Published online Apr 15, 2020. doi: 10.4251/wjgo.v12.i4.467
Peer-review started: December 13, 2019
First decision: February 14, 2020
Revised: March 11, 2020
Accepted: March 26, 2020
Article in press: March 26, 2020
Published online: April 15, 2020
Processing time: 123 Days and 23.8 Hours
The prognosis of intrahepatic cholangiocarcinoma (ICC) patients following surgical resection remains poor. It is necessary to investigate effective biomarkers or prognostic models for ICC patients.
To investigate the prognostic effect of systemic immune-inflammation index (SII) to predict long-term outcomes in ICC patients with undergoing hepatic resection.
Consecutive ICC patients who underwent initial hepatectomy with curative intent from January 2009 to September 2017 were retrospectively reviewed. Receiver-operating characteristic (ROC) curves were used to determine the optimal cut-off values of SII. Kaplan-Meier curves and Cox proportional hazards regression were performed to evaluate the discriminative ability of preoperative SII in predicting overall survival (OS) and recurrence-free survival (RFS).
A total of 530 patients were included and randomly divided into derivation (n = 265) and validation cohort (n = 265). The optimal cut-off value for SII was 450. At a median follow-up of 18 mo (range, 1-115.4 mo), 317 (59.8%) patients died and 381 (71.9%) patients experienced tumor relapse. Low SII level was associated with better OS and RFS (both P < 0.05). Multivariate analyses identified multiple tumors, node invasion and high SII level as independent risk factors for OS, while multiple tumors, node invasion and high SII level were identified as independent risk factors for RFS. Validation cohort confirmed the findings of derivation cohort.
The present study demonstrated the feasibility of preoperative SII as a prognostic indicator for ICC. Patients with increased SII level were associated with worse OS and earlier tumor recurrence. Elevated SII level was an independent risk factor for OS and RFS in patients with ICC after hepatectomy. In the future, the SII could help stratifying patients with ICC, thus guiding therapeutic choices, especially in immunotherapy.
Core tip: Inflammation has been reported to play a crucial role in tumor biology. Systemic immune-inflammation index (SII), an inflammation-based index, was a composite measure of neutrophil, platelet and lymphocyte counts. No data exists until now, has evaluated its prognostic value for intrahepatc cholangiocarcinoma (ICC). This study aimed to investigate the clinical significance of preoperative SII levels in ICC patients undergoing curative resection. Patients with increased SII level were associated with worse overall survival and earlier tumor recurrence. In the future, the SII could help stratifying patients with ICC, thus guiding therapeutic choices, especially in immunotherapy.
- Citation: Li H, Wang JJ, Zhang M, Ren B, Li JX, Xu L, Wu H. Prognostic significance of systemic immune-inflammation index in patients with intrahepatic cholangiocarcinoma undergoing hepatic resection. World J Gastrointest Oncol 2020; 12(4): 467-482
- URL: https://www.wjgnet.com/1948-5204/full/v12/i4/467.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i4.467
Intrahepatic cholangiocarcinoma (ICC) is a subtype of cholangiocarcinoma, representing 15%-20% of all primary liver cancer[1,2]. The incidence of ICC is increasing over the years. The potential etiologic factors for development of ICC include hepatolithiasis, primary sclerosing cholangitis, biliary tree anomalies, hepatobiliary parasitosis and chronic viral hepatitis[3]. ICC is a heterogenous malignancy which carries a dismal long-term survival outcome, with a 5-year survival rate of less than 20%[4]. Among all therapeutic strategies for ICC, surgical resection remains the mainstay, which is regarded as the unique potential curative treatment for patients with early stage tumor[5,6].
Inflammation has been reported to play a crucial role in tumor biology. The systemic inflammatory response (SIR), which could be monitored using some hematologic or biochemical markers including neutrophil, lymphocyte, c-relative protein and platelet count, has been demonstrated to be of major prognostic importance in various cancers[7-9]. Recently, inflammation-based indexes, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and systemic immune-inflammation index (SII), have been used to evaluate the prognosis of patients with diverse cancers[10]. Previous studies have reported that elevated NLR and PLR were associated with poor long-term survival outcomes in patients with ICC[11].
The SII is an index which incorporates platelets, neutrophils and lymphocytes, calculating by neutrophil × platelet/lymphocyte[12]. Previous studies have reported that the SII could help to predict long-term survival outcomes in patients with solid tumors including breast cancer, non-small cell lung cancer, colorectal cancer and pancreatic cancer[13-15]. However, no data exists until now, evaluating the prognostic value of SII for ICC. Thus, the objective of this study was to investigate the clinical significance of preoperative SII levels in ICC patients undergoing curative resection.
This study was approved by the Ethics Committee of the West China Hospital, in accordance with the guidelines of the 1975 Declaration of Helsinki[16]. All consecutive patients undergoing initial hepatic resection with curative intent for ICC from January 2009 to September 2017 were considered for this retrospective study. Written informed consent were obtained from all the eligible patients or their relative. Patients were randomly divided into two groups (derivation and validation cohort). The exclusion criteria were as following: Patients received radiofrequency ablation, transarterial chemoembolization, chemotherapy or other anti-cancer therapies prior to hepatectomy; patients with extrahepatic metastasis or other simultaneous malignancies; patients underwent surgical resection for tumor rupture.
Diagnoses of ICC were confirmed by histological evidence after surgical resection. The tumor-node-metastasis (TNM) stages were stratified according to the 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual. All the clinicopathological data were reviewed and retrieved from the hospital electronic or handwritten medical records, including patient factors, laboratory parameters and histological features of tumors. Among them, macrovascular invasion was defined as radiologically diagnosed vascular invasion of large vessels or macroscopic thrombosis, whereas MVI was defined as histologically identified vascular invasion of small vessels. Patients were followed up according to National Comprehensive Cancer Network (NCCN). Regularly, patients were asked to return hospital for physical examination, tumor markers or contrast-enhanced ultrasonography per 3 month at first year, then every 6 months thereafter. Besides, we contact those who determined not to go back to the hospital to reexamination through telephone follow-up survey. Overall survival (OS) was calculated from the date of liver resection to death or, in those alive, to the date of last follow-up. Recurrence-free survival (RFS) was from the date of hepatectomy to diagnosis of recurrence or last follow-up.
Statistical analyses were performed using the software of Graphpad Prism (version 8.0, San Diego, CA, United States), SPSS (version 23.0, Chicago, IL, United States) and MedCalc (version 15.2.2.0, Ostend, Belgien). The thresholds of SII, NLR and PLR were identified by application of the receiver operating characteristics (ROC) curve, as Youden index attained maximum value. The 5-year survival status was set as the discriminant. χ2 test or Fisher’s exact test was used to analyze categorical variables. Student’s t-test or Kruskal-Wallis test was used to investigate continuous variables. Kaplan-Meier curves were plotted for discovery and validation cohort according to each cut-off value, and their differences were tested using log-rank test. Subsequently, Cox proportional hazards regression model (enter method) was used to identify potential independent prognostic factors for OS and RFS. Potential confounders which were correlated to survival outcomes and those with P values less than 0.05 in univariate regression analyses were selected for multivariate regression models. NLR and PLR were excluded from multivariate analyses. Statistical significance was accepted at a two-tailed P value < 0.05.
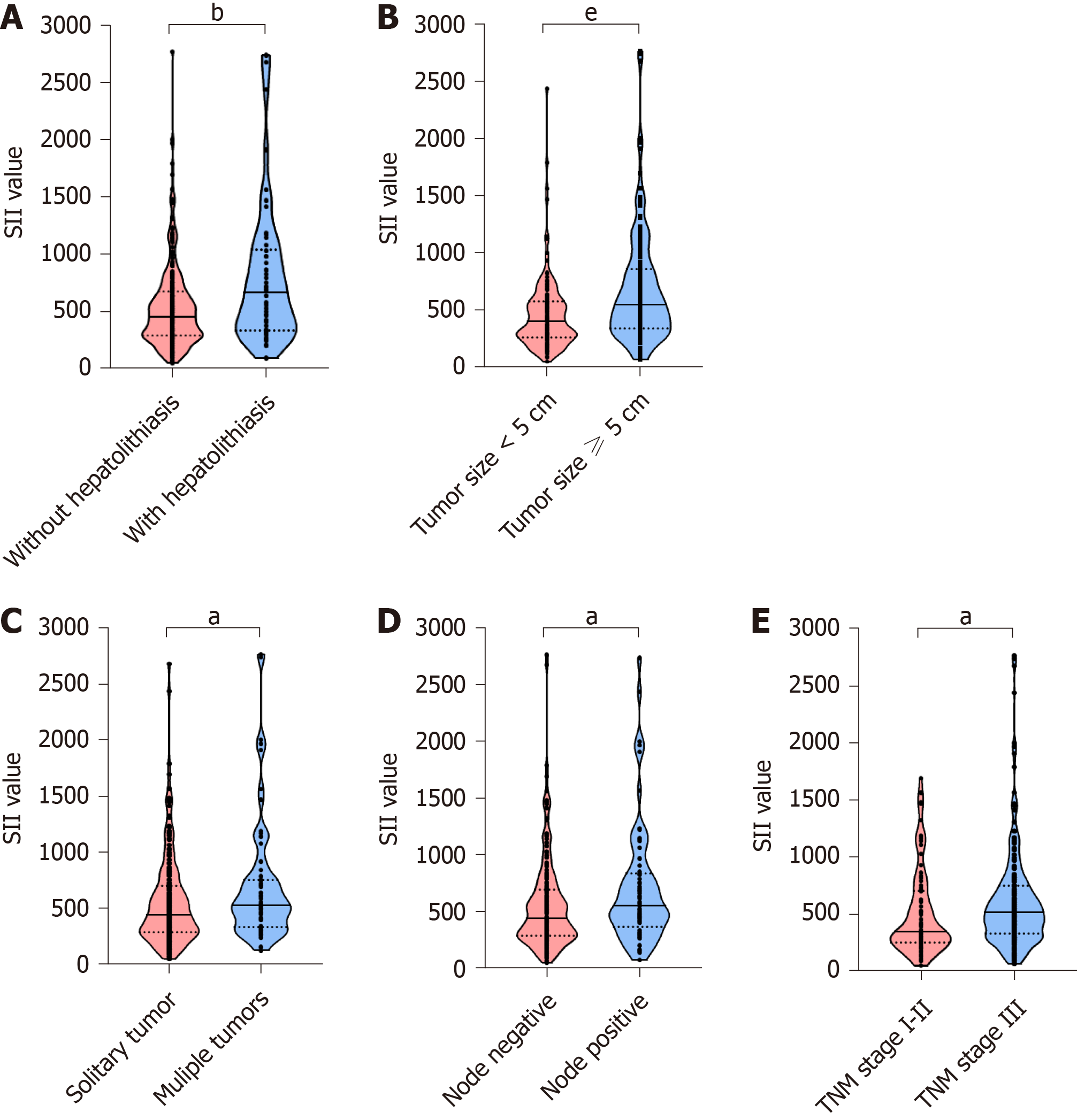
A total of 530 patients (256, 48.3% male), with a mean age of 57.23 years (standard deviation, 10.69 years), were included in this study. Patients were randomly divided into derivation and validation cohort (265 for each group). The demographic and clinicopathological features were summarized in Table 1. The SII level The optimal cut-off values for SII, NLR and PLR were 450, 2.65 and 110, respectively. In the derivation cohort, with the defined cut-off value, 123 (46.4%) patients were stratified into low SII group (SII ≤ 450) and 142 with SII > 450. Elevated SII level was found in ICC patients with hepatolithiasis, tumor size ≥ 5 cm, multiple tumors, node invasion and TNM stage III (Figure 1). As shown in Table 2, patients with elevated SII level was associated with increased serum platelet counts, neutrophil and decreased lymphocytes. Moreover, the maximum tumor size was larger in high SII group (mean, 6.5 cm) than low group (P < 0.001). Patients in high SII group were associated with significantly decreased frequencies of solitary tumor and increased incidence of node invasion. In the validation cohort, 130 (49.1%) patients were stratified into low SII group. Correlation between SII level and clinical characteristics was summarized in Table 2. Patients in high SII group were related to increased serum platelet counts, neutrophil and decreased lymphocytes. Interestingly, a lower frequency of cirrhosis was observed in high SII group (19.3% vs 30%, P = 0.045).
| Variables | All patients (n = 530) | Derivation cohort (n = 265) | Validation cohort (n = 265) | P value |
| Patient factors/laboratory parameters | ||||
| Age, yr | 57.2 (10.7) | 57.9 (10.5) | 56.5 (10.8) | 0.131 |
| Male gender, n (%) | 256 (48.3) | 134 (50.6) | 122 (46.0) | 0.339 |
| HBsAg (positive), n (%) | 153 (29.0) | 80 (30.3) | 73 (27.7) | 0.565 |
| Hepatolithiasis, n (%) | 89 (16.8) | 42 (15.6) | 47 (17.9) | 0.487 |
| CA-199 < 22, n (%) | 150 (29.0) | 77 (30.0) | 73 (28.0) | 0.738 |
| Platelet | 178 (72) | 179 (73) | 177 (71) | 0.778 |
| Neutrophil | 4.6 (2.1) | 4.6 (2.3) | 4.6 (2.0) | 0.976 |
| Lymphocyte | 1.6 (0.9) | 1.6 (1.2) | 1.6 (0.5) | 0.641 |
| NLR | 3.38 (2.95) | 3.34 (2.22) | 3.42 (3.54) | 0.746 |
| PLR | 126 (68) | 127 (65) | 125 (70) | 0.716 |
| SII | 612 (635) | 605 (495) | 619 (751) | 0.803 |
| Histological and gross features of tumors | ||||
| Tumor size, cm | 6.0 (2.7) | 6.0 (2.7) | 6.0 (2.7) | 0.783 |
| Solitary tumor, n (%) | 373 (70.4) | 196 (74.0) | 177 (66.8) | 0.087 |
| Well tumor differentiation, n (%) | 22 (4.2) | 11 (4.2) | 11 (4.2) | 1.000 |
| Macrovascular invasion, n (%) | 123 (23.2) | 71 (26.8) | 52 (19.6) | 0.064 |
| Microvascular invasion, n (%) | 54 (10.2) | 27 (10.2) | 27 (10.2) | 1.000 |
| Node positive, n (%) | 129 (24.3) | 61 (23.0) | 68 (25.7) | 0.544 |
| Perineural invasion, n (%) | 80 (15.1) | 38 (14.3) | 42 (15.8) | 0.716 |
| TNM stage, n (%) | 0.902 | |||
| IA | 63 (11.9) | 33 (12.5) | 30 (11.3) | |
| IB | 38 (7.2) | 21 (7.9) | 17 (6.4) | |
| II | 56 (10.6) | 26 (9.8) | 30 (11.3) | |
| IIIA | 242 (45.7) | 122 (46.0) | 120 (45.3) | |
| IIIB | 131 (24.7) | 63 (23.8) | 68 (25.7) | |
| Follow-up, median (range) | 18.0 (1.0-115.4) | 18.2 (1.0-115.4) | 17.8 (1.2-104.5) | |
| Variables | Derivation | Validation | ||||
| SII ≤ 450 | SII > 450 | P value | SII ≤ 450 | SII > 450 | P value | |
| Total patients | 123 | 142 | 130 | 135 | ||
| Age, yr | 57.89 (9.5) | 57.98 (11.3) | 0.943 | 57.79 (10.7) | 55.41 (10.9) | 0.074 |
| Male gender, n (%) | 67 (54.5) | 67 (47.2) | 0.268 | 61 (46.9) | 61 (45.2) | 0.806 |
| HBsAg, n (%) | 45 (36.9) | 35 (24.6) | 0.033 | 39 (30.2) | 34 (25.2) | 0.413 |
| Hepatolithiasis, n (%) | 15 (12.2) | 27 (19.0) | 0.164 | 22 (16.9) | 25 (18.5) | 0.758 |
| CA-199 < 22, n (%) | 40 (33.1) | 37 (27.2) | 0.010 | 45 (34.9) | 28 (21.2) | 0.002 |
| Platelet | 139 (45) | 214 (75) | < 0.001 | 141 (50) | 213 (70) | < 0.001 |
| Neutrophil | 3.34 (1.0) | 5.65 (2.5) | < 0.001 | 3.46 (1.3) | 5.66 (1.9) | < 0.001 |
| Lymphocyte | 1.81 (1.7) | 1.43 (0.5) | 0.009 | 1.73 (0.5) | 1.41 (0.5) | < 0.001 |
| Tumor size, cm | 5.2 (2.3) | 6.5 (2.9) | < 0.001 | 5.40 (2.2) | 6.36 (3.0) | 0.007 |
| Solitary tumor, n (%) | 99 (80.5) | 97 (68.3) | 0.025 | 92 (70.8) | 86 (63.0) | 0.197 |
| Well tumor differentiation, n (%) | 7 (5.7) | 4 (2.8) | 0.357 | 8 (6.2) | 3 (2.2) | 0.110 |
| Macrovascular invasion, n (%) | 33 (26.8) | 38 (26.8) | 1.000 | 23 (17.7) | 29 (21.5) | 0.449 |
| Microvascular invasion, n (%) | 12 (9.8) | 15 (10.6) | 0.844 | 9 (6.9) | 18 (13.3) | 0.103 |
| Node positive, n (%) | 20 (16.3) | 41 (28.9) | 0.020 | 27 (20.8) | 41 (30.4) | 0.089 |
| Perineural invasion, n (%) | 19 (15.4) | 19 (13.4) | 0.732 | 22 (16.9) | 20 (14.8) | 0.745 |
| TNM stage, n (%) | 0.017 | 0.123 | ||||
| IA | 22 (17.9) | 11 (7.7) | 16 (12.3) | 14 (10.4) | ||
| IB | 11 (8.9) | 10 (7.0) | 8 (6.2) | 9 (6.7) | ||
| II | 14 (11.4) | 12 (8.5) | 11 (8.5) | 19 (14.1) | ||
| IIIA | 56 (45.5) | 66 (46.5) | 68 (52.3) | 52 (38.5) | ||
| IIIB | 20 (16.3) | 43 (30.3) | 27 (20.8) | 41 (30.4) | ||
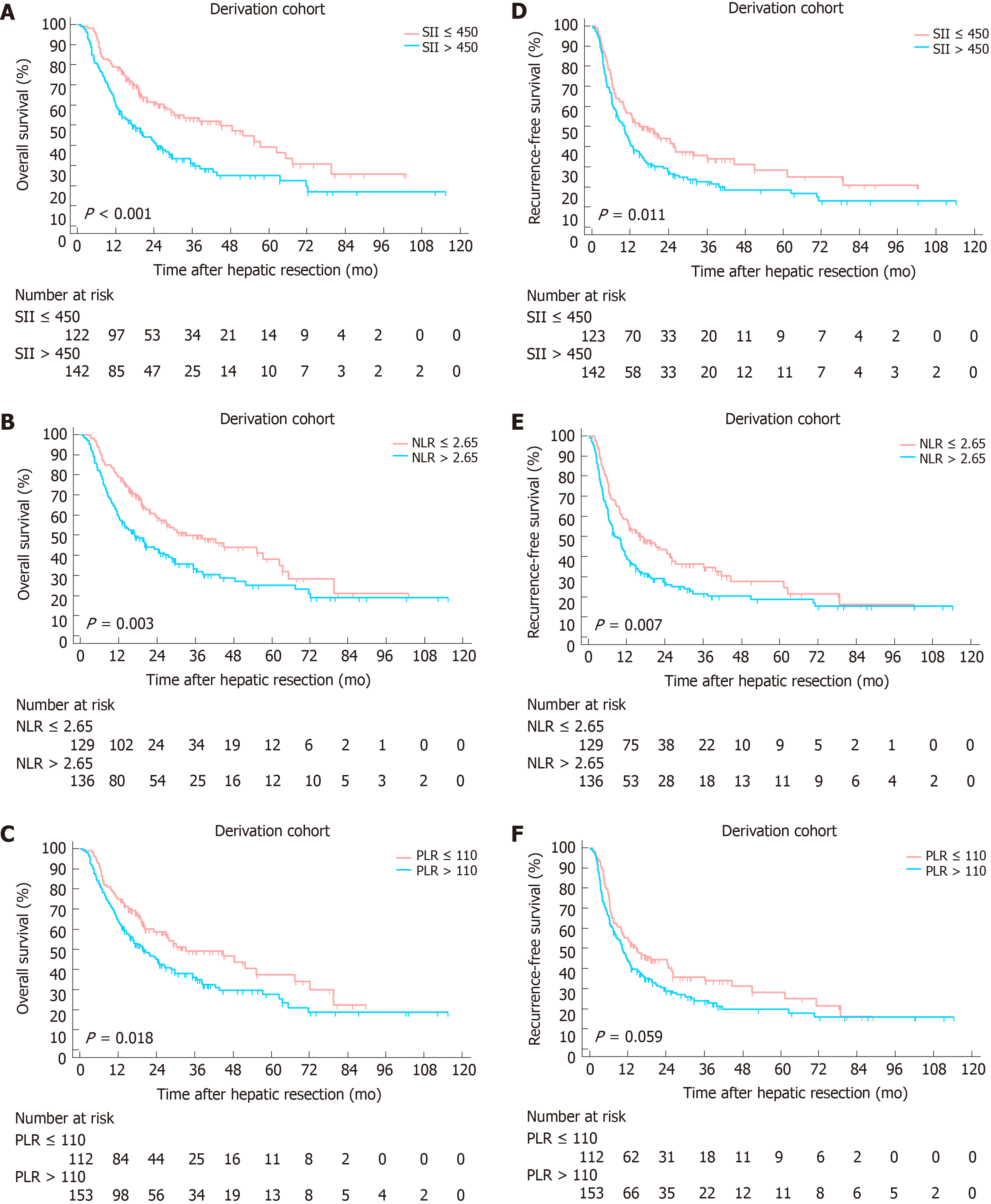
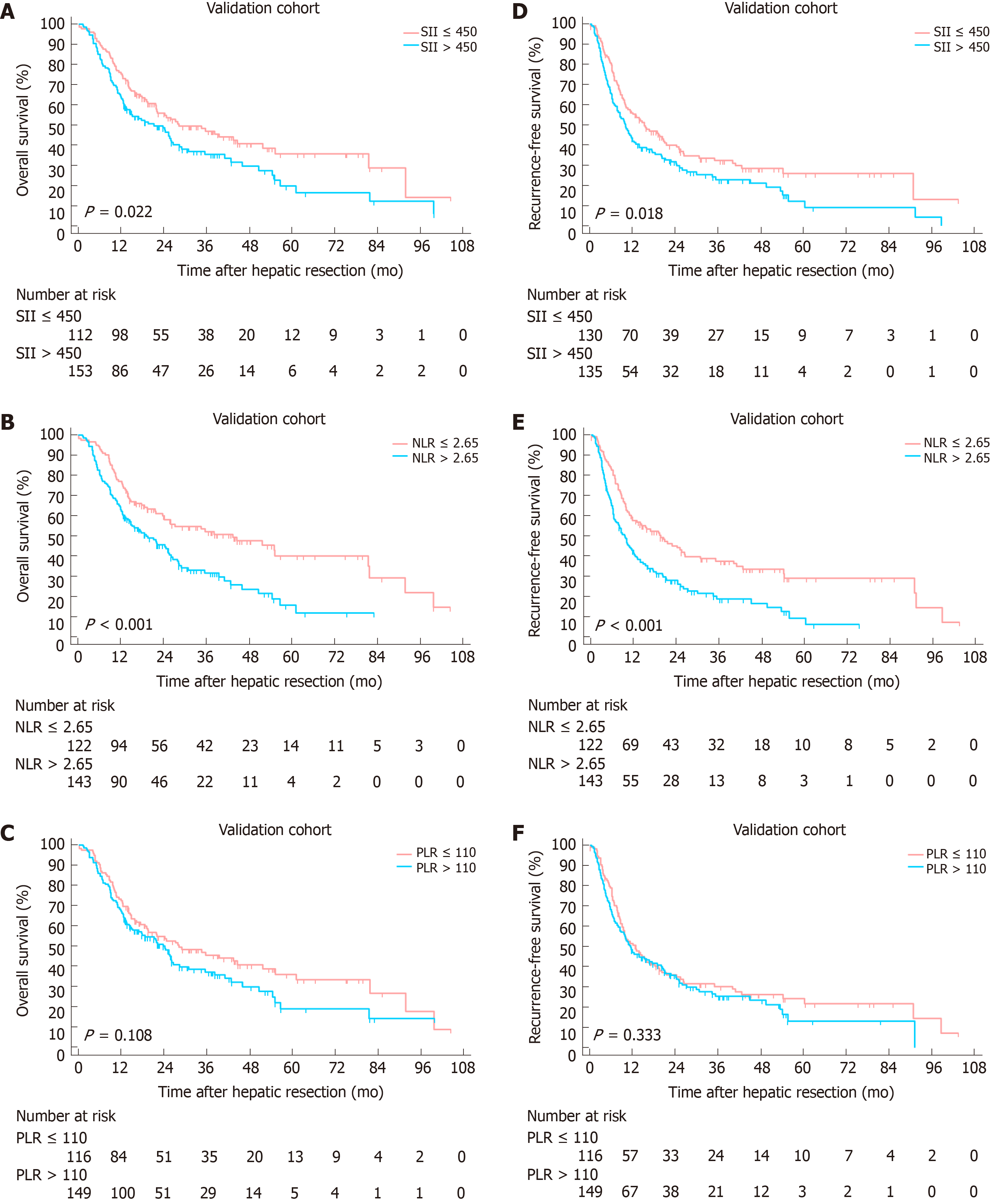
At a median follow-up of 18 mo (range, 1-115.4 mo), 317 (59.8%) patients died and 381 (71.9%) patients experienced tumor relapse. Two independent cohorts were associated with comparable follow-up period. A Kaplan-Meier analysis was performed to detect the correlation between SII, NLR, PLR with OS and RFS. In derivation cohort, increased SII, NLR and PLR were significantly correlated to worse OS (P values were < 0.001, 0.003 and 0.018, respectively, Figure 2A-C). Higher SII and NLR were associated with lower RFS with P values of 0.011 and 0.007, whereas no statistical significance was found between PLR and RFS (P = 0.059, Figure 2D-F). In the validation cohort, increased SII and NLR were significantly correlated to worse OS and RFS (Figure 3). However, no significant association was found between PLR and OS, RFS.
Among 16 clinicopathological features analyzed in univariate Cox regression analysis in the derivation cohort, 10 were identified as potential factors affecting OS. Subsequently, 8 of them were introduced in multivariate Cox regression model, multiple tumors, node invasion and high SII level were identified as independent risk factors for OS (Tables 3 and 4). 6 variables were analyzed using multivariate analysis, multiple tumors, node invasion and high SII level were identified as independent risk factors for RFS (Tables 3 and 4). In the validation cohort, abnormal serum CA-199 level, multiple tumors, node invasion and elevated SII were identified as independent risk factors for both OS and RFS (Supplementary Table 1 and 2).
| Variables | Overall survival | Recurrence-free survival | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr (> 60/≤ 60) | 0.917 | 0.669-1.258 | 0.592 | 0.998 | 0.749-1.330 | 0.991 |
| Gender (F/M) | 0.747 | 0.546-1.023 | 0.069 | 0.791 | 0.594-1.052 | 0.107 |
| HBsAg | 1.347 | 0.966-1.878 | 0.079 | 1.289 | 0.947-1.753 | 0.106 |
| Hepatolithiasis | 1.775 | 1.179-2.675 | 0.006 | 1.357 | 0.913-2.018 | 0.131 |
| CA-199 (≥ 22/< 22) | 1.631 | 1.122-2.370 | 0.010 | 1.357 | 0.981-1.876 | 0.065 |
| Tumor size (≥ 5/< 5) | 1.171 | 0.853-1.608 | 0.328 | 1.359 | 1.016-1.818 | 0.039 |
| Tumor number (multiple/single) | 1.686 | 1.204-2.362 | 0.002 | 1.953 | 1.436-2.658 | < 0.001 |
| Tumor differentiation (moderate-poor/well) | 5.326 | 1.318-21.519 | 0.019 | 4.498 | 1.434-14.110 | 0.010 |
| Macrovascular invasion | 1.076 | 0.753-1.538 | 0.688 | 1.014 | 0.756-1.453 | 0.776 |
| Microvascular invasion | 1.683 | 1.062-2.667 | 0.027 | 2.022 | 1.325-3.088 | 0.001 |
| Node positive | 2.696 | 1.928-3.769 | < 0.001 | 1.955 | 1.424-2.684 | < 0.001 |
| Perineural invasion | 1.174 | 0.832-1.656 | 0.360 | 1.443 | 0.976-2.134 | 0.066 |
| TNM stage (III/I-II) | 1.663 | 1.090-2.539 | 0.018 | 1.529 | 1.130-2.068 | 0.006 |
| NLR (> 2.65/≤ 2.65) | 1.609 | 1.171-2.212 | 0.003 | 1.482 | 1.111-1.978 | 0.007 |
| PLR (> 110/≤ 110) | 1.482 | 1.069-2.055 | 0.018 | 1.325 | 0.989-1.776 | 0.060 |
| SII (> 450/≤ 450) | 1.774 | 1.285-2.449 | < 0.001 | 1.455 | 1.088-1.946 | 0.011 |
| Variables | Overall survival | Recurrence-free survival | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Hepatolithiasis | 1.348 | 0.870-2.088 | 0.182 | |||
| CA-199 (≥ 22/< 22) | 1.458 | 0.979-2.170 | 0.064 | |||
| Tumor size (≥ 5/<5) | 0.873 | 0.556-1.371 | 0.555 | |||
| Tumor number (multiple/single) | 1.666 | 1.140-2.434 | 0.008 | 1.679 | 1.200-2.348 | 0.002 |
| Tumor differentiation (moderate-poor/well) | 3.061 | 0.739-12.675 | 0.123 | 2.813 | 0.877-9.022 | 0.082 |
| Microvascular invasion | 0.938 | 0.556-1.582 | 0.809 | |||
| Node positive | 2.377 | 1.586-3.562 | < 0.001 | 1.881 | 1.316-2.688 | 0.001 |
| TNM stage (III/I-II) | 0.682 | 0.456-1.022 | 0.064 | 0.756 | 0.527-1.085 | 0.129 |
| SII (> 450/≤ 450) | 1.774 | 1.245-2.528 | 0.002 | 1.385 | 1.005-1.909 | 0.046 |
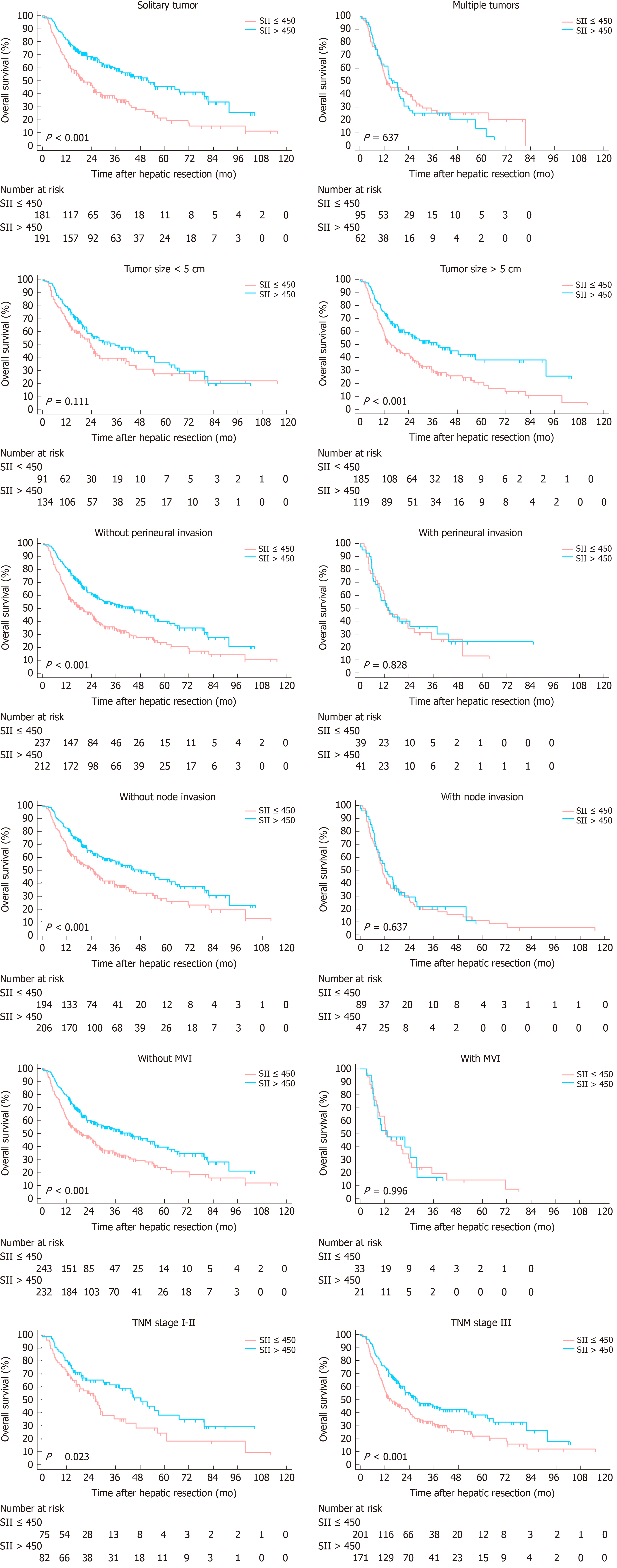
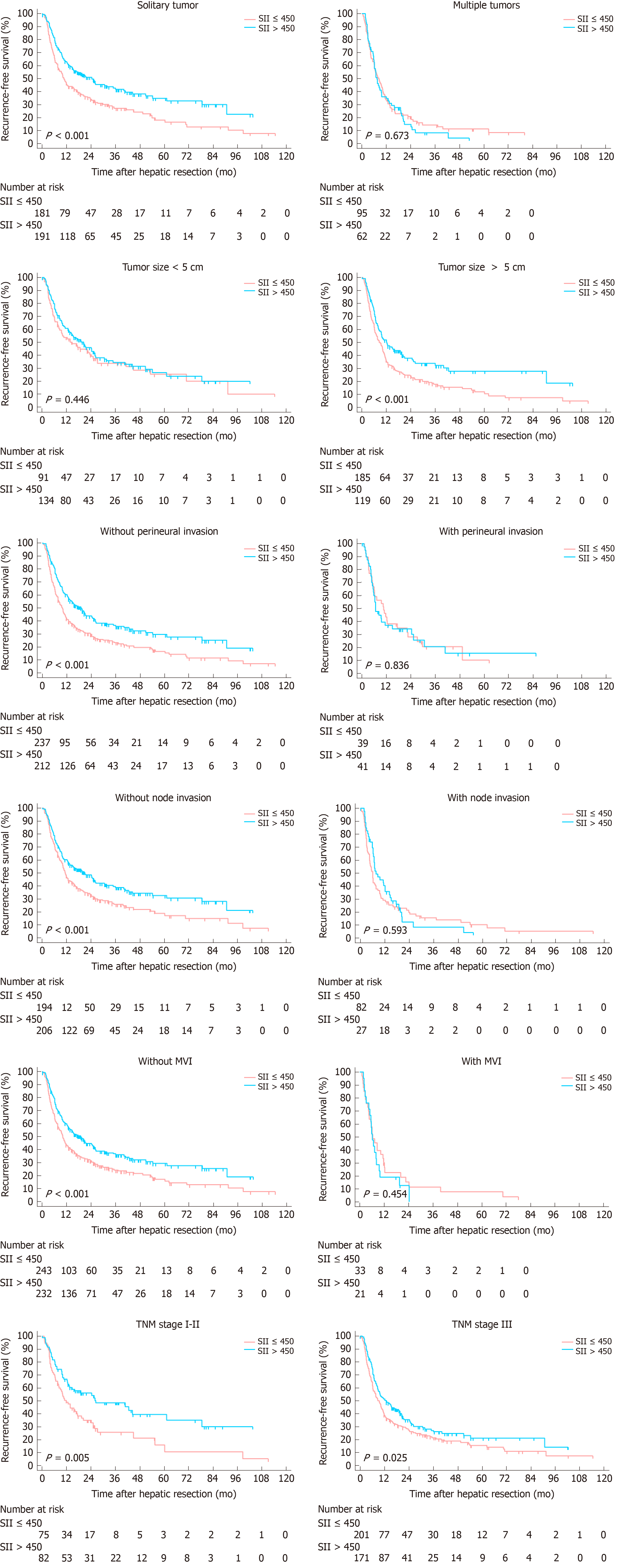
Subgroup analyses were conducted to evaluate prognostic value of SII for patients stratified by potential sources of heterogeneity for the entire cohort. SII showed its prognostic value in ICC regardless of age, status of cirrhosis and TNM stages for OS and RFS. For patients with multiple tumors, node invasion, maximum tumor size more than 5 cm, MVI positive and perineural invasion, no significant correlation was found between SII with OS and RFS (Figures 4 and 5).
Accumulating evidence have indicated that inflammation played an important role in various solid tumors. On one hand, inflammation was thought to promote tumorigenesis and progression, but on the other hand, it might be secondary to SIR to yet-undetected tumor and accumulated DNA-damage[12]. In any cases, the products of inflammation processes could be considered as biomarkers in the application of diagnosis and prognostic prediction. Numerous studies have reported inflammatory-based indexes, with combination of peripheral neutrophil, lymphocyte, monocyte and C-relative protein, were associated with tumor growth, distant metastasis and survival outcomes of patients with various tumors[17-20].
Previous studies have investigated the prognostic value of NLR and PLR in ICC patients, and suggested that NLR and PLR were potential prognostic indicators for long-term survival outcomes[10,21,22]. Consistently, the present study showed that elevated NLR and PLR were associated with worse OS and RFS. A handful of studies have demonstrated that patients with high SII level were associated with poor survival outcomes in various malignancies, suggesting the feasibility of SII in predicting prognosis. Recently, a meta-analysis by Yang et al[23] pooled outcomes from 22 studies and demonstrated that high SII was associated with poor overall outcomes in patients with various cancers. However, Huang and colleagues performed a retrospective study to evaluate the effect of SII to predict recurrence and survival in patients with BCLC stage 0-A of HCC after hepatectomy[24]. They presented an opposite result and revealed that low SII was significantly poor prognostic predictor for OS and recurrence in patients with early stage HCC. We therefore performed the present study to evaluate the effect of SII level for predicting long-term outcomes in patients with ICC after hepatectomy. Importantly, this study demonstrated that an elevated SII (> 450) was associated with larger tumor, increased tumor number, node invasion and worse prognosis in patients with surgically treated ICC. A high preoperative SII level was an independent risk factor for OS and RFS.
To our knowledge, this is the first study with a large population evaluating prognostic significance of SII in ICC patients undergoing hepatic resection. The SII is a composite measure of neutrophil, platelet and lymphocyte counts. The neutrophils altered tumor microenvironment via extrinsic pathway[25]. Additionally, they secreted various cytokines and chemokines to promote tumor cell proliferation and metastasis[26]. The adhesin of platelets to cancer cells was crucial in the formation of metastatic niche[27]. The platelets not only shielded tumor cells against immune cells cytotoxicity, but also released nucleotides to promote the epithelial to mesenchymal transition in cancer cells, leading to migration and invasion[28,29]. Contrary to neutrophils and platelets, the lymphocyte played an antitumor role through its ability of promoting cytotoxic cell death[30]. Previous studies have demonstrated that tumor-infiltrating lymphocyte, essential components of tumor microenvironment, could serve as prognostic biomarkers in various cancers. Patients with high density of tumor-infiltrating lymphocytes were associated with better prognosis and decreased rate of tumor recurrence[31,32]. Therefore, a combination of these parameters might be more comprehensive in reflecting the status of immune response and systemic inflammation. A low SII level, resulting from reduced neutrophil, platelet or increased lymphocyte counts, exhibited activation of systemic immune response and suppression of inflammatory reaction. Consequently, the prognosis of patients with reduced SII level was better than those with increased SII. Moreover, according to our results, more progressive tumor biologic characteristics was observed in patients with high SII.
Several limitations should be taken into consideration when interpreting our findings. Firstly, it was a single-institutional retrospective study. Further large-scale prospective studies are in need to validate our results. Secondly, although sub-set analyses were performed according to potential confounding factors, there still exist factors, such as portal hypertension and smoking, would potentially affect SII. Furthermore, owing to insufficient ward beds, prompt surgical treatments were not available for a subset of ICC patients with operative indication, the referral bias could not be completely avoided.
In summary, the present study analyzed serum inflammation index in a subset of ICC patients undergoing curative resection and demonstrated the feasibility of preoperative SII as a prognostic indicator. Patients with increased SII level were associated with worse long-term survival outcomes. The SII level was an independent risk factor for OS and RFS in patients with ICC after hepatectomy. In the future, the SII could help stratifying patients with ICC, thus guiding therapeutic choices, especially in immunotherapy.
Intrahepatic cholangiocarcinoma (ICC) is a subtype of cholangiocarcinoma, representing 15%-20% of all primary liver cancer. The incidence of ICC is increasing over the years. Among all therapeutic strategies for ICC, surgical resection remains the mainstay. However, the prognosis of ICC patients following surgical resection remains poor. Therefore, it is necessary to investigate effective biomarkers or prognostic models for ICC patients following hepatic resection. Inflammation has been reported to play a crucial role in tumor biology. Recently, inflammation-based indexes, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and systemic immune-inflammation index (SII), have been used to evaluate the prognosis of patients with diverse cancers. However, no data exists until now, evaluating the prognostic value of SII for ICC.
Timely and effective establishment of prognostic models for ICC patients undergoing curative resection is of great value for the long-term outcomes of these patients.
This study aimed to investigate the prognostic significance of SII in patients with ICC undergoing hepatic resection.
We retrospectively reviewed ICC patients who underwent initial hepatectomy with curative intent at West China Hospital between January 2009 and September 2017. Enrolled patients were randomly stratified into derivation and validation cohort. The correlation between SII level and patients’ prognosis were analyzed using Kaplan-Meier curves and Cox proportional hazards regression.
Five hundred and thirty ICC patients were finally included and randomly divided into derivation (n = 265) and validation cohort (n = 265). The baseline characteristics were comparable between two groups. The optimal cut-off value for SII was 450. At a median follow-up of 18 mo (range, 1-115.4 mo), 317 (59.8%) patients died and 381 (71.9%) patients experienced tumor relapse. Low SII level correlated with better OS and RFS (both P < 0.05). Multivariate analyses identified multiple tumors, node invasion and high SII level as independent risk factors for OS, while multiple tumors, node invasion and high SII level were identified as independent risk factors for RFS.
Patients with increased SII level correlated with worse OS and earlier tumor recurrence. Elevated SII level was an independent risk factor for OS and RFS in patients with ICC after hepatectomy.
Future studies focusing on the molecular mechanisms underlying the correlation between SII level and patient clinical outcomes are required.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kang KJ S-Editor: Gong ZM L-Editor: A E-Editor: Qi LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55825] [Article Influence: 7975.0] [Reference Citation Analysis (132)] |
| 2. | Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1141] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 3. | Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 533] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 5. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1073] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 6. | Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, Vibert E, Castaing D, Adam R, Cherqui D, Sa Cunha A. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 7. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8323] [Article Influence: 489.6] [Reference Citation Analysis (0)] |
| 8. | Liu J, Shi Z, Bai Y, Liu L, Cheng K. Prognostic significance of systemic immune-inflammation index in triple-negative breast cancer. Cancer Manag Res. 2019;11:4471-4480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1990] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 10. | Chen Q, Dai Z, Yin D, Yang LX, Wang Z, Xiao YS, Fan J, Zhou J. Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine (Baltimore). 2015;94:e574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Sellers CM, Uhlig J, Ludwig JM, Stein SM, Kim HS. Inflammatory markers in intrahepatic cholangiocarcinoma: Effects of advanced liver disease. Cancer Med. 2019;8:5916-5929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Fest J, Ruiter R, Mulder M, Groot Koerkamp B, Ikram MA, Stricker BH, van Eijck CHJ. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int J Cancer. 2020;146:692-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 13. | De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, Basso U, Mitterer M, Ortega C, Bidoli P, Ferraù F, Crinò L, Frassoldati A, Marchetti P, Mini E, Scoppola A, Verusio C, Fornarini G, Cartenì G, Caserta C, Sternberg CN. Association of Systemic Inflammation Index and Body Mass Index with Survival in Patients with Renal Cell Cancer Treated with Nivolumab. Clin Cancer Res. 2019;25:3839-3846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 14. | Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15:221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Jomrich G, Gruber ES, Winkler D, Hollenstein M, Gnant M, Sahora K, Schindl M. Systemic Immune-Inflammation Index (SII) Predicts Poor Survival in Pancreatic Cancer Patients Undergoing Resection. J Gastrointest Surg. 2020;24:610-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 16. | Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 389] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 17. | Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261-6272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 250] [Cited by in RCA: 471] [Article Influence: 58.9] [Reference Citation Analysis (2)] |
| 18. | Shibutani M, Maeda K, Nagahara H, Ohtani H, Sakurai K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Tanaka H, Muguruma K, Hirakawa K. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol. 2015;21:9966-9973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Lu X, Guo W, Xu W, Zhang X, Shi Z, Zheng L, Zhao W. Prognostic value of the Glasgow prognostic score in colorectal cancer: a meta-analysis of 9,839 patients. Cancer Manag Res. 2019;11:229-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Li H, Wang J, Liu H, Lan T, Xu L, Wang G, Yuan K, Wu H. Existence of intratumoral tertiary lymphoid structures is associated with immune cells infiltration and predicts better prognosis in early-stage hepatocellular carcinoma. Aging (Albany NY). 2020;12:3451-3472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 21. | Gong ZJ, Cheng JW, Gao PT, Huang A, Sun YF, Zhou KQ, Hu B, Qiu SJ, Zhou J, Fan J, Yang XR. Clinical Characteristics and Prognostic Factors of Patients with Intrahepatic Cholangiocarcinoma with Fever: A Propensity Score Matching Analysis. Oncologist. 2019;24:997-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Shi SM, Yang H, Yang LX, Wang Z, Li XD, Yin D, Shi YH, Cao Y, Dai Z, Zhou J, Chen Q. Systemic inflammation score predicts survival in patients with intrahepatic cholangiocarcinoma undergoing curative resection. J Cancer. 2019;10:494-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer. 2018;9:3295-3302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 24. | Huang PY, Wang CC, Lin CC, Lu SN, Wang JH, Hung CH, Kee KM, Chen CH, Chen KD, Hu TH, Tsai MC. Predictive Effects of Inflammatory Scores in Patients with BCLC 0-A Hepatocellular Carcinoma after Hepatectomy. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Felix K, Gaida MM. Neutrophil-Derived Proteases in the Microenvironment of Pancreatic Cancer -Active Players in Tumor Progression. Int J Biol Sci. 2016;12:302-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. 2016;273:312-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Placke T, Kopp HG, Salih HR. The wolf in sheep's clothing: Platelet-derived "pseudo self" impairs cancer cell "missing self" recognition by NK cells. Oncoimmunology. 2012;1:557-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1402] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 29. | Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 474] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 30. | Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 250] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 31. | Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 32. | Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J; University of Michigan Head and Neck SPORE Program. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol. 2015;51:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |