Published online Apr 15, 2020. doi: 10.4251/wjgo.v12.i4.457
Peer-review started: December 23, 2019
First decision: January 19, 2020
Revised: January 29, 2020
Accepted: February 17, 2020
Article in press: February 17, 2020
Published online: April 15, 2020
Processing time: 114 Days and 11.7 Hours
Hilar cholangiocarcinoma (HCCA) often produces perineural invasion (PNI) extending to extra-biliary sites, while significant confusion in the incidence of PNI in HCCA has occurred in the literature, and the mechanism of that procedure remains unclear.
To summarize the incidence of PNI in HCCA and to provide the distribution of nerve plexuses around hepatic portal to clinical surgeons.
Reported series with PNI in HCCA since 1996 were reviewed. A clinicopathological study was conducted on sections from 75 patients with HCCA to summarize the incidence and modes of PNI. Immunohistochemical stains for CD34 and D2-40 in the cancer tissue were performed to clarify the association of PNI with microvessel and lymph duct. Sections of the hepatoduodenal ligament from autopsy cases were scanned and handled by computer to display the distribution of nerve plexuses around the hepatic portal.
The overall incidence of PNI in this study was 92% (69 of 75 patients), while the rate of PNI in HCCA in the literature ranging from 38% to 100%. The incidence of PNI did not show any remarkable differences among various differentiated groups and Bismuth-Corlette classification groups. Logistic regression analysis identified the depth of tumor invasion was the only factor that correlated significantly with PNI (P < 0.01). In spite of finding tumor cells that could invade microvessels and lymph ducts in HCCA, we did not find tumor cells invaded nerves via microvessels or lymph ducts. Three nerve plexuses in the hepatoduodenal ligament and Glisson’s sheath were classified, and they all surrounded the great vessels very closely.
The incidence of PNI of HCCA in Chinese population is around 92% and correlated significantly with a depth of tumor invasion. It also should be considered when stratifying HCCA patients for further treatment.
Core tip: The aim of this study was to summarize the incidence of perineural invasion (PNI) in hilar cholangiocarcinoma (HCCA), and provide the distribution of nerve plexuses around hepatic portal to clinical surgeons. Totally 75 patients with HCCA were investigated to summarize the incidence and modes of PNI. Sections of hepatoduodenal ligament from 5 autopsy cases were scanned to display the distribution of nerve plexuses around hepatic portal. The overall incidence of PNI in this study was 92% and three nerve plexuses in the hepatoduodenal ligament and Glisson’s sheath were classified and they all surrounded the great vessels very closely. PNI is grossly underreported in HCCA and it should be considered when stratifying HCCA patients for further adjuvant treatment.
- Citation: Li CG, Zhou ZP, Tan XL, Zhao ZM. Perineural invasion of hilar cholangiocarcinoma in Chinese population: One center’s experience. World J Gastrointest Oncol 2020; 12(4): 457-466
- URL: https://www.wjgnet.com/1948-5204/full/v12/i4/457.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i4.457
Despite advances in diagnostic modalities and resection rate, the prognosis of hilar cholangiocarcinoma (HCCA) remains dismal[1-4]. Surgical resection is the best therapeutic strategy, while the recurrence rate of tumors after curative resection is more than 53%[5-7]. The spread of tumors through perineural space has been described in a variety of human tumors, and many authors have emphasized nerve dissection together with tumor resection as a more radical approach. Although several studies have shown perineural invasion (PNI) could affect the long-term survival of HCCA[1,3,5], the pathway and mechanism of PNI remain unclear, and it is lack of detailed studies on the distribution of nerve plexuses around the hepatic portal. It is the objective of this study to make clear the incidence of PNI and to highlight its significance in HCCA and to provide the distribution of nerve plexuses around hepatic portal to clinical surgeons. A better understanding of PNI may lend insight into tumor metastasis and recurrence and open doors to improved staging strategies, novel treatment modalities, and perhaps even paradigm shifts in our treatment of patients.
From January 2003 to January 2010, 75 patients with HCCA were treated at the Second Department of Hepatobiliary Surgery, Chinese PLA General Hospital. There were 42 men and 33 women, aged 32-82 (average 57.4 ± 11.9) years. A total of 1106 histological sections were collected from those patients. 5 autopsies, including 3 men and 2 women, were conducted within 24 hours after death between October 2006 and February 2010 in the same hospital.
PNI was defined as described by Liebig et al[8]. Briefly, finding tumor cells within any of the 3 layers of the nerve sheath or tumor foci outside of the nerve with the involvement of 33% of the nerve’s circumference are sufficient features for calling PNI. Images were collected by the BX51 serial microscope (Olympus, Japan) and handled by Image-Pro Plus version 5.0.
Mouse anti-human CD34 and D2-40 monoclonal antibody (Boshide Biotechnology Company, Wuhan, China) and immunohistochemistry SP kit (Boshide Biotechnology Company, Wuhan, China) were used in this study. The paraffin-embedded specimens were serially cut into 3-4 μm slices. The sections were hydrated by alcohol before they were treated by 3% hydrogen peroxide for 30 min to block endogenous peroxidase activity. Heat-induced epitope retrieval was performed by microwaving for 10 min. Then the sections were immunostained by CD34 (monoclonal, 1:100) and D2-40 (monoclonal, 1:100) at 4°C overnight, respectively. Rabbit anti-mouse IgG antibody was added to the sections at 37°C for 30 min before the sections were colored by 3,3’-diaminobenzidine. Positive and negative controls were included in each run.
The hepatoduodenal ligament was intersected at the superior border of duodenum and disconnected to the hepatic portal. The Glisson’s sheath including left hepatic duct and right hepatic duct was carefully separated from the hepatic tissue to the bifurcation of second-order hepatic ducts. The resected specimens were fixed in 10% formalin for 24 h. In the case of hepatoduodenal ligament, transactions vertical to the long axis were cut into 3-5 mm slices. In the case of Glisson’s sheath, transactions cross to the beginning part of left hepatic duct or right hepatic duct were cut respectively. Blocks made from the slices were processed through paraffin and slides were prepared in the usual manner with HE staining. Slides were scanned (×200) by the NanoZoomer Digital Pathology system (HAMAMATSU, Japan) and memorized as JPEG format. The digital images of scanned sections were handled by Photoshop version 9.0 to erase the fibrous connective tissue and adipose tissue. Thus, the position relation of bile duct and blood vessels and nerve fibers could have remained.
Results are expressed as mean ± SD. A χ2 test was used to test for differences in incidence of PNI among various differentiated groups. Univariate analysis was used to test for the correlations of PNI and other histological factors. Statistical analysis was performed by SPSS version 10.0 and a P value less than 0.05 was considered significant.
Reports from 1996 to 2019 showed that the rate of PNI in HCCA was 38.8% to 100%, and the recurrence rate of tumors after curative resection was more than 53% (Table 1)[1,2,4-7,9-15]
| Ref. | Number of patient | Number of PNI (%) | Recurrence after curative resection (%) |
| Hu et al[1], 2019 | 258 | 38.8 (100/258) | 70 (180/258) |
| Kovalenko et al[2], 2018 | 49 | 83.3 (41/49) | - |
| Ercolani et al[4], 2010 | 51 | 75 (33/44) | - |
| Young et al[5], 2010 | 51 | 92 (47/51) | - |
| Aishima et al[6], 2007 | 38 | 76.3 (29/38) | 57.9 (22/38) |
| Robles et al[7], 2004 | 36 | 64 (23/36) | 53.0 (19/36) |
| Nakagohri et al[9], 2003 | 26 | 100 (26/26) | - |
| Seyama et al[10], 2003 | 58 | 84.5 (49/58) | - |
| Nimura et al[11], 2000 | 42 | 59.2 (84142) | 53.7 (58/108) |
| Nagakawa et al[12], 2000 | 47 | 92.5 (43/47) | - |
| Kurosaki et al[13], 1998 | 83 | 88 (73/83) | - |
| Yamaguchi et al[14], 1997 | 24 | 75 (18/24) | - |
| Su et al[15], 1996 | 49 | 73.5 (36/49) | - |
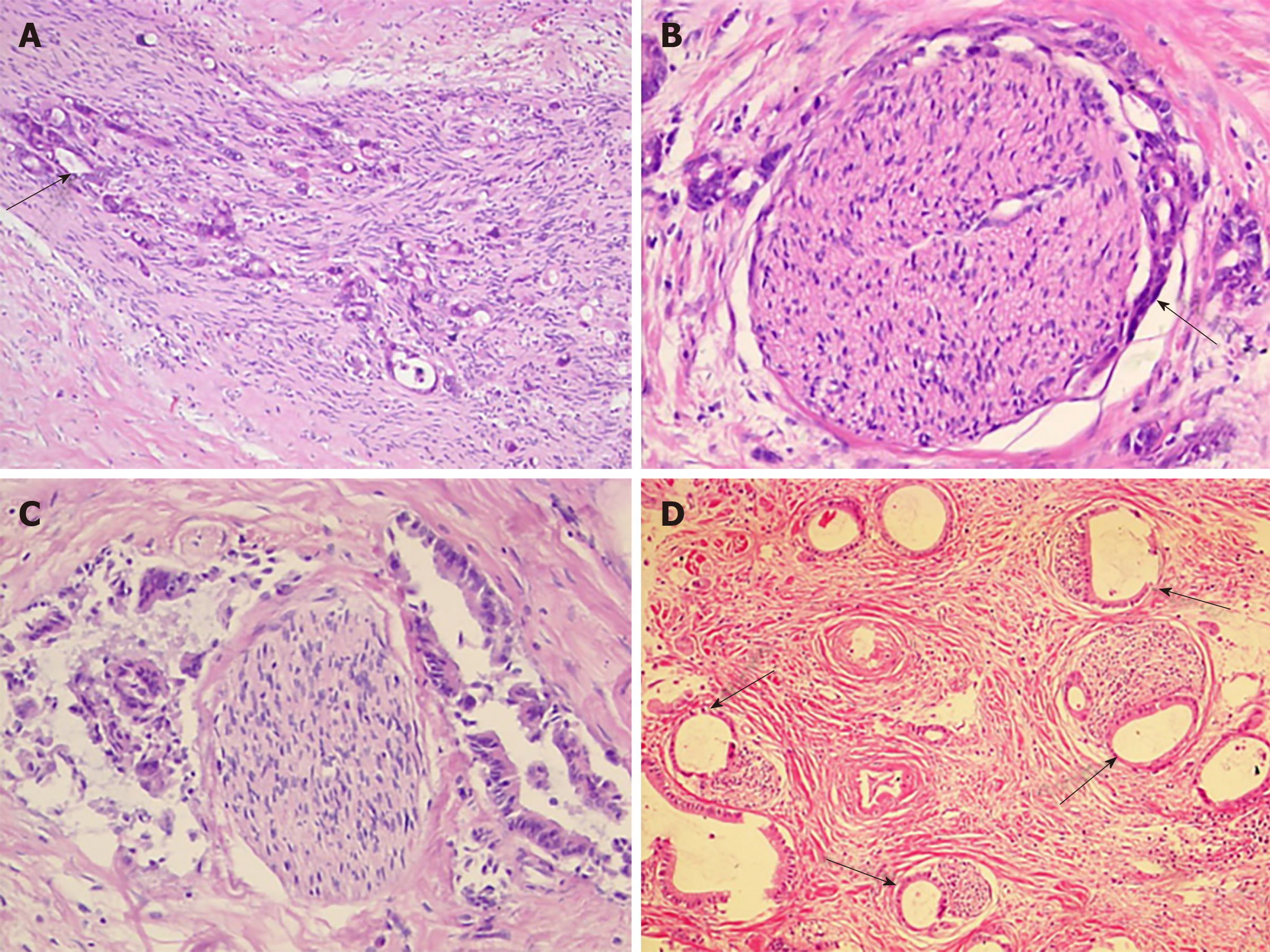
The follow photomicrographs depict PNI in human hilar cholangiocarcinoma specimens. Sections of human hilar cholangiocarcinoma were stained with hematoxylin and eosin and were reviewed by a pathologist for PNI. Tumor cells located within the peripheral nerve sheath either in clusters or forming glandular elements are clear examples of PNI. When tumor cells are not located inside of the nerve sheath but are in close proximity to the nerve in the perineural environment, at least 33% of the circumference of the nerve must be involved by the tumor to diagnose PNI. This is true whether the nerve is located within the main body of the cancer or at a site outside of the primary tumor focus. Although various modes of PNI could be observed, the patterns of invasion in one patient seemed to be similar (Figure 1).
All the 75 patients’ pathologic diagnosis was bile duct adenocarcinoma, 69 (92 percent) had PNI, and 21 (28 percent) had lymph node metastasis (Table 2). The incidence of PNI did not show any remarkable differences among various differentiated groups (χ2 = 0.558, P = 0.757) and Bismuth-Corlette classification groups (χ2 = 3.3141, P = 0.3457).
| Characteristics | n (%) |
| Gender | |
| Female | 33 (44) |
| Male | 42 ( 56) |
| Bismuth-Corlette classification | |
| Type I | 19 (25.33) |
| Type II | 20 (26.67) |
| Type III | 20 (26.67) |
| Type IV | 16 (21.33) |
| Histological differentiation | |
| Well | 9 (12) |
| Moderate | 35 (46.67) |
| Poor | 29 (38.67) |
| Mucinous | 2 (2.67) |
| Lymphatic invasion | |
| No | 54 (72) |
| Yes | 21 (28) |
| Depth of tumor invasion | |
| Within bile duct serosa | 2 (2.67) |
| Beyond bile duct serosa | 73 (97.33) |
| Perineural invasion | |
| No | 6 (8) |
| Yes | 69 (92) |
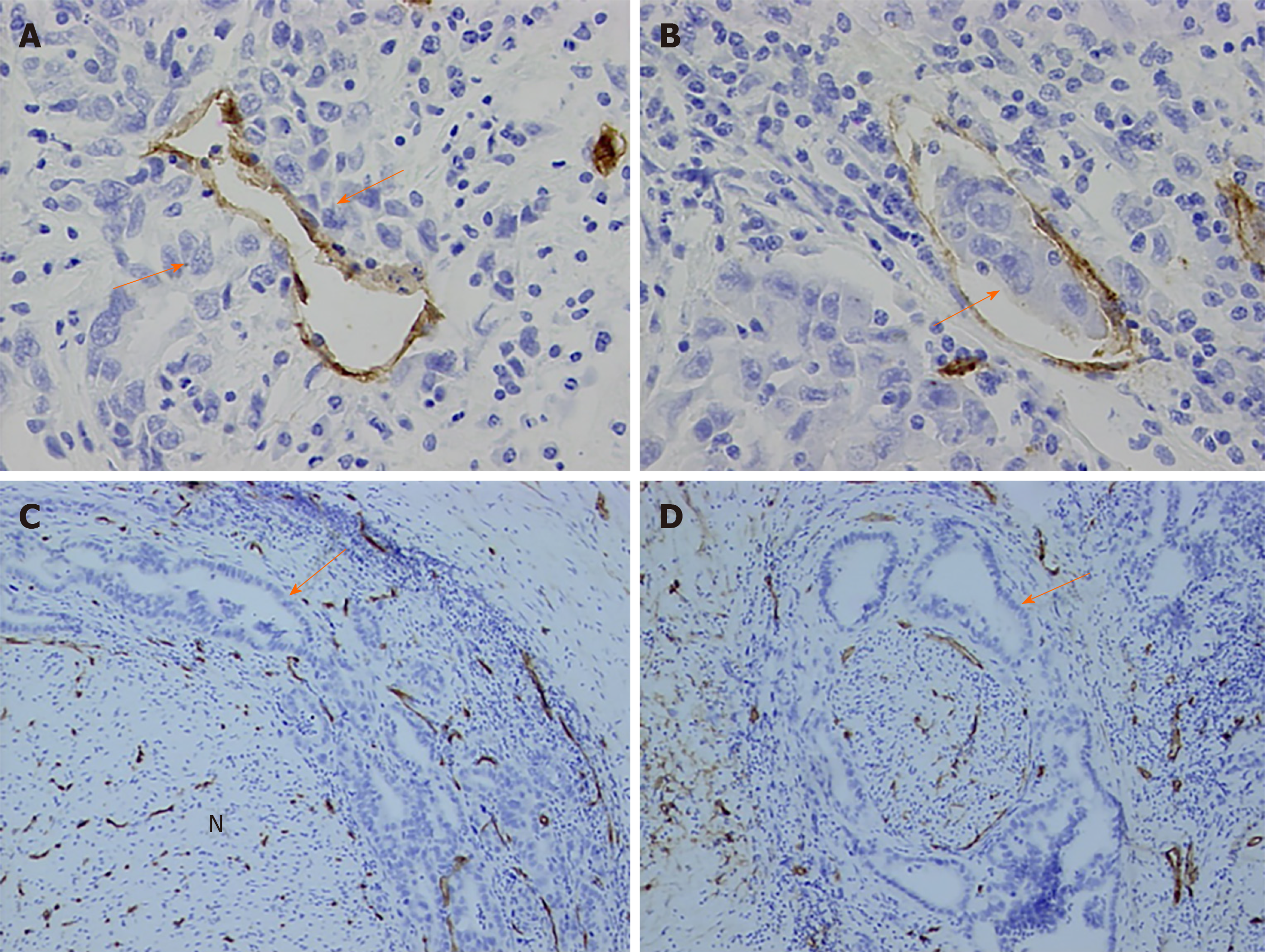
In this study, vascular endothelial cells were specially stained brown by CD34 immunohistochemically. Vascular tumor invasion was detected in the present study (Figure 2A and B). Although vascular proliferation was observed as tumor cells invading the nerve fibers, no evidence showed that tumor cells invaded nerve fibers via microvessels (Figure 2C and D).
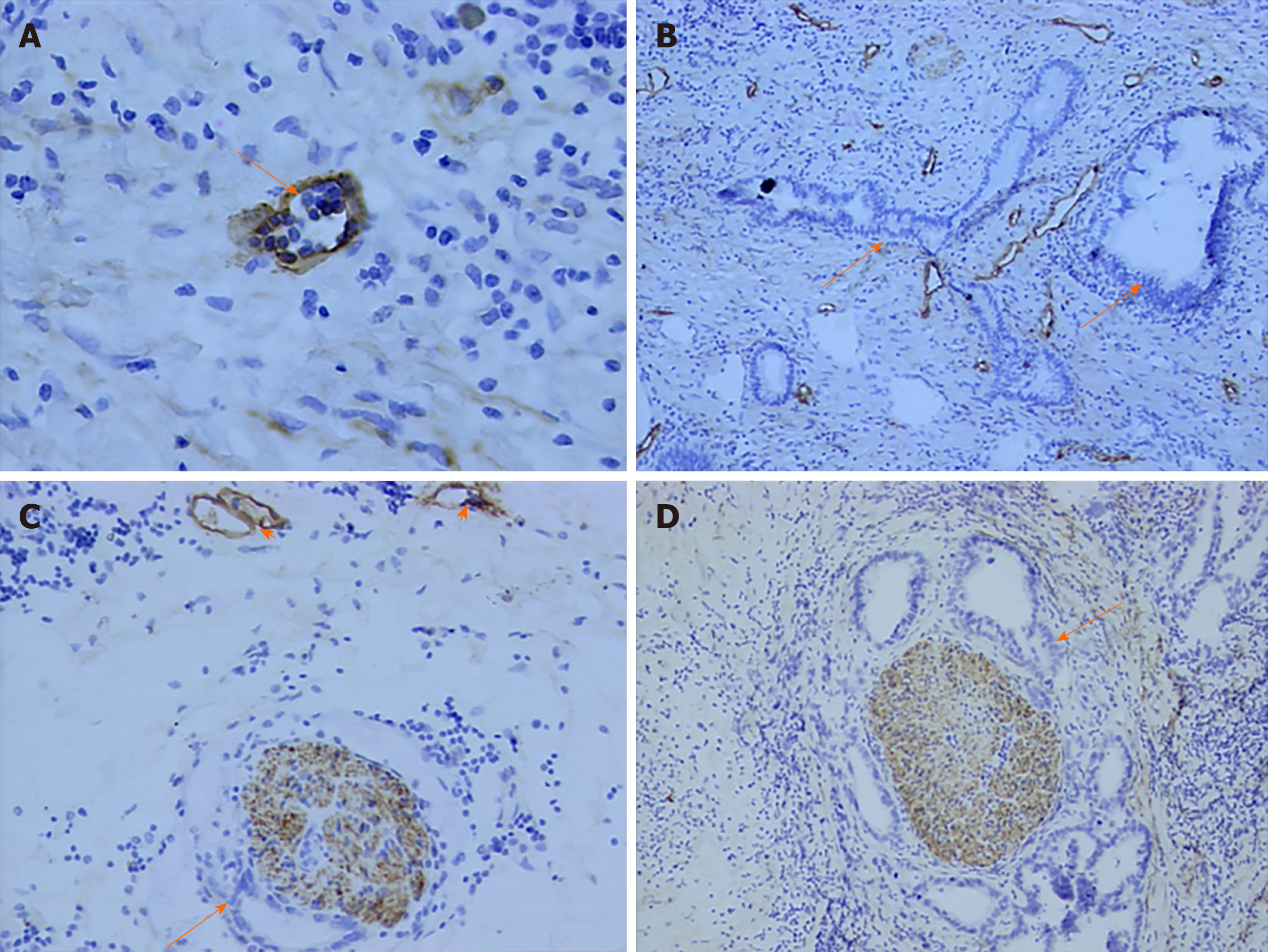
Lymphatic endothelial cell and nerve fibers were stained brown by D2-40 immunohistochemistry stain in this study. A great number of lymphatic microvessels were observed in the primary tumor. In some patients, although regional lymph node metastasis was not detected, tumor cells had invaded the lymphatic microvessels. However, no evidence showed that tumor cells invaded nerve fibers via lymphatic microvessels (Figure 3).
Of the 75 patients, tumor cells invaded within the bile duct subserosa in 2 patients (1 well-differentiated adenocarcinoma and 1 moderately differentiated adenocarcinoma) and they did not have PNI. Tumor cells invaded beyond the bile duct subserosa in the other 73 patients, 67 (94.37 percent) of them had PNI. Logistic regression analysis involving lymph node metastasis, serum CA19-9 content, Bismuth-Corlette type and depth of tumor invasion determined by univariate analysis demonstrated that depth of tumor invasion was the only factor that correlated significantly with PNI (P < 0.001).
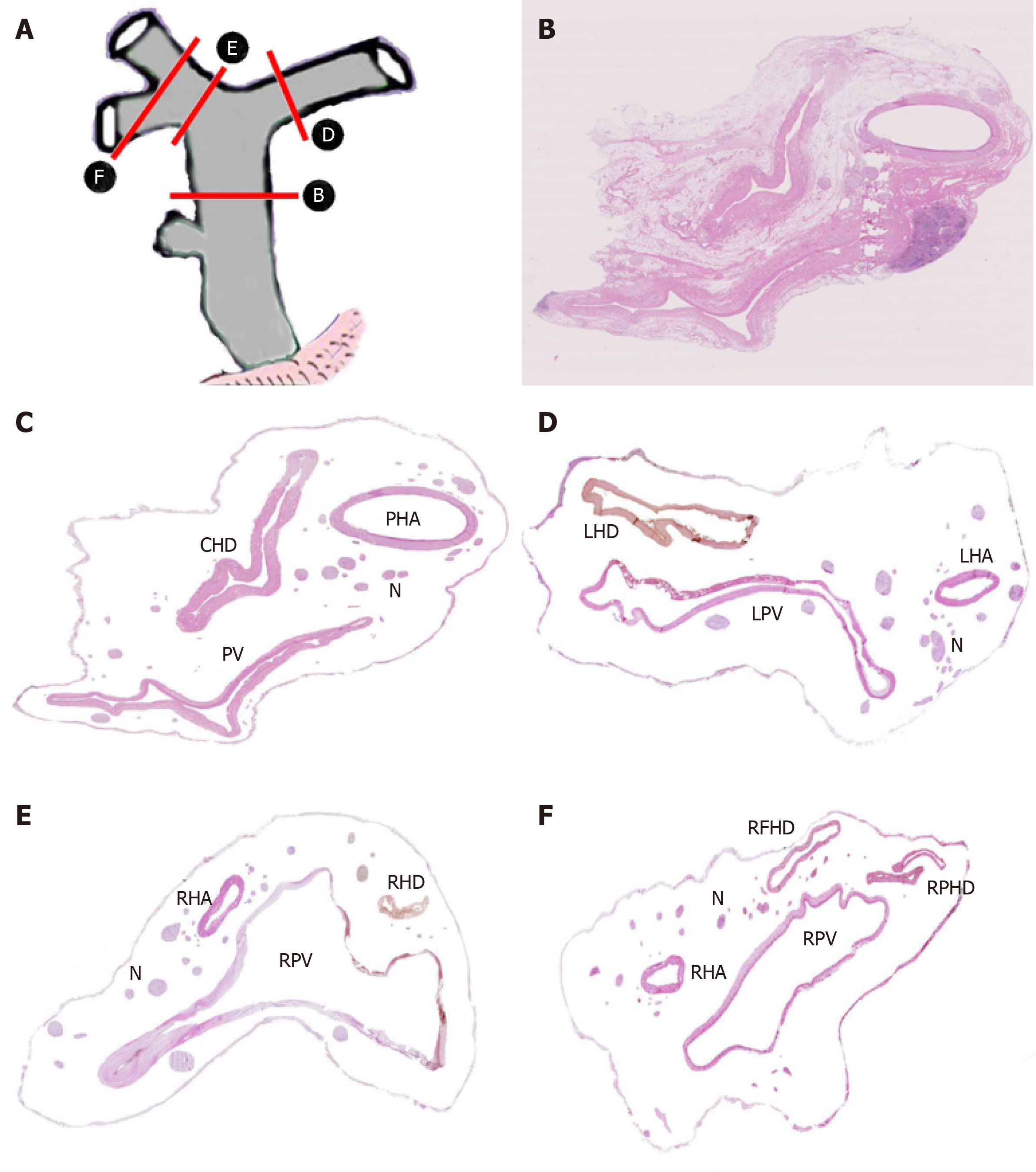
A total of 40 sections from different decks of hepatoduodenal ligament and Glisson’s sheath were scanned and handled by computer to display the location and distribution of nerve plexus around the hepatic portal. The distribution of nerve plexus in the Glisson’s sheath was similar to that in hepatoduodenal ligament. Nerve fibers mainly surrounded the great vessels especially to the hepatic artery, while nerve plexus around bile duct was scarce. Three nerve plexuses were observed. Plexus I was defined as the nerve plexus lateral to hepatic artery. Plexus II was defined as the nerve plexus between hepatic artery and bile duct. Plexus III was described as the nerve plexus posterior portal vein (Figure 4A-F).
Although the pathogenesis and clinical significance of PNI remain unclear, it is considered an under-recognized route of metastatic spread. The clear association between PNI and metastases in several cancers strongly suggests a role for PNI in tumor dissemination. However, the detailed molecular mechanisms with which tumor cells interact with nerve cells are largely unknown. Lacking clear and concrete guidelines on the reporting of PNI have resulted in significant confusion in the incidence of PNI in HCCA in literature[1-9]. In present study, we find 92% of HCCA has PNI similarly to the results of Young et al[5] and Nagakawa et al[12]’s reports. Underreporting of PNI remains an obstacle to gaining an adequate understanding of its real prognostic significance. First, several factors make nerve invasion very difficult to recognize. Inflammatory cells or abundant, mucinous pools may obscure the presence of tumor cells around nerves. Microscopic foci of nerve invasion are common and also may escape detection. According to the latest protocols published by the College of American Pathologists, evaluation of PNI is not a required element in pancreatic, colorectal, or prostate cancer pathology reports[16]. Published reports showed that the rate of PNI in HCCA was 38% to 100%, which should be believed? Therefore, we suggest for clear and concrete guidelines on the reporting of PNI in HCCA to lay out its real prognostic significance.
PNI is a distinct pathologic entity that can be observed in the absence of lymphatic or vascular invasion in HCCA. For the last decades, the predominant theory behind the pathogenesis of PNI has been that tumor cells spreading along neural sheaths are privileged to a low-resistance plane, which serves as a conduit for their migration. However, it has become evident that PNI is not an extension of lymphatic metastasis, as once was suggested. Definitive studies have demonstrated that lymphatic channels do not penetrate the inner sanctum of the nerve sheath[17]. In the present study, we used D2-40 and CD34 antibody to explore lymphatic and vascular invasion in HCCA specimens respectively as previous authors’ reports[18,19]. Although perineural cells can also be stained by D2-40 antibody[20], we could discriminate the tumor cells invaded in perineurial nerves from lymph ducts and microvessels clearly. In the 67 tumor specimens with PNI, we did not find tumor cells invade perineurial nerves via lymphatic or microvessels. In our study, there was no association between PNI and tumor size, differentiation, or lymphovascular invasion, but there was a significant correlation between PNI and depth of tumor invasion. The PNI was encountered more frequently when the tumors invaded beyond the subserosa.
The signaling mechanisms behind PNI likely involve at least 3 different cellular elements, including tumor cells, nerve cells, and stromal cells, and may include autocrine and paracrine mechanisms. There is a growing body of literature implicating neurotrophins and their receptors in PNI of cancer[21-24]. Murakawa et al[25] explored the gene expression profiles characteristic of progression and invasiveness in the cDNA array data in bile duct cancer and could predict PNI with an accuracy of 100%[25]. Question is coming that if we have predicted the patient with PNI, what can we do during the operation, and where are the nerves around hepatic portal?
The local recurrence of bile duct cancer is relatively high even after curative resection of this lesion[26]. An aggressive surgical approach increases the resectability rate and may improve long-term survival even after R1 resection[27]. Therefore, a suitable surgical procedure should be considered for preventing this undesirable outcome. It is helpful for practical application to investigate the distribution of nerve plexuses around hepatic portal. Previous studies only divided the nerve plexuses of hepatoduodenal ligament into two groups, anterior nerve plexus, and posterior nerve plexus. Results from present study showed that the distribution of plexus in the Glisson’s sheath was similar to that in hepatoduodenal ligament. The nerve fibers mainly surrounded the great vessels especially to the hepatic artery, while nerve plexus around bile duct was scarce. These findings suggest that nerve plexuses around the membrane adventitia should be routinely divested completely during the radical excision of HCCA. We would like to emphasize the need of autonomic nerve fiber and plexus dissection around the hepatic and celiac arteries, portal vein, and sometimes right celiac ganglionectomy, together with the lymph nodes, lymphatic, and connective tissues dissection to eradicate the cancer tissue entirely and perform the curative operation for HCCA. The present study concludes that spreading of the bile duct cancer does take place through the perineural space, and the surgeons should always keep their mind on this fact while managing this bile duct cancer.
In conclusion, there is significant confusion in the rate of PNI in HCCA in the literature, and it is an underreported phenomenon in HCCA. We suggest for clear and concrete guidelines on the reporting of PNI in HCCA. Patients with PNI should be considerate for treatment with currently available effective adjuvant therapies. To achieve an R0 resection, surgeons should have knowledge on distribution of nerve plexuses around hepatic portal. Further investigations into the molecular basis of PNI could help develop therapeutic strategies targeted toward this aggressive tumor phenotype.
Hilar cholangiocarcinoma (HCCA) often produces perineural invasion (PNI) extending to extra-biliary sites, while significant confusion in the incidence of PNI in HCCA has occurred in the literature, however, the mechanism of this procedure remains unclear.
A better understanding of PNI may lend insight into tumor metastasis and recurrence and open doors to improved staging strategies, novel treatment modalities, and perhaps even paradigm shifts in our treatment of patients.
This study aimed to summarize the incidence of PNI in HCCA, and the authors try to provide the distribution of nerve plexuses around hepatic portal to clinical surgeons.
A clinicopathological study was conducted on sections from 75 patients with HCCA to summarize the incidence and modes of PNI. Immunohistochemical stains for CD34 and D2-40 in the cancer tissue were performed to clarify the association of PNI with microvessel and lymph duct. Sections of the hepatoduodenal ligament from autopsy cases were scanned and handled by computer to display the distribution of nerve plexuses around the hepatic portal.
The overall incidence of PNI in this study was 92%, while the rate of PNI in HCCA in the literature ranging from 38% to 100%. The incidence of PNI did not show any remarkable differences among various differentiated groups and Bismuth-Corlette classification groups. Logistic regression analysis identified the depth of tumor invasion was the only factor that correlated significantly with PNI. The authors did not find tumor cells invaded nerves via microvessels or lymph ducts.
The incidence of PNI of HCCA in Chinese population is around 92% and correlated significantly with a depth of tumor invasion. This should be considered when stratifying HCCA patients for further treatment.
Further investigations into the molecular basis of PNI could help develop therapeutic strategies targeted toward this aggressive tumor phenotype.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmad Z, Kuniyasu H, Okada S S-Editor: Wang JL L-Editor: A E-Editor: Qi LL
| 1. | Hu HJ, Jin YW, Shrestha A, Ma WJ, Wang JK, Liu F, Zhu YY, Zhou RX, Regmi P, Cheng NS, Li FY. Predictive factors of early recurrence after R0 resection of hilar cholangiocarcinoma: A single institution experience in China. Cancer Med. 2019;8:1567-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Kovalenko YA, Zharikov YO, Kukeev IA, Vishnevsky VA, Chzhao AV. [Predictors of outcomes in surgery for hilar cholangiocarcinoma]. Khirurgiia (Mosk). 2018;5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Bird NTE, McKenna A, Dodd J, Poston G, Jones R, Malik H. Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg. 2018;105:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Ercolani G, Zanello M, Grazi GL, Cescon M, Ravaioli M, Del Gaudio M, Vetrone G, Cucchetti A, Brandi G, Ramacciato G, Pinna AD. Changes in the surgical approach to hilar cholangiocarcinoma during an 18-year period in a Western single center. J Hepatobiliary Pancreat Sci. 2010;17:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Young AL, Prasad KR, Toogood GJ, Lodge JP. Surgical treatment of hilar cholangiocarcinoma in a new era: comparison among leading Eastern and Western centers, Leeds. J Hepatobiliary Pancreat Sci. 2010;17:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Aishima S, Kuroda Y, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007;31:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Robles R, Figueras J, Turrión VS, Margarit C, Moya A, Varo E, Calleja J, Valdivieso A, Valdecasas JC, López P, Gómez M, de Vicente E, Loinaz C, Santoyo J, Fleitas M, Bernardos A, Lladó L, Ramírez P, Bueno FS, Jaurrieta E, Parrilla P. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379-3391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 852] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 9. | Nakagohri T, Asano T, Kinoshita H, Kenmochi T, Urashima T, Miura F, Ochiai T. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg. 2003;27:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, Makuuchi M. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 244] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 11. | Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, Sano T, Yamamoto H, Hayakawa N. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Nagakawa T, Kitagawa H, Kayahara M, Ohta T, Konishi I. [Spreading patterns of hilar bile duct cancer]. Nihon Geka Gakkai Zasshi. 2000;101:399-403. [PubMed] |
| 13. | Kurosaki I, Tsukada K, Watanabe H, Hatakeyama K. Prognostic determinants in extrahepatic bile duct cancer. Hepatogastroenterology. 1998;45:905-909. [PubMed] |
| 14. | Yamaguchi K, Chijiiwa K, Saiki S, Shimizu S, Takashima M, Tanaka M. Carcinoma of the extrahepatic bile duct: mode of spread and its prognostic implications. Hepatogastroenterology. 1997;44:1256-1261. [PubMed] |
| 15. | Su CH, Tsay SH, Wu CC, Shyr YM, King KL, Lee CH, Lui WY, Liu TJ, P'eng FK. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 216] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | College of American Pathologists. Cancer Protocols and Checklists. Northfield, Ill, 2007. Available from: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates. |
| 17. | Reina MA, López A, Villanueva MC, de Andrés JA, León GI. [Morphology of peripheral nerves, their sheaths, and their vascularization]. Rev Esp Anestesiol Reanim. 2000;47:464-475. [PubMed] |
| 18. | Shimizu Y, Jin L, Yamaguchi H, Motosugi U, Sannohe S, Nagata K, Sakurai T, Murata S, Yasuda M, Shimizu M. Detection of lymphatic invasion in resected cases of primary pancreatic cancer based on immunohistochemistry of D2-40. Ann Diagn Pathol. 2009;13:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Saad RS, Kordunsky L, Liu YL, Denning KL, Kandil HA, Silverman JF. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol. 2006;19:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Jokinen CH, Dadras SS, Goldblum JR, van de Rijn M, West RB, Rubin BP. Diagnostic implications of podoplanin expression in peripheral nerve sheath neoplasms. Am J Clin Pathol. 2008;129:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Okada Y, Eibl G, Guha S, Duffy JP, Reber HA, Hines OJ. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Hu HJ, Wu ZR, Jin YW, Ma WJ, Yang Q, Wang JK, Liu F, Li FY. Minimally invasive surgery for hilar cholangiocarcinoma: state of art and future perspectives. ANZ J Surg. 2019;89:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, Wheeler TM, Thompson TC, Rowley D. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082-6090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Murakawa K, Tada M, Takada M, Tamoto E, Shindoh G, Teramoto K, Matsunaga A, Komuro K, Kanai M, Kawakami A, Fujiwara Y, Kobayashi N, Shirata K, Nishimura N, Okushiba S, Kondo S, Hamada J, Katoh H, Yoshiki T, Moriuchi T. Prediction of lymph node metastasis and perineural invasion of biliary tract cancer by selected features from cDNA array data. J Surg Res. 2004;122:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Zharikov YO, Kovalenko YA, Olifir AA, Kalinin DV, Czhao AV. [Clinico-pathological factors and prognosis scale for portal cholangiocarcinoma]. Khirurgiia (Mosk). 2017;27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Juntermanns B, Fingas CD, Sotiropoulos GC, Jaradat D, Dechêne A, Reis H, Kasper S, Paul A, Kaiser GM. [Klatskin tumor: long-term survival following surgery]. Chirurg. 2016;87:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |