene delivery vectors include many cationic nanocarriers that were developed to adsorb nucleic acids by electrostatic interactions, such as cationic liposomes and polymers with positively charged blocks[61,62].
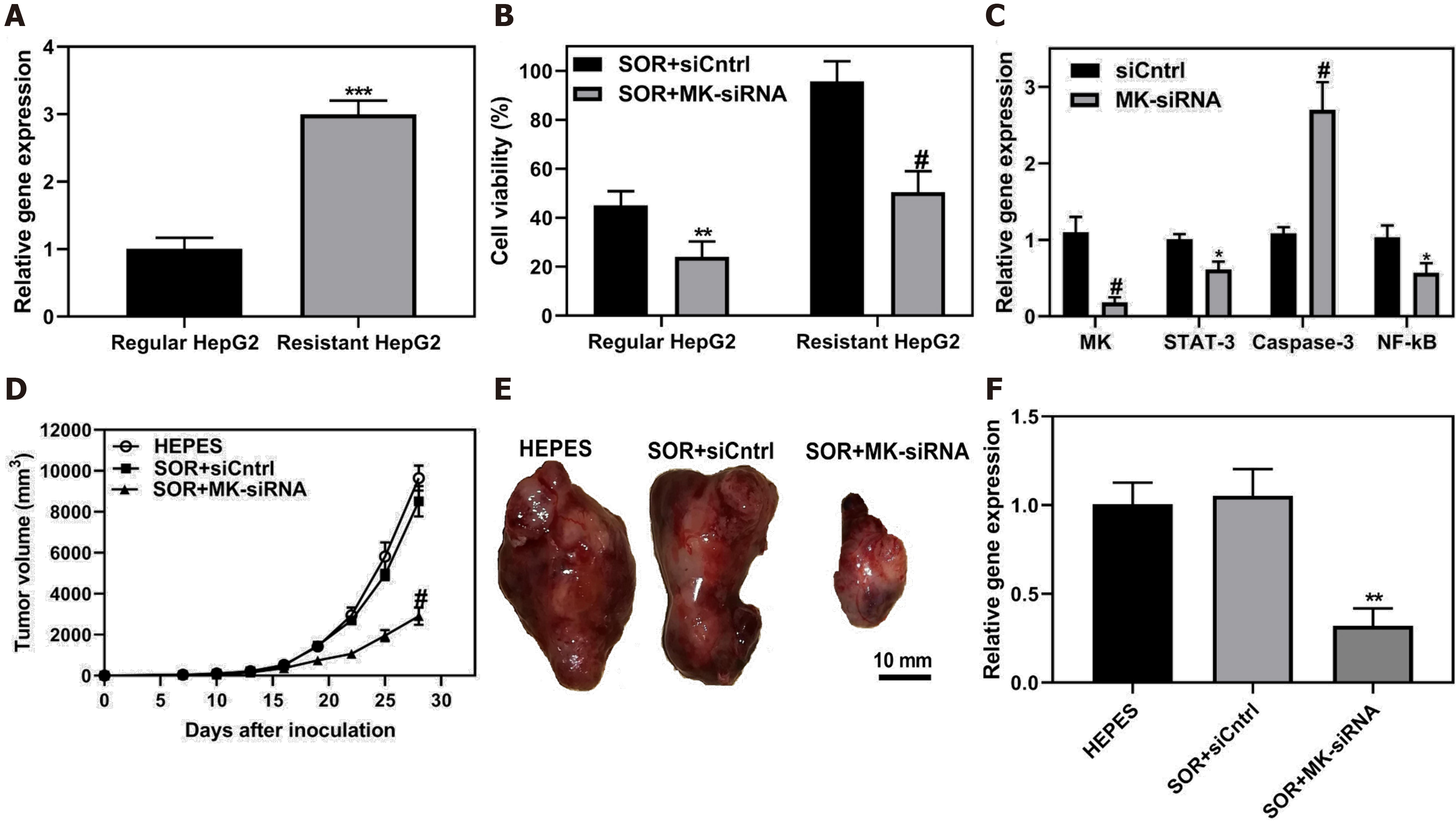
Drug resistance is an essential influence of patient prognosis, especially in HCC. HCC is frequently diagnosed at an advanced stage, and chemotherapy is a standard treatment for patients without surgical options, but drug resistance often occurs. The combination of other novel treatments with chemotherapy might be beneficial for HCC patients. Gene therapy has potential value in chemosensitization. Many studies have reported that delivery of tumor suppressor genes or silencing RNA against oncogenes recovered the sensitivity of tumor cells to chemotherapy drugs. Some studies focused on midkine, which is a biomarker of diagnosis and prognosis of HCC patients, and is involved in the cell proliferation and metastasis[63,64]. Downregulation of midkine to inhibit the progression of HCC has been confirmed in previous studies[65,66]. Harashima et al[67] developed small lipid nanoparticles that encapsulated midkine-siRNA and sorafenib. As shown in Figure 7A, midkine was overexpressed in sorafenib-resistant HepG2 cells, and knockdown of midkine significantly improved the inhibition of cell viability by sorafenib (Figure 7B). They also demonstrated that the silencing of midkine inhibited STAT-3 and NF-κB signaling pathways and promoted Caspase-3 antitumor activity (Figure 7C). The treatment outcome of co-delivery of sorafenib and siRNA against midkine was confirmed in a mouse model, showing significant tumor suppression (Figure 7D-F).
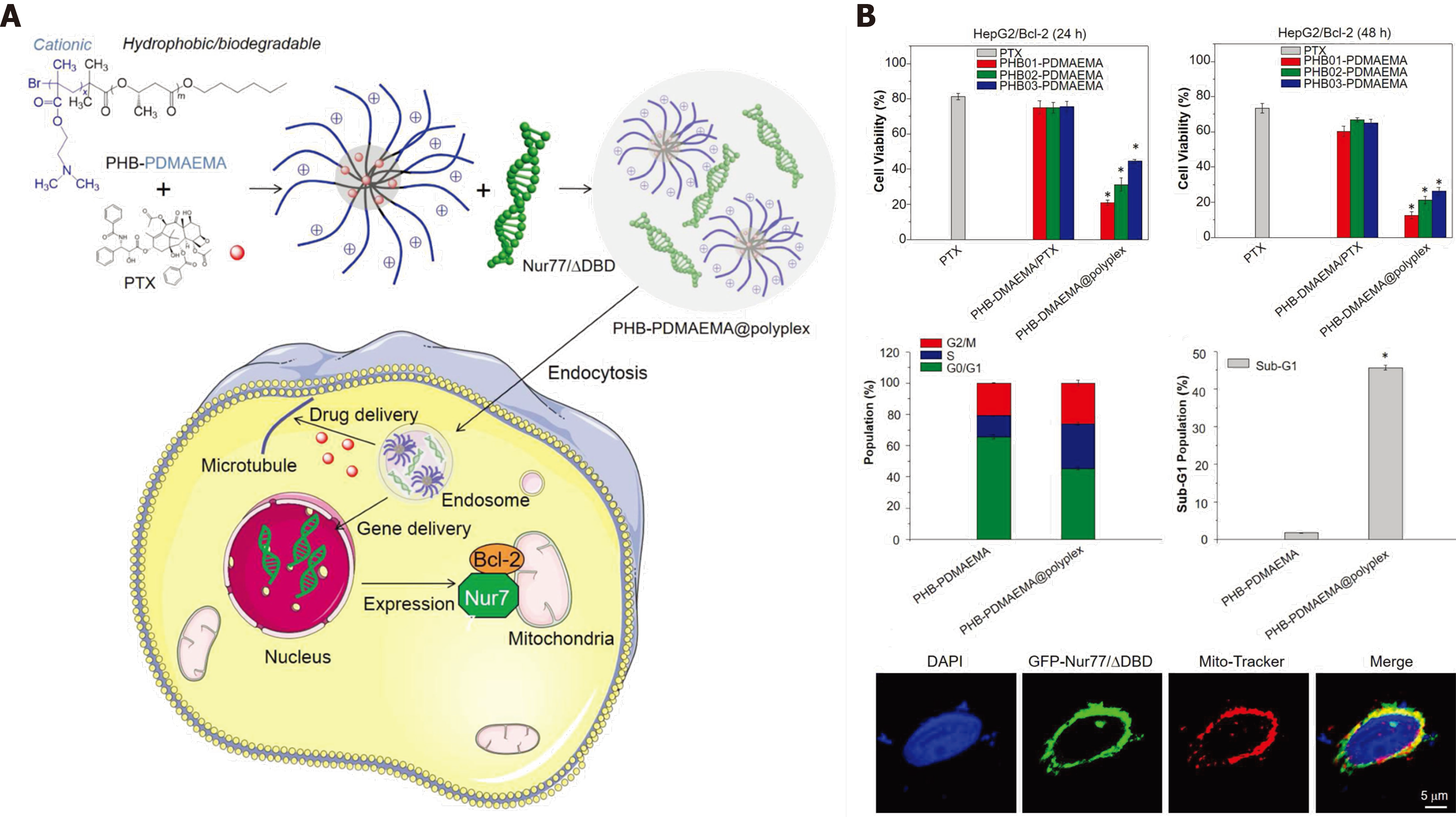
Pump-mediated drug efflux is a mediator of chemotherapy resistance, and P-glycoprotein (MDR1) is a principal regulator in drug efflux. Our team and other research groups have developed some delivery systems to overcome drug efflux-mediated resistance. We previously described thermo-responsive supramolecular polymers that enhanced the cellular uptake of chemotherapeutics by cells that overexpressed multidrug resistance (MDR)1[68,69]. The co-delivery of chemotherapeutics and siRNA against MDR1 resulted in significant inhibition of drug efflux and improved treatment response[70,71]. The findings show that engineered nanosystems with combined treatments have significant advantages to overcome drug efflux-pump resistance. However, non-pump-mediated drug resistance also restricts the therapeutic effect of chemotherapy. Bcl-2, a mitochondrial regulator, functions as an antiapoptotic mediator to interfere with Caspase family apoptotic signaling. Knockdown of Bcl-2 expression with inhibitors or siRNA is a classical strategy to strengthen tumor inhibition by chemotherapeutics[6,72]. However, the stability of siRNA is a notable constraint. Interestingly, Wu et al[9,73-75] developed a strategy to co-deliver Nur77△DBD gene plasmids and chemotherapeutic PTX simultaneously. The Nur77/△DBD interacted with Bcl-2 and changed Bcl-2 from tumor protector to tumor killer. Considering the advantage of DNA on stability over RNA, that strategy might increase the efficiency of gene delivery and enhance the cytotoxicity of chemotherapeutic PTX. The mitochondrial location of Nur77/△DBD promotes reversal of Bcl-2 function (Figure 8). In general, the co-delivery of genes and chemotherapeutic drugs can be applied in both drug efflux pump and non-pump drug resistance. The combination with gene therapy can significantly reverse pro-oncogenic pathways, and improve the performance of chemotherapeutic drugs.
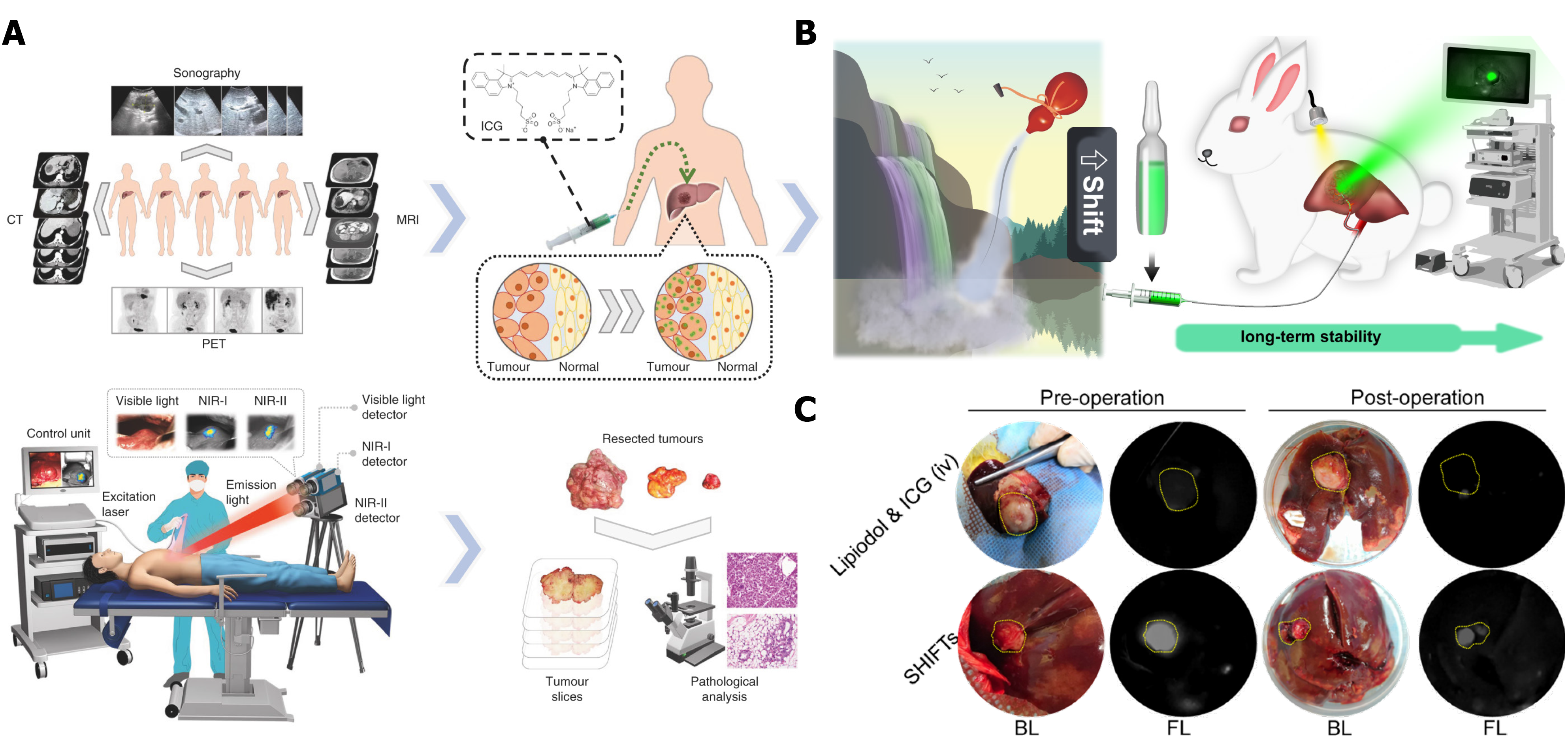
To date, surgical resection is the first choice for HCC treatment, which can significantly prolong the survival of HCC patients. However, because of the difficulty to identify small lesions by the naked eye, incomplete tumor resection contributes to tumor recurrence or metastasis, which significantly affect the clinical outcome. In recent years, imaging-guided tumor resection has shown outstanding performance in HCC surgical treatment. Compared with some classical imaging options including computed tomography and MRI, photo-imaging has better clinical transformation value. Fluorescence imaging (FLI) and photo-acoustic imaging are the two representative solutions with improved efficiency in the detection of small lesions. Tian et al[76] reported indocyanine green (ICG) as an FDA-approved near-infrared (NIR-Ⅱ) probe to guide tumor resection in HCC patients. They reported that intraoperative NIR-II fluorescence imaging had a higher tumor detection sensitivity and a better signal-to-noise ratio to distinguish HCC tumor and normal liver tissue (Figure 9A).
Considering the rapid clearance of ICG in vivo and the time demands of surgery, Liu et al[77] developed a novel embolic formulation that combined an embolic lipiodol agent and ICG. This study was motivated by the clinical demand for TAE to treat HCC. Some insoluble chemotherapeutic drugs cannot fully disperse in lipiodol, which contributes to the instability of the drugs in the local tumor environment. To overcome the problem, a superstable homogeneous iodinated formulation technology (SHIFT) was used to improve the stability of ICG in lipiodol, and allow combined therapy of embolization with fluorescence-guided surgical resection, which is suitable for patients with advanced HCC (Figure 9B). Interestingly, the rabbit VX2 tumor model results showed remarkable fluorescence intensity in the tumor after 2 wk of embolization therapy (Figure 9C). Intravenous injection of free ICG 24 h before surgery resulted in no significant fluorescence intensity. The evidence shows that transcatheter embolization synergistic fluorescence imaging-assisted surgical resection can enhance tumor detection, and achievement of complete tumor resection to avoid tumor recurrence and metastasis. Additionally, precise fluorescence imaging can decrease the resection risk of normal tissues. In general, the strategy of imaging-guided surgical resection has clinical potential for HCC patients, even for those with progressive disease.
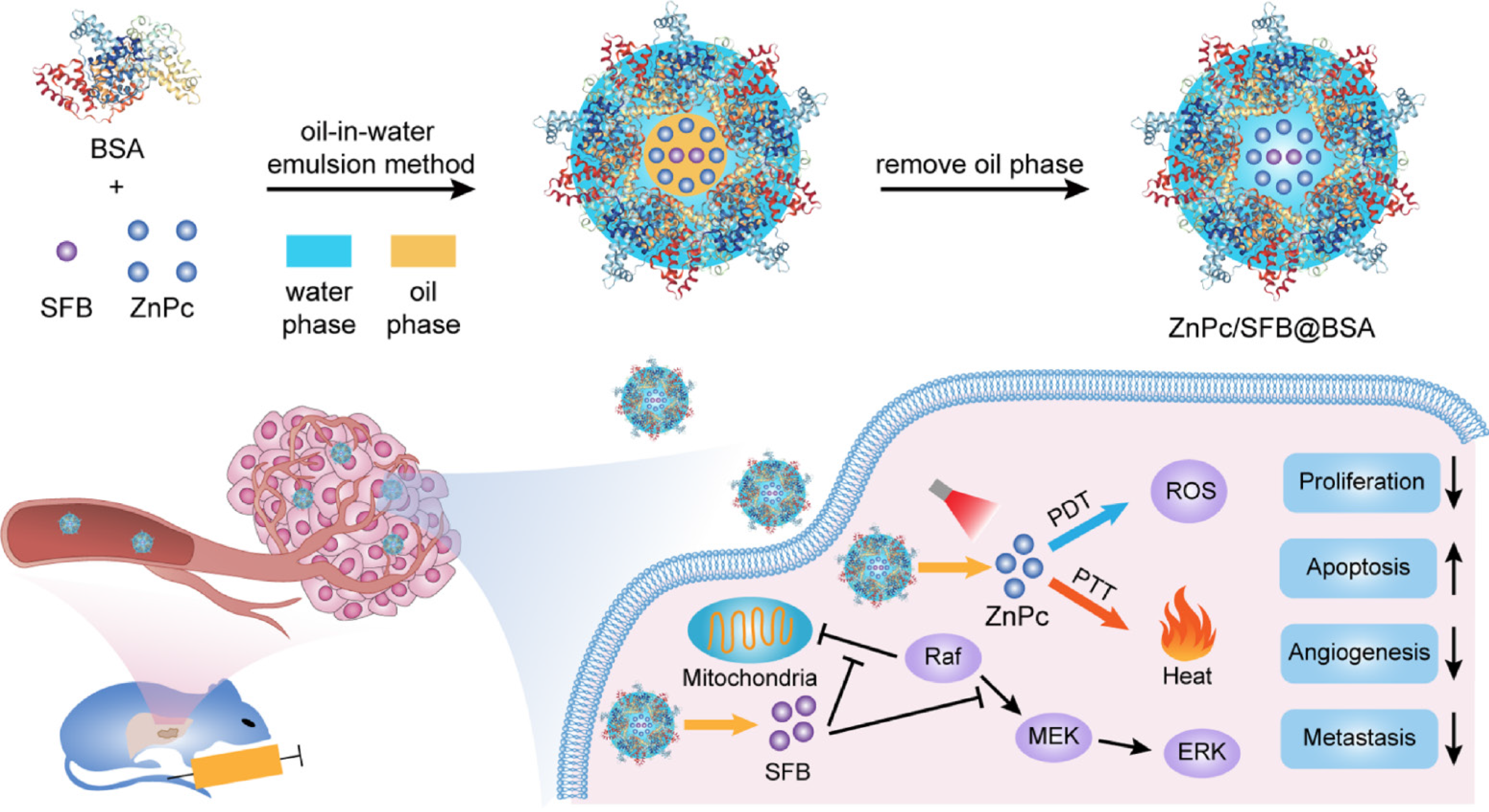
Photothermal therapy (PTT) and photodynamic therapy (PDT) are the two main phototherapies. Simply, photosensitizers are exploited by PTT and PDT under laser irradiation, the heat or reactive oxygen species (ROS) that are produced kill tumors[78,79]. Some studies have reported the potential application of PTT and PDT in HCC. Yu et al[80] evaluated bovine serum albumin (BSA)-coated zinc phthalocyanine (ZnPc) and chemotherapeutic sorafenib (SFB) nanoparticles in an oil-in-water emulsion (Figure 10). Zinc phthalocyanine was added as photosensitizer to achieve PTT and PDT effect. Based on the advantages of nanoparticles for drug delivery, sorafenib significantly inhibited Raf/MEK/ERK signaling, which is essential for cell proliferation, angiogenesis, and metastasis. Efficient heat and ROS generation could remarkably induce tumor apoptosis, and enhance the sensitivity of tumor cells to sorafenib chemotherapy. The combination of phototherapy and chemotherapy is a potential strategy to address the shortcomings of chemotherapy, such as acquired drug tolerance.
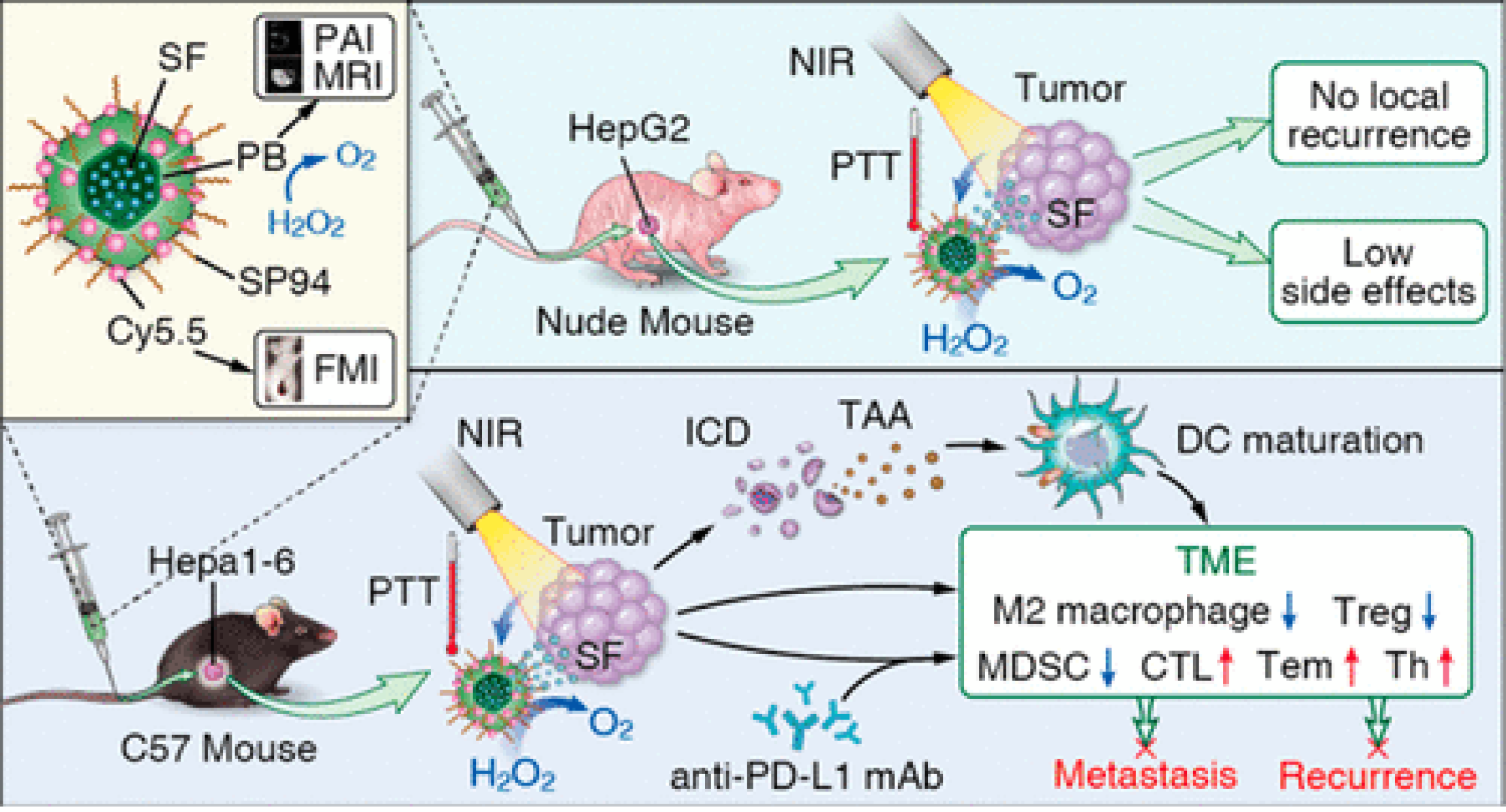
Immunotherapy as a revolutionary cancer treatment that include immune checkpoint blockade, such as CTLA-4, PD-1 or PD-L1 and chimeric antigen receptor T cell (CAT-T)[81,82]. However, off-target toxicity and low efficiency are the main shortcomings. Many studies have confirmed that immunotherapy slightly improves the survival of HCC patients[26,83]. Considering the difficulties of immunotherapy in solid tumors, the potential value of nanomaterial-assisted efficient drug delivery, and synergistic treatment effects, many studies have investigated ways to increase the therapeutic effect of immunotherapy. Some synergistic strategies have achieved therapeutic effects[84,85]. Tian’s group[10] reported that SP94-coated Prussian blue nanoparticles effectively delivered sorafenib to liver tumor cells (Figure 11). The Prussian blue color showed that the nanoparticles mediated an efficient PTT effect under laser irradiation, and the complementary treatment with sorafenib induced immunogenic cell death, released tumor-associated antigen, and promoted dendritic cell (DC) maturation, which significantly enhanced the therapeutic response of anti-PD-L1 monoclonal antibody (mAb). Synergistic phototherapy and checkpoint blockade immunotherapy strategy can restructure tumor immunosuppression microenvironments, making HCC patients more sensitive to immunotherapy. Overall, synergistic strategy opens the door for immunotherapy of HCC.
In conclusion, this frontier focuses on the prominent problems in the clinical treatment of liver cancer, especially in the discussion of key factors that restrict the early diagnosis and create a poor prognosis of liver cancer, and further explores nanotechnology-based solutions. With smart nano-design, the efficacy of drug delivery is achieved by active or passive targeting strategies. Combined strategies with current curative or palliative treatments of liver cancer can strengthen the therapeutic effect of surgery, ablation, chemotherapy, gene therapy, and phototherapy. In clinical practice, combination therapy is commonly used for liver cancer to overcome the shortcomings of single treatment that are subject to acquired drug resistance and toxic side effects. The specific structure of many nanotheranostic strategies improves the performance of combination therapy, which significantly improves the prognosis of liver cancer patients and prolongs survival. Overall, development of the engineering nanotheranostic strategy could revolutionize the current treatment of liver cancer.
The authors thank the previous publisher or copyright holder for giving permission of figure to be re-published.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: China Anticancer Association, No. M160205336A.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen S S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Xing YX
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11931] [Article Influence: 2982.8] [Reference Citation Analysis (4)] |
| 2. | Li Z, Di C, Li S, Yang X, Nie G. Smart Nanotherapeutic Targeting of Tumor Vasculature. Acc Chem Res. 2019;52:2703-2712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 3. | Jiang X, Fan X, Xu W, Zhao C, Wu H, Zhang R, Wu G. Self-assembled peptide nanoparticles responsive to multiple tumor microenvironment triggers provide highly efficient targeted delivery and release of antitumor drug. J Control Release. 2019;316:196-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Tang X, Chen L, Li A, Cai S, Zhang Y, Liu X, Jiang Z, Liang Y, Ma D. Anti-GPC3 antibody-modified sorafenib-loaded nanoparticles significantly inhibited HepG2 hepatocellular carcinoma. Drug Deliv. 2018;25:1484-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Shen J, Cai W, Ma Y, Xu R, Huo Z, Song L, Qiu X, Zhang Y, Li A, Cao W, Zhou S, Tang X. hGC33-Modified and Sorafenib-Loaded Nanoparticles have a Synergistic Anti-Hepatoma Effect by Inhibiting Wnt Signaling Pathway. Nanoscale Res Lett. 2020;15:220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 6. | Kim J, Shim MK, Yang S, Moon Y, Song S, Choi J, Kim J, Kim K. Combination of cancer-specific prodrug nanoparticle with Bcl-2 inhibitor to overcome acquired drug resistance. J Control Release. 2021;330:920-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Zheng L, Li C, Huang X, Lin X, Lin W, Yang F, Chen T. Thermosensitive hydrogels for sustained-release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials. 2019;216:119220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | Li L, Guo X, Peng X, Zhang H, Liu Y, Li H, He X, Shi D, Xiong B, Zhao Y, Zheng C, Yang X. Radiofrequency-responsive dual-valent gold nanoclusters for enhancing synergistic therapy of tumor ablation and artery embolization. Nano Today. 2020;35:100934. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Li Z, Liu X, Chen X, Chua MX, Wu YL. Targeted delivery of Bcl-2 conversion gene by MPEG-PCL-PEI-FA cationic copolymer to combat therapeutic resistant cancer. Mater Sci Eng C Mater Biol Appl. 2017;76:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Zhou T, Liang X, Wang P, Hu Y, Qi Y, Jin Y, Du Y, Fang C, Tian J. A Hepatocellular Carcinoma Targeting Nanostrategy with Hypoxia-Ameliorating and Photothermal Abilities that, Combined with Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano. 2020;14:12679-12696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 11. | Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, Zhao R, Duan Y, Zeng Z, Li X, Li G, Xiong W, Zhou M. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 12. | Pilleron S, Sarfati D, Janssen-Heijnen M, Vignat J, Ferlay J, Bray F, Soerjomataram I. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int J Cancer. 2019;144:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 13. | Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, Lencioni R, Greten TF, Kudo M, Mandrekar SJ, Zhu AX, Finn RS, Roberts LR; AASLD Panel of Experts on Trial Design in HCC. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology. 2021;73 Suppl 1:158-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 14. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1380] [Article Influence: 197.1] [Reference Citation Analysis (0)] |
| 15. | Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, Yin Z, Xie X, Wang X, Chen W, Zhou L, Li Y, Chen H, Gao L, Lu X, Wu T, Wang H, Niu J, Xu G. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology. 2018;67:662-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 16. | Inchingolo R, Posa A, Mariappan M, Spiliopoulos S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J Gastroenterol. 2019;25:4614-4628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. Liver cancer: Approaching a personalized care. J Hepatol. 2015;62:S144-S156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 18. | Hackl C, Schlitt HJ, Renner P, Lang SA. Liver surgery in cirrhosis and portal hypertension. World J Gastroenterol. 2016;22:2725-2735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4097] [Article Influence: 585.3] [Reference Citation Analysis (6)] |
| 20. | Marroni CA, Fleck AM Jr, Fernandes SA, Galant LH, Mucenic M, de Mattos Meine MH, Mariante-Neto G, Brandão ABM. Liver transplantation and alcoholic liver disease: History, controversies, and considerations. World J Gastroenterol. 2018;24:2785-2805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (3)] |
| 21. | Line PD. Frontiers in liver transplantation. Br J Surg. 2020;107:790-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Thuluvath PJ, Thuluvath AJ, Hanish S, Savva Y. Liver transplantation in patients with multiple organ failures: Feasibility and outcomes. J Hepatol. 2018;69:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Hu J, Dong Y, Ding L, Mao F, Cao J, Sun Y, Wang W, Duan Y. Localized Chemotherapy Prevents Lung Metastasis After Incomplete Microwave Ablation of Hepatic VX2 Tumor. J Biomed Nanotechnol. 2019;15:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Sparchez Z, Mocan T, Radu P, Mocan LP, Sparchez M, Leucuta DC, Al Hajjar N. Prognostic Factors after Percutaneous Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma. Impact of Incomplete Ablation on Recurrence and Overall Survival Rates. J Gastrointestin Liver Dis. 2018;27:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Niessen C, Thumann S, Beyer L, Pregler B, Kramer J, Lang S, Teufel A, Jung EM, Stroszczynski C, Wiggermann P. Percutaneous Irreversible Electroporation: Long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci Rep. 2017;7:43687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1339] [Article Influence: 267.8] [Reference Citation Analysis (0)] |
| 27. | Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: Looking outside the box. J Hepatol. 2020;72:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 28. | Chen X, Zhang X, Zhang L, Gao Y, Wang C, Hong W, Zhao G, Li L, Liu R. Amphiphilic Janus nanoparticles for imaging-guided synergistic chemo-photothermal hepatocellular carcinoma therapy in the second near-infrared window. Nanoscale. 2021;13:3974-3982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: Improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Zhang J, Wang T, Mu S, Olerile LD, Yu X, Zhang N. Biomacromolecule/Lipid hybrid nanoparticles for controlled delivery of sorafenib in targeting hepatocellular carcinoma therapy. Nanomedicine (Lond). 2017;12:911-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Wu M, Zhong C, Zhang Q, Wang L, Liu Y, Zhang X, Zhao X. pH-responsive delivery vehicle based on RGD-modified polydopamine-paclitaxel-loaded poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanoparticles for targeted therapy in hepatocellular carcinoma. J Nanobiotechnology. 2021;19:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 32. | Fu Y, Urban DJ, Nani RR, Zhang YF, Li N, Fu H, Shah H, Gorka AP, Guha R, Chen L, Hall MD, Schnermann MJ, Ho M. Glypican-3-Specific Antibody Drug Conjugates Targeting Hepatocellular Carcinoma. Hepatology. 2019;70:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 33. | Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 545] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 34. | Ebrahim Attia AB, Oh P, Yang C, Tan JP, Rao N, Hedrick JL, Yang YY, Ge R. Insights into EPR effect vs lectin-mediated targeted delivery: biodegradable polycarbonate micellar nanoparticles with and without galactose surface decoration. Small. 2014;10:4281-4286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Kang H, Rho S, Stiles WR, Hu S, Baek Y, Hwang DW, Kashiwagi S, Kim MS, Choi HS. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv Healthc Mater. 2020;9:e1901223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 290] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 36. | Tong X, Wang Z, Sun X, Song J, Jacobson O, Niu G, Kiesewetter DO, Chen X. Size Dependent Kinetics of Gold Nanorods in EPR Mediated Tumor Delivery. Theranostics. 2016;6:2039-2051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 37. | Zhou Y, Chen X, Cao J, Gao H. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J Mater Chem B. 2020;8:6765-6781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 38. | Piñeiro Fernández J, Luddy KA, Harmon C, O'Farrelly C. Hepatic Tumor Microenvironments and Effects on NK Cell Phenotype and Function. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 39. | Cai M, Li B, Lin L, Huang J, An Y, Huang W, Zhou Z, Wang Y, Shuai X, Zhu K. A reduction and pH dual-sensitive nanodrug for targeted theranostics in hepatocellular carcinoma. Biomater Sci. 2020;8:3485-3499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Zhang M, Guo X, Wang M, Liu K. Tumor microenvironment-induced structure changing drug/gene delivery system for overcoming delivery-associated challenges. J Control Release. 2020;323:203-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, Shah S, Almader-Douglas D, Uson Junior PLS, Mahipal A, Ma WW, Jin Z, Mody K, Starr J, Borad MJ, Ahn DH, Murad MH, Bekaii-Saab T. Systemic Therapy and Sequencing Options in Advanced Hepatocellular Carcinoma: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2020;6:e204930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 42. | Qian K, Ma Y, Wan J, Geng S, Li H, Fu Q, Peng X, Kan X, Zhou G, Liu W, Xiong B, Zhao Y, Zheng C, Yang X, Xu H. The studies about doxorubicin-loaded p(N-isopropyl-acrylamide-co-butyl methylacrylate) temperature-sensitive nanogel dispersions on the application in TACE therapies for rabbit VX2 Liver tumor. J Control Release. 2015;212:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Li L, Liu Y, Li H, Guo X, He X, Geng S, Zhao H, Peng X, Shi D, Xiong B, Zhou G, Zhao Y, Zheng C, Yang X. Rational design of temperature-sensitive blood-vessel-embolic nanogels for improving hypoxic tumor microenvironment after transcatheter arterial embolization. Theranostics. 2018;8:6291-6306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: Current state of the art. World J Gastroenterol. 2018;24:161-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 45. | Chan BQY, Cheng H, Liow SS, Dou Q, Wu YL, Loh XJ, Li Z. Poly(carbonate urethane)-Based Thermogels with Enhanced Drug Release Efficacy for Chemotherapeutic Applications. Polymers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Hu B, Owh C, Chee PL, Leow WR, Liu X, Wu YL, Guo P, Loh XJ, Chen X. Supramolecular hydrogels for antimicrobial therapy. Chem Soc Rev. 2018;47:6917-6929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 47. | Liu X, Li Z, Loh XJ, Chen K, Wu YL. Targeted and Sustained Corelease of Chemotherapeutics and Gene by Injectable Supramolecular Hydrogel for Drug-Resistant Cancer Therapy. Macromol Rapid Commun. 2019;40:e1800117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 48. | Ning P, Lü S, Bai X, Wu X, Gao C, Wen N, Liu M. High encapsulation and localized delivery of curcumin from an injectable hydrogel. Mater Sci Eng C Mater Biol Appl. 2018;83:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Habibollahi P, Sheth RA, Cressman ENK. Histological Correlation for Radiofrequency and Microwave Ablation in the Local Control of Hepatocellular Carcinoma (HCC) before Liver Transplantation: A Comprehensive Review. Cancers (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Luo W, Zhang Y, He G, Yu M, Zheng M, Liu L, Zhou X. Effects of radiofrequency ablation vs other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Miyauchi Y, Himoto T, Kimura Y, Nakai S, Deguchi A, Yoneyama H, Yoshida S, Kuriyama S. Comparison between combination therapy of percutaneous ethanol injection and radiofrequency ablation and radiofrequency ablation alone for patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Reghupaty SC, Sarkar D. Current Status of Gene Therapy in Hepatocellular Carcinoma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Huang KW, Hsu FF, Qiu JT, Chern GJ, Lee YA, Chang CC, Huang YT, Sung YC, Chiang CC, Huang RL, Lin CC, Dinh TK, Huang HC, Shih YC, Alson D, Lin CY, Lin YC, Chang PC, Lin SY, Chen Y. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci Adv. 2020;6:eaax5032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 54. | Huang KW, Lai YT, Chern GJ, Huang SF, Tsai CL, Sung YC, Chiang CC, Hwang PB, Ho TL, Huang RL, Shiue TY, Chen Y, Wang SK. Galactose Derivative-Modified Nanoparticles for Efficient siRNA Delivery to Hepatocellular Carcinoma. Biomacromolecules. 2018;19:2330-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 55. | Wang Y, Chen M, Wu Z, Tong C, Dai H, Guo Y, Liu Y, Huang J, Lv H, Luo C, Feng KC, Yang QM, Li XL, Han W. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology. 2018;7:e1440169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 56. | Li D, Li N, Zhang YF, Fu H, Feng M, Schneider D, Su L, Wu X, Zhou J, Mackay S, Kramer J, Duan Z, Yang H, Kolluri A, Hummer AM, Torres MB, Zhu H, Hall MD, Luo X, Chen J, Wang Q, Abate-Daga D, Dropulic B, Hewitt SM, Orentas RJ, Greten TF, Ho M. Persistent Polyfunctional Chimeric Antigen Receptor T Cells That Target Glypican 3 Eliminate Orthotopic Hepatocellular Carcinomas in Mice. Gastroenterology. 2020;158:2250-2265.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 57. | Olden BR, Cheng Y, Yu JL, Pun SH. Cationic polymers for non-viral gene delivery to human T cells. J Control Release. 2018;282:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 58. | Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release. 2016;240:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 59. | Van Bruggen C, Hexum JK, Tan Z, Dalal RJ, Reineke TM. Nonviral Gene Delivery with Cationic Glycopolymers. Acc Chem Res. 2019;52:1347-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 60. | Kargaard A, Sluijter JPG, Klumperman B. Polymeric siRNA gene delivery - transfection efficiency vs cytotoxicity. J Control Release. 2019;316:263-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Wu Y, Xiong Y, Wang L, Zhou Q, Li L, Levkin PA, Davidson G, Gao L, Deng W. Development of new self-assembled cationic amino liposomes for efficient gene delivery. Biomater Sci. 2020;8:3021-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Chountoulesi M, Pippa N, Chrysostomou V, Pispas S, Chrysina ED, Forys A, Otulakowski L, Trzebicka B, Demetzos C. Stimuli-Responsive Lyotropic Liquid Crystalline Nanosystems with Incorporated Poly(2-Dimethylamino Ethyl Methacrylate)-b-Poly(Lauryl Methacrylate) Amphiphilic Block Copolymer. Polymers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573-10583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 294] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (6)] |
| 64. | Omran MM, Farid K, Omar MA, Emran TM, El-Taweel FM, Tabll AA. A combination of α-fetoprotein, midkine, thioredoxin and a metabolite for predicting hepatocellular carcinoma. Ann Hepatol. 2020;19:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Dai LC, Wang X, Yao X, Lu YL, Ping JL, He JF. Enhanced therapeutic effects of combined chemotherapeutic drugs and midkine antisense oligonucleotides for hepatocellular carcinoma. World J Gastroenterol. 2007;13:1989-1994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Younis MA, Khalil IA, Abd Elwakil MM, Harashima H. A Multifunctional Lipid-Based Nanodevice for the Highly Specific Codelivery of Sorafenib and Midkine siRNA to Hepatic Cancer Cells. Mol Pharm. 2019;16:4031-4044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Younis MA, Khalil IA, Elewa YHA, Kon Y, Harashima H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J Control Release. 2021;331:335-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 68. | Fan X, Cheng H, Wang X, Ye E, Loh XJ, Wu YL, Li Z. Thermoresponsive Supramolecular Chemotherapy by "V"-Shaped Armed β-Cyclodextrin Star Polymer to Overcome Drug Resistance. Adv Healthc Mater. 2018;7:e1701143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Cheng H, Fan X, Wang X, Ye E, Loh XJ, Li Z, Wu YL. Hierarchically Self-Assembled Supramolecular Host-Guest Delivery System for Drug Resistant Cancer Therapy. Biomacromolecules. 2018;19:1926-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | Wang C, Guan W, Peng J, Chen Y, Xu G, Dou H. Gene/paclitaxel co-delivering nanocarriers prepared by framework-induced self-assembly for the inhibition of highly drug-resistant tumors. Acta Biomater. 2020;103:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 71. | Gao Y, Jia L, Wang Q, Hu H, Zhao X, Chen D, Qiao M. pH/Redox Dual-Responsive Polyplex with Effective Endosomal Escape for Codelivery of siRNA and Doxorubicin against Drug-Resistant Cancer Cells. ACS Appl Mater Interfaces. 2019;11:16296-16310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 72. | Ghaffari M, Dehghan G, Baradaran B, Zarebkohan A, Mansoori B, Soleymani J, Ezzati Nazhad Dolatabadi J, Hamblin MR. Co-delivery of curcumin and Bcl-2 siRNA by PAMAM dendrimers for enhancement of the therapeutic efficacy in HeLa cancer cells. Colloids Surf B Biointerfaces. 2020;188:110762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 73. | Cheng H, Wu Z, Wu C, Wang X, Liow SS, Li Z, Wu YL. Overcoming STC2 mediated drug resistance through drug and gene co-delivery by PHB-PDMAEMA cationic polyester in liver cancer cells. Mater Sci Eng C Mater Biol Appl. 2018;83:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 74. | Wang X, Liow SS, Wu Q, Li C, Owh C, Li Z, Loh XJ, Wu YL. Codelivery for Paclitaxel and Bcl-2 Conversion Gene by PHB-PDMAEMA Amphiphilic Cationic Copolymer for Effective Drug Resistant Cancer Therapy. Macromol Biosci. 2017;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 75. | Liu X, Chen X, Chua MX, Li Z, Loh XJ, Wu YL. Injectable Supramolecular Hydrogels as Delivery Agents of Bcl-2 Conversion Gene for the Effective Shrinkage of Therapeutic Resistance Tumors. Adv Healthc Mater. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 76. | Hu Z, Fang C, Li B, Zhang Z, Cao C, Cai M, Su S, Sun X, Shi X, Li C, Zhou T, Zhang Y, Chi C, He P, Xia X, Chen Y, Gambhir SS, Cheng Z, Tian J. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat Biomed Eng. 2020;4:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 613] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 77. | Chen H, Cheng H, Dai Q, Cheng Y, Zhang Y, Li D, Sun Y, Mao J, Ren K, Chu C, Liu G. A superstable homogeneous lipiodol-ICG formulation for locoregional hepatocellular carcinoma treatment. J Control Release. 2020;323:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 78. | Denkova AG, de Kruijff RM, Serra-Crespo P. Nanocarrier-Mediated Photochemotherapy and Photoradiotherapy. Adv Healthc Mater. 2018;7:e1701211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 79. | Moole H, Tathireddy H, Dharmapuri S, Moole V, Boddireddy R, Yedama P, Uppu A, Bondalapati N, Duvvuri A. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis. World J Gastroenterol. 2017;23:1278-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 80. | Yu XN, Deng Y, Zhang GC, Liu J, Liu TT, Dong L, Zhu CF, Shen XZ, Li YH, Zhu JM. Sorafenib-Conjugated Zinc Phthalocyanine Based Nanocapsule for Trimodal Therapy in an Orthotopic Hepatocellular Carcinoma Xenograft Mouse Model. ACS Appl Mater Interfaces. 2020;12:17193-17206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 81. | Johnston MP, Khakoo SI. Immunotherapy for hepatocellular carcinoma: Current and future. World J Gastroenterol. 2019;25:2977-2989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 82. | Jiang Y, Han QJ, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 83. | Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, Dhainaut M, Villacorta-Martin C, Singhi AD, Moghe A, von Felden J, Tal Grinspan L, Wang S, Kamphorst AO, Monga SP, Brown BD, Villanueva A, Llovet JM, Merad M, Lujambio A. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019;9:1124-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 620] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 84. | Da Silva CG, Camps MGM, Li TMWY, Zerrillo L, Löwik CW, Ossendorp F, Cruz LJ. Effective chemoimmunotherapy by co-delivery of doxorubicin and immune adjuvants in biodegradable nanoparticles. Theranostics. 2019;9:6485-6500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 85. | Goldberg MS. Improving cancer immunotherapy through nanotechnology. Nat Rev Cancer. 2019;19:587-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 400] [Article Influence: 66.7] [Reference Citation Analysis (0)] |