Published online Dec 15, 2019. doi: 10.4251/wjgo.v11.i12.1231
Peer-review started: February 22, 2019
First decision: June 4, 2019
Revised: September 1, 2019
Accepted: September 26, 2019
Article in press: September 26, 2019
Published online: December 15, 2019
Processing time: 292 Days and 21.5 Hours
Follicular dendritic cell (FDC) sarcoma/tumor is a rare malignant tumor of follicular dendritic cells, which is considered a low-grade sarcoma that can involve lymph nodes or extranodal sites. Conventional FDC sarcomas are negative for Epstein-Barr virus (EBV), whereas the inflammatory pseudotumor-like variant consistently shows EBV in the neoplastic cells.
We report two cases of inflammatory pseudotumor-like FDC sarcoma in the liver that received 3D laparoscopic right hepatectomy and open right hepatectomy separately.
EBV probe-based in situ hybridization and detection of immunohistochemical markers of FDC play an important role in the diagnosis and differential diagnosis of inflammatory pseudotumor-like FDC sarcoma. Complete surgical excision combined with regional lymphadenectomy may be effective in reducing the postoperative recurrence and metastasis and improving long-term survival rates.
Core tip: There have been 48 previously reported cases of inflammatory pseudotumor-like follicular dendritic cell (FDC) sarcoma, which occurs almost exclusively in the liver and spleen. Here we report two cases of inflammatory pseudotumor-like FDC sarcoma in the liver that were treated by 3D laparoscopic right hepatectomy and open right hepatectomy separately.
- Citation: Zhang BX, Chen ZH, Liu Y, Zeng YJ, Li YC. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: A brief report of two cases. World J Gastrointest Oncol 2019; 11(12): 1231-1239
- URL: https://www.wjgnet.com/1948-5204/full/v11/i12/1231.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i12.1231
Follicular dendritic cell (FDC) sarcoma/tumor is a rare malignant tumor of follicular dendritic cells, which are mesenchymal cells in the lymphoid follicles with antigen presenting ability. It is considered a low-grade sarcoma that can involve lymph nodes or extranodal sites[1-5]. In 1996, Shek et al[6] reported the first case of primary FDC sarcoma in the liver. The histology was similar to an inflammatory pseudotumor and it was related to Epstein-Barr virus (EBV)-related clonal proliferation[6]. Inflammatory pseudotumor-like FDC sarcoma was first described as a distinctive variant of FDC sarcoma and associated with EBV in 2001[7]. There have been 48 previously reported cases of inflammatory pseudotumor-like FDC sarcoma, which occurs almost exclusively in the liver and spleen (Table 1). Ancillary tests, including detection of immunohistochemical markers of FDC such as CD21, CD23, or CD35 and EBV probe-based in situ hybridization, are required for this diagnosis. Here we report two cases of inflammatory pseudotumor-like FDC sarcoma in the liver that were treated by 3D laparoscopic right hepatectomy and open right hepatectomy separately.
| Ref. | Sex/age | Location | Maximum diameter (cm) | Symptom | Treatment | Follow-up (mo) | Outcome |
| Li et al[13] | F/64 | Spleen | 7.2 | Upper abdominal pain | Laparoscopic splenectomy | 8 | NED |
| M/61 | Spleen | 6.2 | Asymptomatic | Laparoscopic splenectomy | 16 | NED | |
| F/42 | Spleen | 4 | Left-sided flank pain | Laparoscopic splenectomy | 9 | NED | |
| F/57 | Spleen | 13.3 | Upper abdominal pain | Laparoscopic splenectomy | 4 | LWD, pulmonary metastasis | |
| M/52 | Spleen | 2 masses: 3.7, 2.9 | Back pain | Laparoscopic splenectomy | 5 | LWD, bone metastasis | |
| Hang et al[14] | M/57 | Spleen | 2.7 | Asymptomatic | Laparoscopic partial splenectomy | 9 | NED |
| Ge et al[15] | F/54 | Spleen | 3.5 | Left-sided flank pain | Splenectomy | 10 | NED |
| M/79 | Spleen | 6 | Asymptomatic | Splenectomy | 18 | NED | |
| Pan et al[16] | F/78 | Colon | 3.9 | Abdominal discomfort, bloody stool | Polypectomy | 5 | NED |
| Choe et al[17] | F/64 | Spleen | 5.5 | Asymptomatic | Splenectomy | 78 | NED |
| F/72 | Spleen | 7.2 | Asymptomatic | Splenectomy | 18 | NED | |
| F/53 | Spleen | 3.2 | Asymptomatic | Splenectomy | 13 | NED | |
| M/76 | Spleen | 3.2 | Asymptomatic | Splenectomy | 8 | NED | |
| M/72 | Spleen | 6 | Asymptomatic | Splenectomy | 18 | NED | |
| M/75 | Spleen | 3.5 | Abdominal pain | Splenectomy | 30 | NED | |
| Granados et al[18] | F/57 | Liver | 13 | Abdominal pain, vomiting | Partial hepatectomy | 24 | NED |
| Cheuk et al[7] | F/19 | Liver | 12 | Right upper quadrant pain, abdominal mass, weight loss | Partial hepatectomy | 40 | NED |
| F/56 | Liver | 15 | Abdominal discomfort | Partial hepatectomy | 56 | LWD, recurrence in liver | |
| F/40 | Liver | 12.5 | Upper abdominal pain, weight loss | Partial hepatectomy | 108 | LWD, intraabdominal recurrence | |
| F/49 | Liver | 4.2 | Asymptomatic | Partial hepatectomy | 9 | NED | |
| M/37 | Liver | 15 | Abdominal mass, weight loss | Partial hepatectomy | 42 | NED | |
| F/35 | Liver | 20 | Abdominal discomfort, fever, weight loss | Partial hepatectomy | 95 | DOD, disseminated in liver and peritoneum | |
| F/31 | Liver | 15 | Abdominal distension, weight loss | Partial hepatectomy | 60 | NED | |
| F/58 | Spleen | 22 | Abdominal mass | Splenectomy | 4 | NED | |
| F/39 | Spleen | 7.5 | Weight loss, fever | Splenectomy | 2 | LWD, persistent fever | |
| F/61 | Spleen | 3.5 | Asymptomatic | Splenectomy | NA | NA | |
| F/49 | Peri-pancreas | 15 | Abdominal distension | Whipple’s operation | NA | NA | |
| Li et al[19] | F/49 | Spleen | 4.7 | Asymptomatic | Splenectomy | NA | NA |
| F/56 | Spleen | 8 | Abdominal pain | Splenectomy | 17 | NED | |
| M/38 | Liver | 8.5 | Anorexia | Partial hepatectomy | 11 | NED | |
| F/42 | Liver | 2 masses: 2, 1.7 | Abdominal pain | Partial hepatectomy | 36 | NED | |
| M/50 | Spleen and liver | Spleen: 10 Liver: 3 | Abdominal bloating | Splenectomy and partial hepatectomy | 17 | NED | |
| F/39 | Liver | 9 | Asymptomatic | Partial hepatectomy | 84 | NED | |
| Chen et al[20] | F/28 | Liver | 6 | Abdominal pain, fatigue, anorexia | Partial hepatectomy | 48 | LWD, recurrence in liver |
| M/39 | Spleen | 7.4 | Asymptomatic | Splenectomy | 40 | NED | |
| M/48 | Liver | 23.3 | Abdominal pain, fever, fatigue | Partial hepatectomy | 23 | NED | |
| M/65 | Spleen and liver | Spleen: 22.3 Liver: 5.8 (multi masses) | Abdominal pain, fever, fatigue, anorexia, weight loss | Splenectomy | 2 | DOD | |
| M/51 | Spleen | 8.5 | Weight loss | Splenectomy | 19 | NED | |
| M/68 | Spleen | 2.3 | Asymptomatic | Splenectomy | 6 | NED | |
| F/51 | Spleen | 5.3 | Abdominal discomfort | Splenectomy | 5 | NED | |
| M/67 | Spleen | 7.5 | Asymptomatic | Splenectomy | 5 | NED | |
| M/60 | Liver | 3 | Asymptomatic | Partial hepatectomy | 3 | NED | |
| F/52 | Spleen | 0.9 | Asymptomatic | Splenectomy | 12 | NED | |
| Kitamura et al[21] | F/74 | Spleen | 3.6 | Asymptomatic | Splenectomy | 24 | NED |
| Bui et al[22] | F/50 | Spleen | 6 | Abdominal pain | Splenectomy | NA | NA |
| Vardas et al[23] | M/61 | Spleen | 10 | Abdominal pain | Splenectomy | 12 | NED |
| Kim et al[24] | M/76 | Spleen | 3.2 | Asymptomatic | Splenectomy | NA | NA |
| Horiguchi et al[25] | F/77 | Spleen | 8.5 | Abdominal pain | Splenectomy | 36 | NED |
| Present case | F/31 | Liver | 2 masses: 3.5, 2.5 | Anorexia | 3D laparoscopic right hepatectomy | 10 | NED |
| M/48 | Liver and hepatoduodenal ligament lymph node | Liver: 10 Lymph node: 3.5 | Asymptomatic | Open right hepatectomy, lymph node excision | 2 | NED |
Case 1: A 31-year-old woman was admitted to hospital for evaluation of a four-week history of anorexia.
Case 2: A 48-year-old man stumbled across a liver mass through a routine ultrasound examination.
Unremarkable.
Case 1: Her past medical history was chronic hepatitis B for more than 10 years without antiviral treatment.
Case 2: Unremarkable.
Unremarkable.
Case 1: Physical examination revealed mild tenderness to palpation in the right upper quadrant.
Case 2: Physical examination was unremarkable.
Case 1: Laboratory tests showed seropositivity for HBsAg, HBeAb, and HBcAb. Furthermore, serum level of hepatitis B virus-DNA was lower than detection limit.
Case 2: Laboratory tests were unremarkable.
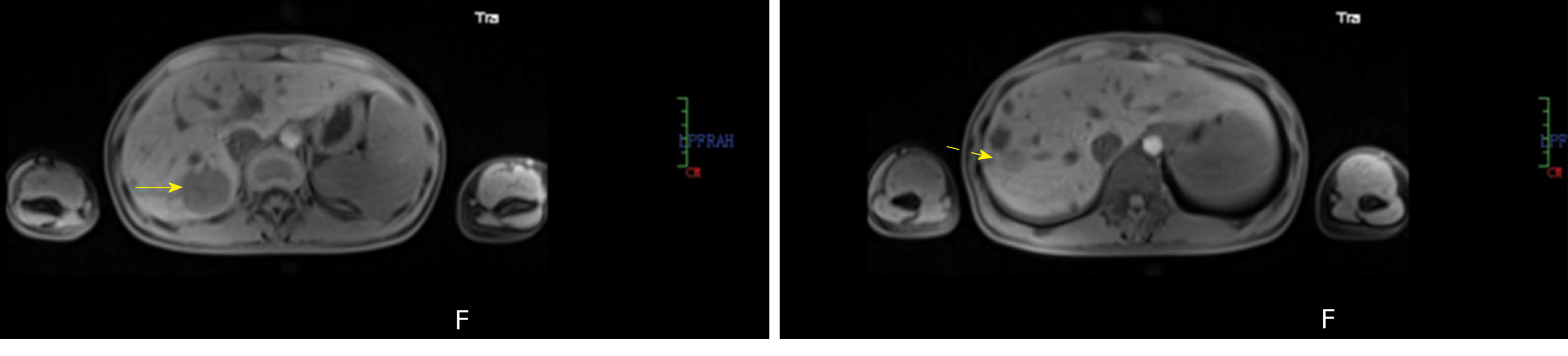
Case 1: Abdominal magnetic resonance imaging revealed two well-circumscribed masses in the right posterior lobe of the liver (Figure 1).
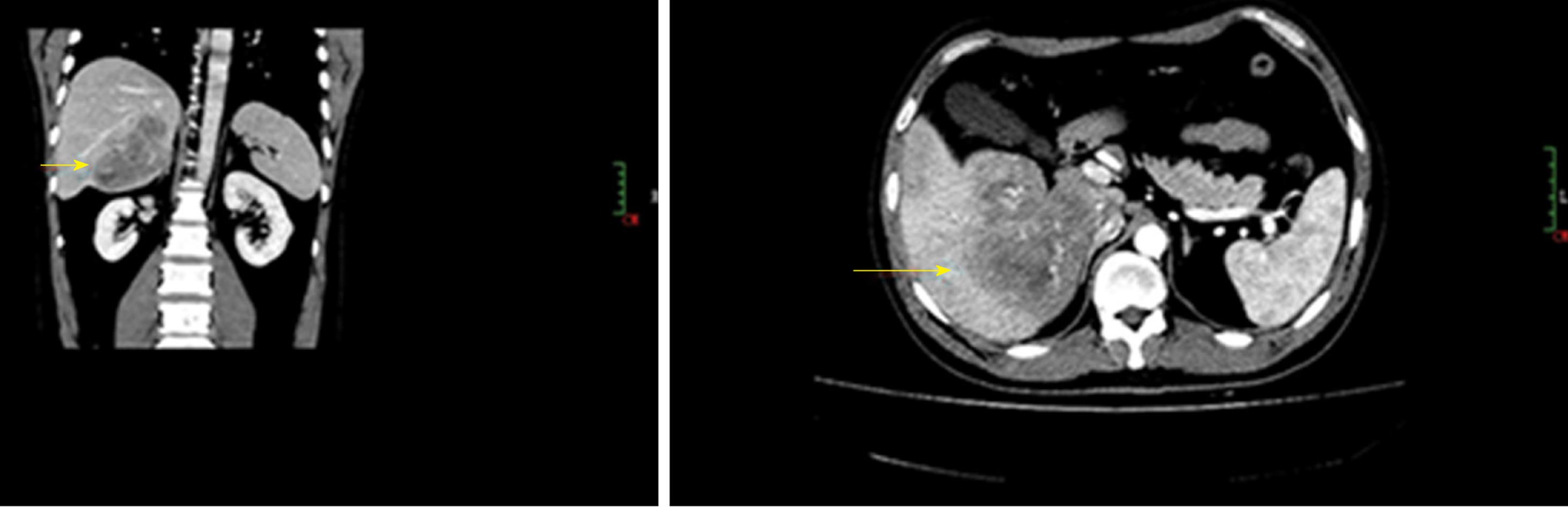
Case 2: An abdominal computed tomography examination revealed an ill-defined 10 cm mass in the right lobe of the liver accompanied with enlargement of hepatic portal lymph nodes (Figure 2).
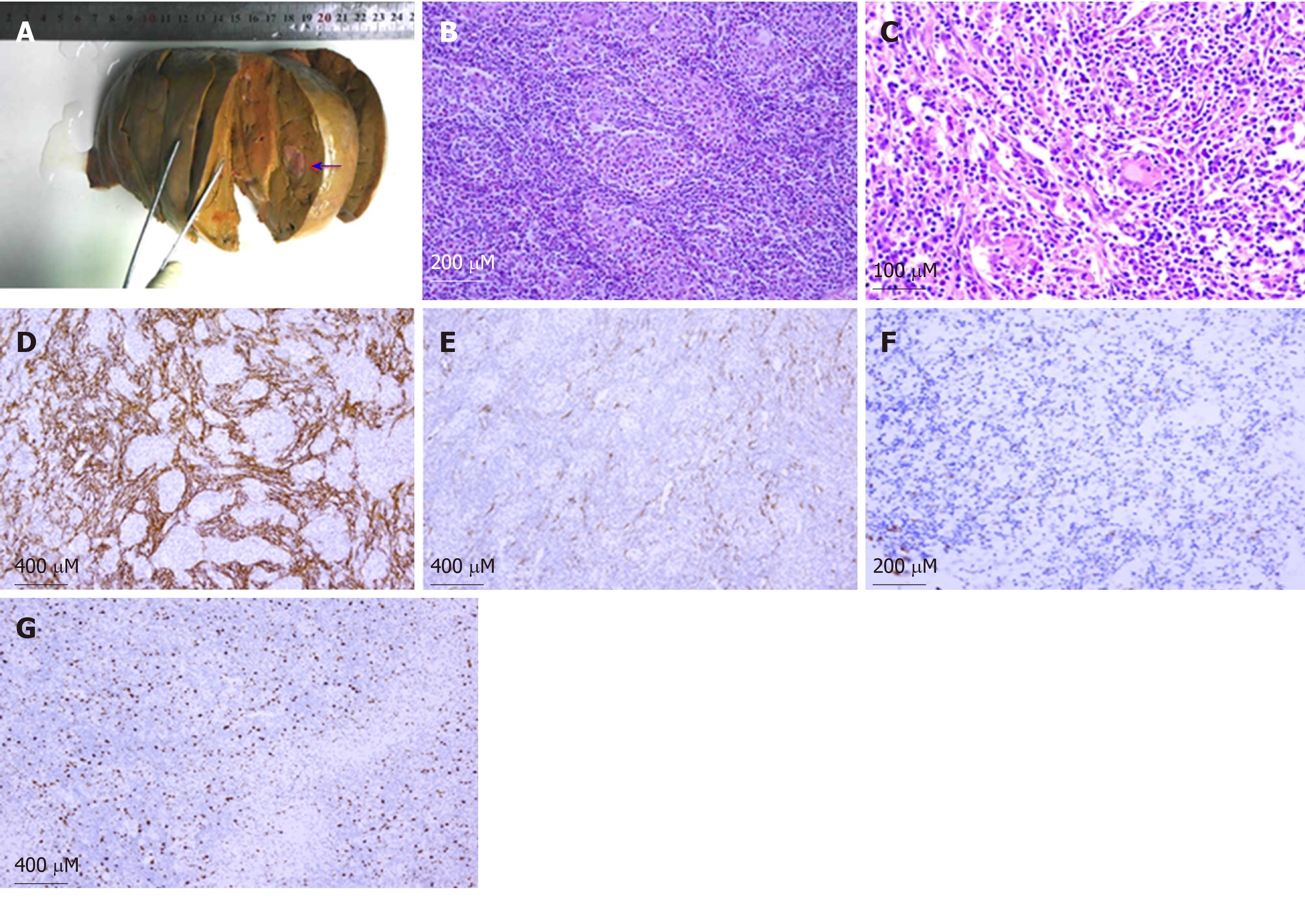
EBV-positive inflammatory pseudotumor-like FDC sarcoma in the liver (Figure 3).
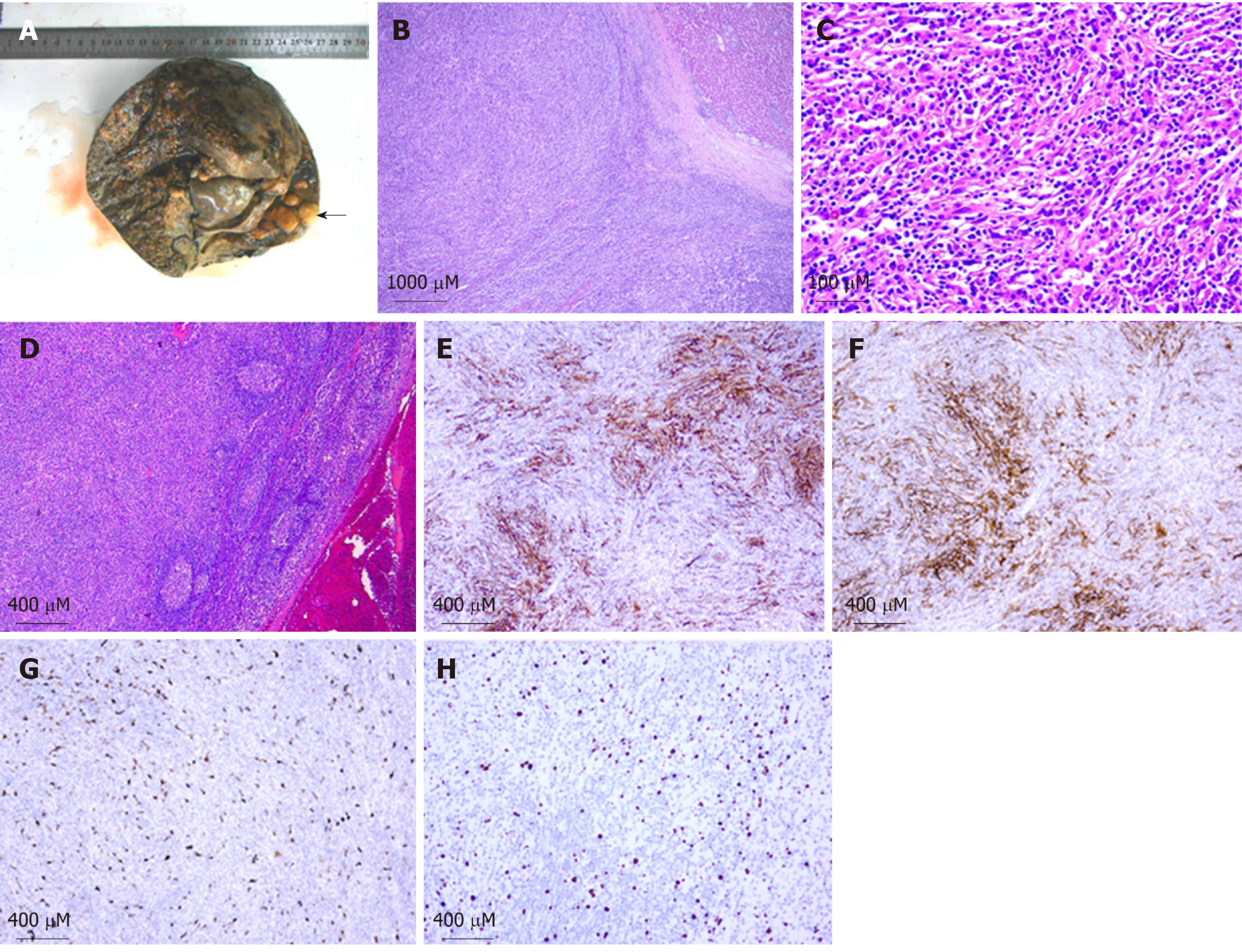
EBV-positive inflammatory pseudotumor-like FDC sarcoma in the liver with hepatoduodenal ligament lymph node involvement (Figure 4).
3D laparoscopic right hepatectomy.
Open right hepatectomy combined with regional lymphadenectomy.
Follow-up for 10 mo showed no recurrence or metastasis.
Follow-up for 2 mo showed no recurrence or metastasis.
FDC sarcoma is a neoplastic proliferation of spindled to ovoid cells exhibiting morphological and immunophenotypic features of FDCs. Histologically, FDC sarcomas are classified into two types: (A) Conventional FDC sarcoma consisting of spindled to ovoid cells forming fascicles, storiform arrays, whorls, diffuse sheets, or vague nodules with an array of small lymphocytes; and (B) Inflammatory pseudotumor-like FDC sarcoma composed of neoplastic spindled cells that are dispersed within a prominent lymphoplasmacytic infiltrate[3]. To date, 48 cases of inflammatory pseudotumor-like FDC sarcoma have been reported in the English-language literature, located in the liver (16/48), spleen (32/48), colon (1/48), and peri-pancreas (1/48), respectively. These cases included 19 males and 29 females (male/female ratio of 1: 1.5), with a mean age of 55 years (range, 19-79 years). Clinical manifestations include abdominal pain, abdominal bloating, abdominal mass, weight loss, fever, fatigue, and anorexia, but most cases are asymptomatic (Table 1).
The origin of FDC sarcoma remains controversial. Phenotypic marker studies and in vitro experiments with fibroblast-like cell lines have developed FDCs from fibroblast-like cells[8]. The neoplastic cells are often positive for FDC markers, such as CD21, CD23, and CD35, with the staining ranging from extensive to very local. FDCs appear to be closely related to bone marrow stromal progenitors, with several myofibroblast features[9]. Two studies examining the transcriptional profile of FDC sarcoma have revealed: (A) A peculiar immunological microenvironment enriched in follicular helper T cells and Treg populations, with special relevance to the inhibitory immune receptor programmed cell death protein 1 and its ligands, programmed cell death-Ligand 1 and programmed cell death-Ligand 2; and (B) The highly specific expression of the genes encoding for FDC secreted peptide and serglycin[10-11].
Conventional FDC sarcomas are negative for EBV, whereas the inflammatory pseudotumor-like variant consistently shows EBV in the neoplastic cells[7]. EBV-encoded small RNA was detected in both of the present cases by in situ hybridization. EBV-encoded latent membrane protein 1, which has been found to have an oncogenic role, has been identified in 74% (26/35) cases of inflammatory pseudotumor-like FDC sarcomas by immunohistochemical staining[7,17,19-21,25]. Recently, Takeuchi et al[12] reported increased numbers of EBV-infected cells in IgG4-related lymphadenopathy, compared with other reactive lymphadenopathy or extranodal IgG4-related disease, which suggests that there may be a relationship between IgG4-related disease and EBV[12]. Interestingly, Choe et al[17] reported that significant numbers of IgG4-positive plasma cells were found in six cases of EBV-positive inflammatory pseudotumor-like FDC sarcoma of the spleen, suggesting that EBV plays a critical role in inflammatory pseudotumor-like FDC sarcoma and IgG4-related sclerosing disease[17]. Generally, the pathogenic mechanism of EBV in inflammatory pseudotumor-like FDC sarcoma remains unclear and further investigation is required.
FDC sarcoma is usually treated by complete surgical excision, with or without adjuvant radiotherapy or chemotherapy. A pooled analysis of the literature revealed local recurrence and distant metastasis rates of 28% and 27%, respectively. Large tumor size (≥ 6 cm), coagulative necrosis, high mitotic count (≥ 5 mitoses per 10 high-power fields), and significant cytological atypia are associated with a worse prognosis[2,5]. Regarding the prognosis of patients with inflammatory pseudotumor-like FDC sarcoma, based on the literature reports of inflammatory pseudotumor-like FDC sarcoma with a median follow-up period of 17 mo, 35 patients had no evidence of disease. Five patients exhibited distant metastasis and two had local recurrence, with traits similar to large tumors and multiple masses. One of the current cases presented with liver and hepatoduodenal ligament lymph node involvement, suggesting that inflammatory pseudotumor-like FDC sarcoma presents an increased risk of lymph node metastasis. Complete surgical excision combined with regional lymphadenectomy may be effective in reducing the postoperative recurrence and metastasis and improving the long-term survival rates.
In conclusion, there is little specificity in the clinical manifestations of inflammatory pseudotumor-like FDC sarcoma. EBV probe-based in situ hybridization and detection of immunohistochemical markers of FDC play important roles in the diagnosis and differential diagnosis of inflammatory pseudotumor-like FDC sarcoma. Radical surgical resection is the main therapeutic intervention for inflammatory pseudotumor-like FDC sarcoma, especially for cases with lymph node involvement, and patients require long-term post-surgical follow-up.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ebrahimifar M, Grizzi F, Pandey A, Souto Nacif LS-Editor: Zhang L L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122:562-572. [PubMed] |
| 2. | Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer. 1997;79:294-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Steven HS, Elias C, Nancy LH, Elaine SJ, Stefano AP, Harald S, Jurgen T, Daniel AA, Robert PH, Michelle MLB, Attilio O, Reiner S. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC press. 2017;476-479. |
| 4. | Duan GJ, Wu F, Zhu J, Guo DY, Zhang R, Shen LL, Wang SH, Li Q, Xiao HL, Mou JH, Yan XC. Extranodal follicular dendritic cell sarcoma of the pharyngeal region: a potential diagnostic pitfall, with literature review. Am J Clin Pathol. 2010;133:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Saygin C, Uzunaslan D, Ozguroglu M, Senocak M, Tuzuner N. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013;88:253-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Shek TW, Ho FC, Ng IO, Chan AC, Ma L, Srivastava G. Follicular dendritic cell tumor of the liver. Evidence for an Epstein-Barr virus-related clonal proliferation of follicular dendritic cells. Am J Surg Pathol. 1996;20:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 124] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | van Nierop K, de Groot C. Human follicular dendritic cells: function, origin and development. Semin Immunol. 2002;14:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Muñoz-Fernández R, Blanco FJ, Frecha C, Martín F, Kimatrai M, Abadía-Molina AC, García-Pacheco JM, Olivares EG. Follicular dendritic cells are related to bone marrow stromal cell progenitors and to myofibroblasts. J Immunol. 2006;177:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Hu ZQ, Zhao WH. Critical role of PD-1/PD-L1 pathway in generation and function of follicular regulatory T cells. Cell Mol Immunol. 2013;10:286-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Lorenzi L, Döring C, Rausch T, Benes V, Lonardi S, Bugatti M, Campo E, Cabeçadas J, Simonitsch-Klupp I, Borges A, Mehta J, Agostinelli C, Pileri SA, Facchetti F, Hansmann ML, Hartmann S. Identification of novel follicular dendritic cell sarcoma markers, FDCSP and SRGN, by whole transcriptome sequencing. Oncotarget. 2017;8:16463-16472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Takeuchi M, Sato Y, Yasui H, Ozawa H, Ohno K, Takata K, Gion Y, Orita Y, Tachibana T, Itoh T, Asano N, Nakamura S, Swerdlow SH, Yoshino T. Epstein-Barr virus-infected cells in IgG4-related lymphadenopathy with comparison with extranodal IgG4-related disease. Am J Surg Pathol. 2014;38:946-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Li X, Shi Z, You R, Li Y, Cao D, Lin R, Huang X. Inflammatory Pseudotumor-Like Follicular Dendritic Cell Sarcoma of the Spleen: Computed Tomography Imaging Characteristics in 5 Patients. J Comput Assist Tomogr. 2018;42:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Hang JF, Wang LC, Lai CR. Cytological features of inflammatory pseudotumor-like follicular dendritic cell sarcoma of spleen: A case report. Diagn Cytopathol. 2017;45:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Ge R, Liu C, Yin X, Chen J, Zhou X, Huang C, Yu W, Shen X. Clinicopathologic characteristics of inflammatory pseudotumor-like follicular dendritic cell sarcoma. Int J Clin Exp Pathol. 2014;7:2421-2429. [PubMed] |
| 16. | Pan ST, Cheng CY, Lee NS, Liang PI, Chuang SS. Follicular Dendritic Cell Sarcoma of the Inflammatory Pseudotumor-like Variant Presenting as a Colonic Polyp. Korean J Pathol. 2014;48:140-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Choe JY, Go H, Jeon YK, Yun JY, Kim YA, Kim HJ, Huh J, Lee H, Shin DH, Kim JE. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: a report of six cases with increased IgG4-positive plasma cells. Pathol Int. 2013;63:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 18. | Granados R, Aramburu JA, Rodríguez JM, Nieto MA. Cytopathology of a primary follicular dendritic cell sarcoma of the liver of the inflammatory pseudotumor-like type. Diagn Cytopathol. 2008;36:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Li XQ, Cheuk W, Lam PW, Wang Z, Loong F, Yeong ML, Browett P, McCall J, Chan JK. Inflammatory pseudotumor-like follicular dendritic cell tumor of liver and spleen: granulomatous and eosinophil-rich variants mimicking inflammatory or infective lesions. Am J Surg Pathol. 2014;38:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Chen Y, Shi H, Li H, Zhen T, Han A. Clinicopathological features of inflammatory pseudotumour-like follicular dendritic cell tumour of the abdomen. Histopathology. 2016;68:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Kitamura Y, Takayama Y, Nishie A, Asayama Y, Ushijima Y, Fujita N, Morita K, Baba S, Kubo Y, Shirabe K, Honda H. Inflammatory Pseudotumor-like Follicular Dendritic Cell Tumor of the Spleen: Case Report and Review of the Literature. Magn Reson Med Sci. 2015;14:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Bui PL, Vicens RA, Westin JR, Jensen CT. Multimodality imaging of Epstein-Barr virus-associated inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen: case report and literature review. Clin Imaging. 2015;39:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Vardas K, Manganas D, Papadimitriou G, Kalatzis V, Kyriakopoulos G, Chantziara M, Exarhos D, Drakopoulos S. Splenic inflammatory pseudotumor-like follicular dendritic cell tumor. Case Rep Oncol. 2014;7:410-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Kim HJ, Kim JE, Kang GH, Kim JY, Park K. Inflammatory Pseudotumor-like Follicular Dendritic Cell Tumor of the Spleen with Extensive Histiocytic Granulomas and Necrosis: A Case Report and Literature Review. Korean J Pathol. 2013;47:599-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Horiguchi H, Matsui-Horiguchi M, Sakata H, Ichinose M, Yamamoto T, Fujiwara M, Ohse H. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen. Pathol Int. 2004;54:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |